Abstract
Water management is known to be a key factor on methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O) emissions from paddy soils. A field experiment was conducted to study the effect of continuous irrigation (CI) and intermittent irrigation (II) on these emissions. Methane, CO2, and N2O emissions from a paddy soil were sampled weekly using a semi-static closed chamber and quantified with the photoacoustic technique from May to November 2011 in Amposta (Ebro Delta, NE Spain). Intermittent irrigation of rice paddies significantly stimulated (N2O + N2)–N emission, whereas no substantial N2O emission was observed when the soil was re-wetted after the dry phase. The cumulative emission of (N2O + N2)–N was significantly larger from the II plots (0.73 kg N2O–N ha−1 season–1, P < 0.05) than from the CI plots (−1.40 kg N2O–N ha−1 season−1). Draining prior to harvesting increased N2O emissions. Draining and flooding cycles controlled CO2 emission. The cumulative CO2 emission from II was 8416.35 kg CO2 ha−1 season−1, significantly larger than that from CI (6045.26 kg CO2 ha−1 season−1, P < 0.05). Lower CH4 emission due to water drainage increased CO2 emissions. The soil acted as a sink of CH4 for both types of irrigation. Neither N2O–N nor CH4 emissions were affected by soil temperature. Global warming potential was the highest in II (4738.39 kg CO2 ha−1) and the lowest in CI (3463.41 kg CO2 ha−1). These findings suggest that CI can significantly mitigate the integrative greenhouse effect caused by CH4 and N2O from paddy fields while ensuring the highest rice yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global warming induced by increasing greenhouse gases (GHG) concentration in the atmosphere is a matter of great environmental concern. Methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O) are important long-living GHG, which have attracted considerable attention during the last decades because of their contribution to global warming. The agroecosystem plays a significant role in the global budget of GHG (Hou et al. 2012). Agriculture is responsible for about 50 % of the global anthropogenic CH4, and for 60 % of N2O (IPCC 2007), and can be an important source of trace gases or can act as a major sink. Agricultural CH4 and N2O emissions have increased by nearly 17 % from 1990 to 2005 (IPCC 2007), and agricultural N2O emissions are predicted to increase between 23 and 60 % by 2030 due to increased chemical and manure nitrogen inputs (FAO 2010). Paddy fields are considered to be an important source of anthropogenic CH4, N2O (Hou et al. 2012), and CO2 (Liu et al. 2013). Atmospheric CH4 from rice fields will further increase with the increasing rice harvested area in the years to come (Cai et al. 2007).
Rice is the staple food of nearly 50 % of the world’s population. Rice planting areas account for about 20 % of the world total. In Spain, rice covers about 3 % of the Spanish irrigated area (about 11,0785 ha rice) (http://www.magrama.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/esyrce/, accessed 12 January 2015). Hence, it is interesting to study CH4 and N2O emissions from Spanish paddy fields.
Water management has been recognized as one of the most important practices that affect CH4, CO2, and N2O emission from paddy fields (Xiong et al. 2007; Liu et al. 2010; Hadi et al. 2010; Hou et al. 2012). In Spain, the most typical water management of paddy fields involves continuous flooding to improve rice growth and increase yields. The influence of water management on CH4 and N2O emission from paddy fields under continuous flooding and intermittent irrigation has been well documented in the climatic conditions of China, Japan, and India (Panthak et al. 2003; Zheng et al. 2004; Zheng et al. 2006; Hadi et al. 2010; Hou et al. 2012; Suryavanshi et al. 2013) but not in the Mediterranean climate, especially in the Ebro Delta (Spain). Continuously flooded rice fields have a high potential for CH4 emission, while N2O emissions are negligible. However, Nugroho et al. (1994) reported that intermittent drainage did not affect CH4 emission or, inversely, sometimes resulted in higher CH4 emission than continuously flooded soil in Indonesia. Nitrous oxide emissions during intermittent irrigation depend greatly on whether or not water logging is present in paddy soils (Zou et al. 2005). Intermittent drainage has been proposed as a water management technique to reduce CH4 emission from paddy soils; it is also useful for the removal of hazardous organic components in rice rhizosphere and to increase the availability of some nutrients (Hadi et al. 2010). Intermittent irrigation has been shown to mitigate CH4 emissions compared with continuous flooding (Zou et al. 2009; Liu et al. 2010). Draining a paddy soil prior to harvesting increased N2O emissions (Hadi et al. 2010).
Intermittent drainage can have a strong effect on soil CO2 emissions, increasing them considerably (Miyata et al. 2000; Saito et al. 2005). However, the mechanism of CO2 exchange between rice paddies and the atmosphere is not fully understood (Miyata et al. 2000). For example, using eddy covariance measurements, Miyata et al. (2000) found a significantly larger net CO2 flux from rice paddy soil to atmosphere when the field was drained compared to when it was flooded. These differences in the CO2 flux were mainly due to increased CO2 emissions from the soil surface under drained conditions resulting from the removal of the diffusion barrier caused by floodwater. The existence of floodwater, anaerobic soil, and changes in the micrometeorological environment influence root activity, photosynthesis, and respiration of the rice plant (Liu et al. 2013).
Soil properties (such as soil moisture, soil oxygen status, soil redox potential, and soil temperature) and microbial activity in continuous irrigation (CI) paddy fields are very different from those in intermittent irrigation (II) rice fields, and induce changes in CH4, CO2, and N2O emissions. To our knowledge, very few studies have been made on the quantification of CH4, CO2, and N2O emissions from CI and II paddy fields in Spain. In the present study, the CH4, CO2, and N2O–N and (N2O + N2)–N emissions from paddy fields with two types of water management (CI and II) were measured during one cropping season (from May to November 2011). The aim of this study was to determine the effect of irrigation frequency (continuous irrigation and intermittent irrigation) on the emission of greenhouse gases (CH4, CO2, N2O–N), N2, and the global warming potential (GWP) from a paddy soil at the Ebro Delta (NE Spain) to know the environmentally sound irrigation practice.
Materials and methods
Experimental design
The experiment was conducted in 2011 on experimental plots at the Institute for Food and Agricultural Research and Technology (IRTA), in its Amposta station (40°39′19.02″N, 00°47′01.2″W), Catalonia, Spain. This region has a Mediterranean climate with an average annual temperature of 17 °C and a mean annual precipitation of 550 mm. Soil texture in the experimental site is silty clay loam (3.5 % total sand, 61.7 % total silt, and 34.8 % total clay), which is representative of the soil type in this region. The main (0–20 cm depth) properties of this soil are 2.34 % organic matter, 3 mg \({\text{NO}}_{3}^{ - }{-}{\text{N}}{\text{ }}{\text{kg}^{ -1 }}\), 6 mg \({\text{NH}}_{4}^{ + }{-}{\text{N}}{\text{ }}{\text{kg}}^{ -1 }\), 44 mg total P-Olsen kg−1, 158 mg total K kg−1, and pH 8.1. The experiment involved two irrigation treatments: continuous irrigation (CI) and intermittent irrigation (II). Each treatment had three replicates established in a randomized block design in six plots of an approximate size of 30 m2 (5 × 6 m) each. Irrigation started on 15th March and the field was flooded until 1st June.
In the CI treatment, a water layer of 10 cm was kept until 12th September when the field was drained. Harvesting took place on 20th September. In the II treatment, soil was flooded as in the continuous flooding system (10 cm water layer), but the irrigation was suspended until the water layer disappeared, at that moment it was irrigated again. Ten irrigations were done during the growing season to the II treatment, and each irrigation lasted for approximately 1 day. The final drainage was applied on 12th September and harvesting took place on 16th September. In Spain, rice is permanently flooded for most of the growing season, although short drainage periods may be needed to correct specific crop disorders dependent on herbicide application, etc. Rice (Oryza sativa L.) cultivar Gleva was sown directly on site on 9th May in both treatments (II and CI) at a density of 170 kg ha−1. Fertilizers, herbicides, and pesticides were applied in accordance with local conventional practice. To minimize the impact of weeds on yield, Molinate and Bentazona herbicides combined with cultural practices were used. Puddling before sowing and weeding by hand (until the rice plant has become so tall that cannot be weeded without damage) were performed, especially for the so-called wild rice, red rice or “crodo” as it is called in Italy. Two applications of fungicide (Benzotiazol at a dose of 300 g ha−1) were done on 26th July and on 19th August. Nitrogen fertilizer was applied at a rate of 170 kg N ha−1 as ammonium sulfate (21 % N richness). To improve N utilization, N was applied 33 % on the 6th May, 33 % on the 10th June, and 33 % on the 12th July. Phosphorous and K were applied on 6th May to all the plots, at 57 kg P2O5 ha−1, and 57 kg K2O ha−1.
Sampling and measurements
Gas samples were collected using the closed chamber method at an interval of 7 days throughout the period of rice growth (Peng et al. 2011; Hou et al. 2012). The cylindrical (20-cm diameter and 60-cm high) static chamber was made of polyvinyl chloride (PVC) coated with an epoxy resin and was inserted 18 cm into the soil each sampling day on the 6 plots. This cylinder was closed with a vented screwed lid with a three-way key. Air samples from inside the chamber were taken in duplicate immediately after closing the chamber, and 20 and 40 min later. Samples were taken through a Teflon tube connected to the three-way key and into 100-ml plastic syringes adapted with a valve. Before sampling, air within the chamber was mixed by filling and emptying the syringe six times before withdrawing the sample. After taking the gas sample, the syringes were closed by the valve. After 40 min of sampling, the three-way keys were left open until the sampling with acetylene.
The acetylene (C2H2) inhibition method (Balderston et al. 1976; Yoshinari and Knowles 1976) was used to inhibit the last step of denitrification (N2O–N2). Ten percent (v/v) of the air enclosed in the chamber was replaced by C2H2. After C2H2 was allowed to diffuse into the soil for 20 min, samples were taken as described in the previous paragraph. After 40 min of sampling, the three-way keys were left open until the following sampling and the chambers were removed from the field and cleaned properly with water.
The syringes were transported to the laboratory and the concentrations of N2O, CO2 and CH4 in the sampled air were analyzed using the photoacoustic technique (Innova 1312 Photoacoustic Multigas Monitor). The N2O, CO2, and CH4 emission fluxes were determined from the linear increase of gas concentration at each sampling time (0, 20, and 40 min) during the time of chamber closure. The cumulative emission throughout the study period was calculated by integrating the emission curve through time. During N2O, CO2, and CH4 emission monitoring, soil temperature at a depth of 5 cm was determined by means of a thermometer. In this study, water-filled pore space (WFPS) was calculated according to the following equation (Peng et al. 2011):
where total soil porosity = (1 − soil bulk density)/2.65, with 2.65 g cm−3 as the assumed particle density of the soil (Porta et al. 2008).
Nitrogen gas emission was obtained by subtracting N2O emission without acetylene from N2O emission with acetylene (Ryden et al. 1979), and then the N2O–N/(N2O + N2)–N ratio was calculated.
Since the chambers were not transparent, it cannot be assumed that the CO2 flux was the net flux, as photosynthesis was ignored.
Global warming potential (GWP)
Global warming potential (GWP) is an index defined as the cumulative radiative forcing between the present and some chosen later time ‘‘horizon’’ caused by a unit mass of gas emitted now. In GWP estimation, CO2 is typically taken as the reference gas, and an increase or reduction in emission of CH4 and N2O is converted into ‘‘CO2-equivalents’’ through their GWPs. The GWP for CH4 (based on a 100-year time horizon) is 25, whereas that for N2O is 298, when the GWP value for CO2 is taken as 1 (IPCC 2007). GWP of CH4, N2O, and CO2 emissions was calculated using the following equation (IPCC 2007): GWP = cumulative CO2 emission + cumulative CH4 emission × 25 + cumulative N2O emission × 298.
Statistical analysis
Statistical analyses of data were carried out using the JMP ver. 10 (SAS Institute Inc., Cary, USA). Daily emission fluxes, as well as the estimated seasonal emission data, were checked for normal distribution. A t test was used to examine the statistical significance of the parameter estimates. An analysis of variance (ANOVA) F test partitioned the total variation of the results. A one-way ANOVA was used to test whether the cumulative emissions depended on the water regime.
Results and discussion
The coefficients of variation (CV%) of N2O–N and (N2O + N2)–N ranged from 199 to 477 %. Such high uncertainties are unfortunately common in this kind of study.
Grant and Pattey (2003) obtained coefficients of variation from 30 to 100 %. Our CV was in the same range as that reported by Flessa et al. (1995) and Thornton et al. (1996) (CV > 150 %), but lower than described by Teira-Esmatges et al. (1998). A review on N2O studies with a much greater number of replicates than the one reported here was done by Mosier et al. (1996), revealing that it is not the measurement technique that provides most of the uncertainty in N2O flux values in the literature but rather the diverse combination of physical and biological factors that control gas fluxes.
Nitrous oxide and molecular nitrogen emission
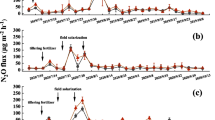
The measured N2O fluxes differed considerably from day to day, probably due to the changes in soil temperature and water content. The measured emission fluxes ranged from −71 to 384 g (N2O + N2)–N ha−1 day−1 in CI, and from −113 to 526 g (N2O + N2)–N ha−1 day−1 in II (Fig. 1), which is broader than many of the ranges reported in the literature (Peng et al. 2011; Hou et al. 2012).
Daily flux of (N2O + N2)–N from the paddy soils under different water management practices through the sampling season. The vertical arrow on day 112 of measurement indicates the harvest of rice in the II treatment, and that on day 116 indicates the harvest of rice in the CI treatment. II intermittent irrigation, CI continuous irrigation. Vertical lines indicate the standard error of the average (n = 3)
Emission from the soil depends on (Teira-Esmatges et al. 1998) (i) the formation of N2O during denitrification and nitrification and its diffusion to the headspace, and (ii) the consumption of N2O through its reduction to N2 during denitrification and the diffusion rate of N2O from the headspace into the soil. When N2O consumption was larger than its emission, the N2O concentration in the headspace decreased resulting in negative fluxes of N2O. Negative fluxes indicate that the soil acted as a sink for N2O.
The evolution of the daily (N2O + N2)–N fluxes from the CI is quite parallel to that from the II treatment (Fig. 1). The (N2O + N2)–N emission fluxes from the II paddy plots were higher than those from the CI plots for most of the rice-growing season. The average daily flux of (N2O + N2)–N in the II fields (178.2 g ha−1 day−1) was 1.5 times higher than in the CI fields (115.18 g ha−1 day−1). The highest peak of (N2O + N2)–N emission flux from the II paddy fields (526.44 g ha−1 day−1, 1.72 times that from the CI paddy fields) was observed on measurement day 91 in the physiological maturity period, which coincides with the day when the soil temperature was the highest (27.0 °C in the CI plots and 27.8 °C in the II plots). Other four peaks of (N2O + N2)–N emission flux from the II fields were observed (Fig. 1). In the CI plots, another three peaks of (N2O + N2)–N emission were observed (Fig. 1). The peaks in II are mainly due to mineral fertilizer application (days 14 and 62 of sampling) and to the high soil water content, while peaks in CI are mainly due to the application of mineral fertilizer (day 62 of sampling) and to draining (days 42, 91 and 118 of sampling).
After fertilization, on measurement days 14 and 48, the (N2O + N2)–N flux increased along with soil temperature, probably because the concentration of \({\text{NH}}_{4}^{ + }\)–N and of \({\text{NO}}_{3}^{ - }\)–N in soil solution increased. Toshiaki et al. (2007) reported that the development of denitrifying bacteria activity along with the rise of soil temperature might account for the high N2O emissions.
Under CI, the (N2O + N2)–N flux was lower than under II. One possible reason for this may be the dissolution of N2O in the flooding water. Toshiaki et al. (2007) reported that the N2O concentration in flood water was as high as between 0.65 and 10.4 µg l−1. He proposed that the (N2O + N2)–N emission can be mitigated considerably by a thin film of flooding water on paddy soils, which is already common practice in the Ebro Delta.
Intermittent irrigation clearly increased the (N2O + N2)–N fluxes from the rice paddy. Smith and Patrick (1983) observed that alternate anaerobic and aerobic conditions considerably increased N2O fluxes relative to continuous anaerobic and aerobic conditions, and that the net N2O flux increased when the duration of both the anaerobic and aerobic periods was increased from 7 to 14 days.
In this experiment, the time between the disappearance of the floodwater layer and reflooding was usually about 1 week. The results are consistent with those reported by Cai et al. (1997) and Toshiaki et al. (2007) who showed that the soil water content associated with maximum N2O emissions was normally close to field capacity. At about this soil water content, either nitrifiers or denitrifiers may be N2O generators. Yagi et al. (1996) showed that water management with very short anaerobic–aerobic cycling induced a very low N2O emission in a Japanese paddy field.
The results of the present study suggest that II of rice paddies significantly stimulated (N2O + N2)–N emission. The cumulative emissions during the sampling period were 25.05 kg (N2O + N2)–N ha−1 season−1 in CI and 34.12 kg (N2O + N2)–N ha−1 season−1 in II (Table 1). The cumulative (N2O + N2)–N emission from the II plots was 1.36 times greater than that from the CI plots. Felber et al. (2012) estimated that the total denitrification loss of nitrogen ((N2O + N2)–N) is in the range of 6–26 kg N ha−1 year−1, albeit with uncertainties close to 100 %.
The measured daily N2O–N emission fluxes cover a shorter range of emissions than the (N2O + N2)–N emissions though broader than many of the ranges reported in the literature (Aulakh et al. 2001; Ma et al. 2012). They ranged from −119.66 to 116.07 g N2O–N ha−1 day−1 in CI, and from −114 to 79 g N2O–N ha−1 day−1 in II (Fig. 2). Ma et al. (2012) and Hadi et al. (2010) also reported negative N2O–N fluxes. In CI, 48 % of the N2O–N flux measurements were negative, and 8.3 % were >100 g of N2O–N ha−1 day−1. In II, 27 % of the N2O–N flux measurements were negative and 6.25 % were >50 g of N2O–N ha−1 day−1. The drain applied before harvest determined increased N2O–N in both treatments, though this was much clearer in II (Fig. 2).
Daily flux of N2O–N from the paddy soils under different water management practices through the sampling season. The vertical arrow on day 112 of measurement indicates the harvest of rice in the II treatment, and that on day 116 indicates the harvest of rice in the CI treatment. II intermittent irrigation, CI continuous irrigation. Vertical lines indicate standard errors of three replications for each treatment
The cumulative (N2O + N2)–N and N2O–N emissions from the studied irrigation types were significantly different. The cumulative N2O–N emissions during the sampling period were 0.73 kg N2O–N ha−1 season−1 from II and −1.40 kg N2O–N ha−1 season−1 from CI (Table 1). The cumulative N2O–N ha−1 emissions with similar management practices found by Hadi et al. (2010) were between −50.3 and −14.8 kg N2O–N ha−1 season−1. Aulakh et al. (2001) reported cumulative emissions between 2 and 36.8 kg (N2O + N2)–N ha−1 season−1.
Ratio of N2O–N:(N2O + N2)–N
The N2O–N:(N2O + N2)–N ratio is an indicator of the extent to which denitrification proceeds to N2. In general, this ratio varied a lot indicating that the production of the two gases reacted differently to changes in conditions and that their production is partly independent. This is in agreement with what Ciarlo et al. (2007) reported.
The total N2O produced in the presence of acetylene may be a good indicator of total denitrification; the production of N2O in the absence of acetylene may not be solely a product of denitrification but may include nitrification and other aerobic processes as well. In the II plots, WFPS ranged between 73 and 93 % (Fig. 3), suggesting complete reduction of N2O–N2.
The cumulative N2O–N:(N2O + N2)–N ratio was 0.055 for CI and 0.021 for II. The low N2O–N:(N2O + N2)–N ratio is probably due to stronger anaerobic conditions created by the presence of a surface water layer, which promoted N2O reduction to N2, as suggested by Xu et al. (2004). In addition, the surface water layer probably limited N2O upward diffusion, as suggested by Yan et al. (2000), and this probably stimulated N2O reduction to N2. Another possible explanation would be the greater sensitivity of the N2O reductase than the other denitrifying enzymes to oxygen (Knowles 1982). Ciarlo et al. (2007) found an average N2O–N:(N2O + N2)–N ratio of 0.051–0.17 when WFPS was between 70 and 120 %. Other researchers have also noted that in continuously wet soil denitrification proceeds rapidly to N2 and little N2O is released (Abdirashid et al. 2003).
It is known that high soil nitrate content inhibits N2O reduction to N2 (Ciarlo et al. 2007). Knowles (1982) explained this phenomenon by stating that nitrate is preferred as an electron acceptor with respect to nitrous oxide. However, neither N2O nor the N2O–N:(N2O + N2)–N ratio was related to soil nitrate contents, probably because of the strong control that moisture exerted on these variables.
Carbon dioxide emission
The daily CO2 flux is shown in Fig. 4. The measured emission fluxes ranged from −5664.0 to 104140.8 g CO2 ha−1 day−1 in CI, and from −1574.4 to 158923.2 g CO2 ha−1 day−1 in II, exhibiting a wide fluctuation during the rice-growing season. The results are consistent with those reported by Li et al. (2004), who found that rice fields may act as a sink for CO2, with net CO2 fluxes of −2191.8 to 6849.3 kg CO2 ha−1 day−1. Liu et al. (2008) found a range of CO2 emission fluxes from 6571.2 to 443760.0 mg CO2 ha−1 day−1. The cumulative CO2 emissions during the sampling period were 8416.3 kg CO2 ha−1 season−1 from II and 6045.2 kg CO2 ha−1 season−1 from CI.
Daily flux of CO2 from the paddy soils under different water management practices through the sampling season. The vertical arrow on day 112 of measurement indicates the harvest of rice in the II treatment, and that on day 116 indicates the harvest of rice in the CI treatment. II intermittent irrigation, CI continuous irrigation. Vertical lines indicate standard errors of three replications for each treatment
The CO2 fluxes during the rice seedling stage ranged from 5241.6 to 18403.2 g CO2 ha−1 day−1 in CI, and from 17841.3 to 19012.8 g CO2 ha−1 day−1 in II. The first drainage in II plots took place on 1st June (day 6 of sampling), until which every field had remained flooded. In both treatments, fluxes of CO2 increased gradually until the mid-seedling stage when two peaks were clearly observed (on day 21). In II plots, the peak appears due to draining and mineral fertilization (ammonium sulfate), while in the CI plots the peak appears due only to the application of ammonium sulfate. In the first part of the tillering stage, two other peaks were observed (Fig. 4), associated also to fertilization (II and CI) and drainage (II).
Fertilization has shown contradictory effects on soil CO2 flux: enhancement (Fisk and Fahey, 2001; Iqbal et al. 2009; Bhattacharyya et al. 2012), or no effect (Lee et al. 2007). Fertilizer application enhances biomass production and C input. Many experiments have shown a positive effect of N additions on soil organic carbon content, a consequence of the higher biomass generated which, in turn, returns the C in the form of CO2 to the atmosphere (Iqbal et al. 2009). The results of this experiment are in agreement with those of Xiao et al. (2005) who reported an increased soil CO2 flux in response to N fertilization from rice paddies in suburban Shanghai, China. The higher CO2 flux as a response to fertilization can be interpreted in two ways. One interpretation is that the microbial use of C increases when applying nitrogen fertilizer (Fisk and Fahey 2001). Another is that microbial biomass assimilates carbon less efficiently and so respires a greater proportion of C when applying nitrogen fertilizers (Iqbal et al. 2009).
In II plots, the soil CO2 fluxes increased immediately after flooding and exceeded pre-flooding values by two-thirds. This increase was abrupt (Fig. 4). Replacement of soil air by water must have caused an enriched CO2 pulse. Subsequently, the CO2 flux rapidly decreased after the water pulse. In the following days, CO2 remained at minimum levels (about 2875.2 g CO2 ha−1 day−1) during flooding. As standing water declined and eventually disappeared, the CO2 fluxes gradually increased and finally reached maximum levels (about 158923.2 g CO2 ha−1 day−1). This indicates that draining and flooding cycles play vital roles in controlling CO2 emissions in paddy soils.
Average soil CO2 daily flux under flooded conditions was 38651.66 g CO2 ha−1 day−1 whereas under drained conditions it was 6382.16 g CO2 ha−1 day−1. It is likely that floodwater decreased topsoil diffusivity and may have decreased soil CO2 fluxes (Maier et al. 2010). Reduction of oxidizing activity under anoxic conditions may be another reason for low soil CO2 fluxes during the flooding period (Kogel-Knabner et al. 2010). Miyata et al. (2000), Cai et al. (2003), and Liu et al. (2013) found that that the water content of paddy soils had a strong effect not only on CH4 emissions but also on CO2 emissions. Lower CH4 emissions due to water drainage may increase CO2 emission. However, during the submerged period of paddy rice cultivation, CO2 production in the soil is severely restricted. This effect can be explained with two basic mechanisms (Liu et al. 2013). Firstly, when flooding biological activity reduction under anoxic conditions slows down rather than completely inhibits CO2 production. Secondly, when flooding a field for subsequent rice cultivation, water replaces the gaseous phase in the soil pores. Since the CO2 diffusion rate in water is four orders of magnitude lower than that in air, part of the produced CO2 is stored in the soil. Hence, the soil CO2 fluxes can be dramatically reduced by flooding during rice cultivation (Miyata et al. 2000; Campbell et al. 2001; Saito et al. 2005). Results from the present study provide indirect support for this conclusion, since the soil CO2 flux rates under flooded conditions were significantly lower than those observed under drained conditions.
The soil CO2 fluxes from both treatments were generally low during the post-harvest period and reached their maximum negative values at the end of the sampling period (Fig. 4). Low or negative fluxes of CO2 in the post-harvest period coincide with the period of drainage and a low temperature. This indicates that draining (when included as an integral part of the irrigation treatment) and temperature play vital roles in controlling CO2 emissions in a paddy soil.
Methane emission
The daily fluxes of CH4 emission from paddy soils under continuous and intermittent irrigation are shown in Fig. 5. The measured emission fluxes ranged from −4810.93 to 8041.06 g CH4 ha−1 day−1 in CI, and from −5082.06 to 3285.86 g CH4 ha−1 day−1 in II, exhibiting a wide seasonal fluctuation during the rice-growing season. The negative fluxes indicate that the soil acts as a net sink. Negative fluxes have also been found by Muhr et al. (2008), Yao et al. (2012), and Feike et al. (2013).
Daily flux of CH4 from the paddy soils under different water management practices through the sampling season. The vertical arrow on day 112 of measurement indicates the harvest of rice in the II treatment, and that on day 116 indicates the harvest of rice in the CI treatment. II intermittent irrigation, CI continuous irrigation. Vertical lines indicate standard errors of three replications for each treatment
The average daily flux of CH4 in the II fields was −1063.87 and −63.05 g ha−1 day−1 in the CI fields. The first CH4 efflux peak in continuous flooding (6432.6 g CH4 ha−1 day−1) was very high and was observed at the end of the vegetative phase (day 42 of sampling). In the CI, a second CH4 efflux peak (8041.06 g CH4 ha−1 day−1) during the reproductive phase (day 69 of sampling) was observed. Near flowering, high CH4 efflux probably occurs due to large root exudation serving as a source of carbon (C) (Wassmann and Aulakh 2000). In addition, at this stage, aerenchymas are fully mature throughout the entire plant, working as continuous channels transporting CH4 into the atmosphere (Wassmann and Aulakh 2000). The decrease in CH4 flux during maturation and until harvest is due to the adverse conditions for CH4 production caused by the end of irrigation and plant senescence, when labile organic C compounds are no longer released by roots (Cai et al. 1997). In the post-harvest period, the daily fluxes of CH4 emissions were very low. Water management in the field is one of the determinants for CH4 production in paddy soils, because methanogenesis takes place under strict anaerobic reducing conditions. Methane emission under CI was significantly higher than under II. The results of this study are consistent with the results obtained by Moterle et al. (2013) and Suryavanshi et al. (2013), who observed that there was a pronounced difference in CH4 production between paddy soils under intermittent irrigation and continuous flooding. The relationship between the period of drainage and the change of CH4 flux is important to evaluate how long the fields should be drained to mitigate CH4 emission (Yagi et al. 1996). Draining lasting at least 10 days was needed to reduce CH4 flux appreciably in the field studied by Yagi et al. (1996). This is probably due to the fact that longer drainage allows oxygen to diffuse into deeper layers of the soil column, in which the redox components are converted from their reduced form to their oxidized form (Fe2+ to Fe3+, Mn2+ to Mn4+, \({\text{NH}}_{4}^{ +}\) to \({\text{NO}}_{3}^{ - }\)), as shown by Patrick and Jugsujinda (1992). Once the redox components in soil are converted to their oxidized form, it takes a certain period to reduce these oxidized components and to decrease soil Eh to a level suitable for CH4 production after the field is reflooded.
Although some authors such as Dong et al. (2011) argue that the already high N applications to paddy fields will have to increase, because this is the most limiting factor in rice productivity, this is not the case in the Ebro Delta, as fertilizer doses are already well optimized. Low emissions of CH4 in the two treatments may be due to the use of ammonium sulfate and soil salinity. Linquist et al. (2012) summarized that sulfate can reduce overall CH4 emissions by both suppressing methanogenesis as well as contributing to anaerobic CH4 oxidation. Nitrogen fertilization may directly or indirectly affect the processes involved in the CH4 budget of rice paddies, i.e., the production, oxidation, and transport of CH4. However, studies investigating N fertilizer effects on these processes have yielded contradictory results. For example, after fertilization with urea or \(( {\text{NH}}_{4}^{ + } )_{2} {\text{SO}}_{4}\), lower CH4 emissions were detected and this was attributed to the direct inhibition of methanogenesis by these fertilizers (Lindau et al. 1990; Cai et al. 1997; Ma et al. 2007). However, higher CH4 emissions were also observed from paddy fields after applications of ammonium-based non-sulfate fertilizers (e.g., urea, \(\left( {{\text{NH}}_{4}^{ + } } \right)_{ 2} {\text{HPO}}_{ 4}\), which may increase plant growth and carbon supply and thus provide more methanogenic substrates and enhance the efficiency of CH4 transport to the atmosphere (Singh et al. 1996; Schimel, 2000; Zheng et al. 2006). Dan et al. (2001) and Cai et al. (2007) reported no difference in CH4 emissions between N fertilized and unfertilized rice paddies. These conflicting findings regarding CH4 emissions as affected by N fertilizer underscore the need for more research, especially for non-sulfate, non-nitrate N fertilizers, because CH4 production is generally inhibited by sulfate, as described in previous studies (Schütz et al. 1989; Minami 1995; Scheid et al. 2003), and also because nitrate-based fertilizers are not recommended for use in paddy rice production in order to avoid intensive N2 loss by denitrification. The above contrasting effects of N fertilizer on CH4 emissions from paddy fields indicate that soil N availability interacts with other site-specific factors when controlling CH4 production processes.
The negative cumulative emissions of CH4 (soil acts as a sink) in both treatments may be due to soil salinity and to a high sulfate \(( {\text{SO}}_{4}^{2 - } )\) content in irrigation water. In this study, the soil salinity was 4.65 dS m−1 (measured in the soil saturated paste extract). In the study area (Ebro Delta) groundwater is highly salty, sometimes more than sea water. Over extensive areas the groundwater electrical conductivity at 5 m depth varies from 16 to 60 dS m−1 with maximum values over 100 dS m−1 (Casanova 1998). It is possible that salinity caused a reduction in the total microbial activity, thereby reducing CH4 production (Pattanaik et al. 2000). Biswas et al. (2006) have studied CH4 emission from the saline rice fields of Sunderban mangroves of the Indian East coast and reported a significant reduction of CH4 emission from the rice fields reclaimed from mangrove swamp compared to upland rice fields and mangrove forest area. In the present study, the high concentrations of sulfate in the irrigation water (about 150 ppm \({\text{SO}}_{4}^{2 - }\), Casanova 1998) may lead to the suppression of CH4 emission. In natural systems, Pennock et al. (2010) found that annual CH4 emissions from a freshwater wetland declined when the concentration of sulfate in the water increased. Segers (1998) summarized that sulfate can reduce overall CH4 emissions by both suppressing methanogenesis as well as contributing to anaerobic CH4 oxidation. Three possible mechanisms as to how sulfate (and other electron acceptors) could suppress methanogenesis were proposed. First, the reduction of electron acceptors could reduce substrate concentrations to a value that is too low for methanogenesis. Second, the presence of electron acceptors could result in a redox potential that is too high for methanogenesis. Third, electron acceptors could be toxic for methanogens. In a supra-optimal concentration of sulfate, this element could possibly decrease CH4 emission, which is presumably due to saturation of the relevant enzyme surfaces, competition for electrons between methanogens and sulfate reducers, and the development of toxicity (Banik et al. 1995). So, the soil salinity together with a high sulfate concentration in the irrigation water could inhibit CH4 emission.
Global warming potential
The integrative GWP of CO2, CH4, and N2O on a 100-year horizon for the CI treatment was 3463.41 kg CO2-eq ha−1, which was 26.9 % lower than that for the II paddy fields. The soil acted as a sink for N2O which resulted in the reduction of the GWP for the CI treatment. In addition, the mean rice yield of the CI paddy fields was 9572.23 kg ha−1, which was higher than that of II paddies by 34.2 % and the difference was significant (P > 0.05) (Table 1). These results suggest that CI can significantly mitigate the integrative greenhouse effect caused by CH4 and N2O from paddy fields while ensuring the highest rice yield.
Conclusions
From this study, it can be concluded that (1) intermittent irrigation (II) led to significantly higher (N2O + N2)–N (1.36 times greater) and CO2 emissions than continuous irrigation (CI); (2) draining prior to harvesting increased N2O–N emissions; (3) draining and flooding cycles controlled CO2 emissions; (4) lower CH4 emissions due to water drainage (II) may increase CO2 and (N2O + N2)–N emission, and (5) the soil acted as a CH4 sink for both types of irrigation.
The integrative GWP of CO2, CH4 and N2O on a 100-year horizon decreased by 34.2 % in the CI paddy fields compared with the II fields. These results suggest that CI can significantly mitigate the integrative greenhouse effect caused by CH4 and N2O from paddy fields while ensuring the highest rice yield.
The present study makes it clear how flooding and drainage affect the exchanges of (N2O + N2)–N, CO2 and CH4 from rice paddies in the short term. Further measurements throughout rice cultivation to assess the long-term effect of an intermittent drainage practice on the exchange of these gases from rice paddies are needed.
References
Abdirashid AE, Madramootoo C, Hammel C, Liu A (2003) Denitrification and nitrous oxide to nitrous oxide plus dinitrogen ratios in the soil profile under three tillage systems. Biol Fertil Soils 38:340–348
Aulakh MS, Khera TS, Doran JW, Bronson KF (2001) Denitrification, N2O and CO2 fluxes in rice-wheat cropping systems affected by crop residues, fertilizer N and legume green manure. Biol Fertil Soils 34:375–389
Balderston WL, Sherr B, Payne WJ (1976) Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol 31:504–508
Banik A, Sen M, Sen SP (1995) Methane emission from inundated saline paddy field of West Bengal. Indian J Radio Space Phys 24:64–68
Bhattacharyya P, Roy KS, Neogi S, Adhya TK, Rao KS, Manna MC (2012) Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil Tillage Res 124:119–130
Biswas H, Mukhopdhayay SK, De TK, Sen S, Jana TK (2006) Methane emission from wetland rice fields in Sagar Island, Ne Cost of Bay of Bengal India. Int J Agric Res 1:76–84
Cai Z, Xing G, Yan X, Xu H, Tsuruta H, Yagi K, Minami K (1997) Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilizers and water management. Plant Soil 196:7–14
Cai ZC, Tsuruta H, Gao M, Xu H, Wei CF (2003) Options for mitigating methane emission from a permanently flooded rice field. Glob Change Biol 9:37–45
Cai ZC, Shan YH, Xu H (2007) Effects of nitrogen fertilization on CH4 emissions from rice fields. Soil Sci Plant Nutr 53:353–361
Campbell CS, Heilman JL, McInnes KJ, Wilson LT, Medley JC (2001) Diel and seasonal variation in CO2 flux of irrigated rice. Agric For Meteorol 108:15–27
Casanova D (1998) Quantifying the effects of land conditions on rice growth: a case study in the Ebro Delta (Spain) using remote sensing. Thesis Landbouw Universiteit Wageningen. Gràfiques Pacific, Barcelona.
Ciarlo E, Conti M, Bartoloni N, Rubio G (2007) The effect of moisture on nitrous oxide emissions from soil and the N2O/(N2O + N2) ratio under laboratory conditions. Biol Fertil Soils 43:675–681
Dan JG, Kruger M, Frenzel P, Conrad R (2001) Effect of late season urea fertilization on methane emission from a rice field in Italy. Agric Ecosyst Environ 83:191–199
Dong H, Yao Z, Mei B, Xie B, Wang R, Deng J, Cui F, Zhu J (2011) Effect of ammonium-based, non-sulfate fertilizers on CH4 emissions from a paddy field with a typical Chinese water management regime. Atmos Environ 45:1095–1101
FAO (2010) FAO STATS. Food and Agriculture Organization, Rome, Italy. http://www.faostat.fao.org/default.aspx. Accessed July 2013
Feike AD, Morgan JA, Follett RF, Lecain DR (2013) Climate change reduces the net sink of CH4 and N2O in a semiarid grassland. Glob Change Biol 19:1816–1826
Felber R, Conen F, Flechard CR, Neftel A (2012) The acetylene inhibition technique to determine total denitrification (N2 + N2O) losses from soil samples: potentials and limitations. Biogeosci Discuss 9:2851–2882. doi:10.5194/bgd-9-2851
Fisk MC, Fahey TJ (2001) Microbial biomass and nitrogen cycling responses to fertilization and litter removal in young northern hardwood forests. Biogeochemistry 53:201–223
Flessa H, Dorsch P, Beese F (1995) Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany. J Geophys Res 100:23115–23124
Grant RF, Pattey E (2003) Modeling variability in N2O emissions from fertilized agricultural fields. Soil Biol Biochem 35:225–243
Hadi A, Inubushi K, Yagi K (2010) Effect of water management on greenhouse gas emissions and microbial properties of paddy soils in Japan and Indonesia. Paddy Water Environ 8:319–324
Hou H, Peng S, Xu J, Yang S, Mao Z (2012) Seasonal variations of CH4 and N2O emissions in response to water management of paddy fields located in Southeast China. Chemosphere 89:889–892
IPCC (2007) Agriculture. In: Metz B, Davidson OR, Bosch PR (eds) Climate change 2007: mitigation, contribution of working group iii to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Iqbal J, Hu R, Lin S, Hatano R, Feng M, Lu L, Ahamadou B, Du L (2009) CO2 emission in a subtropical red paddy soil (Ultisol) as affected by straw and N-fertilizer applications: A case study in Southern China. Agric Ecosyst Environ 131:292–302
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70
Kogel-Knabner I, Amelung W, Cao ZH, Fiedler S, Frenzel P, Frenzel P, Jahn R, Kalbitz K, Kölbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14
Lee DK, Doolittle JJ, Owens VN (2007) Soil carbon dioxide fluxes in established switch grass land managed for biomass production. Soil Biol Biochem 39:178–186
Li C, Mosier A, Wassmann R, Cai Z, Zheng X, Huang Y, Tsuruta H, Boonjawat J, Lantin R (2004) Modeling greenhouse gas emissions from rice-based production systems: sensitivity and upscaling. Glob Biogeochem Cycles 18:GB1043. doi:10.1029/2003GB002045
Lindau CW, Delaune RD, Patrik WH, Bollich PK (1990) Fertilizer effects on dinitrogen, nitrous oxide, and methane emissions from lowland rice. Soil Sci Soc Am J 54:1789–1794
Linquist BC, Adviento-Borbe MA, Pitelkow CM, van Kessel C, Jan van Groenigen K (2012) Fertilizer management practices and greenhouse gas emissions from rice systems: a quantitative review and analysis. Field Crops Res 135:10–21
Liu H, Zhao P, Lu P, Wang YS, Lin YB, Rao XQ (2008) Greenhouse gas fluxes from soils of different land-use types in a hilly area of South China Agriculture. Ecosyst Environ 124:125–135
Liu SW, Qin YM, Zou JW, Liu QH (2010) Effects of water regime during rice growing season on annual direct N2O emission in a paddy rice–winter wheat rotation system in southeast China. Sci Total Environ 408:906–913
Liu Y, Wan K, Tao Y, LiZ ZG, LiS CF (2013) Carbon dioxide flux from rice paddy soils in Central China: effects of intermittent flooding and draining cycles. PLoS One 8(2):e56562. doi:10.1371/journal.pone.0056562
Ma J, Li XL, Xu H, Han Y, Cai ZC, Yagi K (2007) Effects of nitrogen fertilizer and wheat straw application on CH4 and N2O emissions from a paddy rice field. Aust J Soil Res 45:359–367
Ma Y, Wang J, Zhou W, Yan X, Xiong Z (2012) Greenhouse gas emissions during the seedling stage of rice agricultural as affected by cultivar type and crop density. Biol Fertil Soils. doi:10.1007/s00374-011-0656-z
Maier M, Schack-Kirchner H, Hildebrand EE, Holst J (2010) Pore-space CO2 dynamics in a deep, well-aerated soil. Eur J Soil Sci 61:877–887
Minami K (1995) The effect of nitrogen fertilizer use and other practices on methane emission from flooded rice. Fertil Res 40:71–84
Miyata A, Leuning R, Denmead OT, Kim J, Harazono Y (2000) Carbon dioxide and methane fluxes from an intermittently flooded paddy field. Agric For Meteorol 102:287–303
Mosier AR, Duxbury JN, Freney JR, Heinemeyer O, Minami K (1996) Nitrous oxide emission from agricultural fields: assessment measurement and mitigation. Plant Soil 181:95–108
Moterle DF, Souza da Silva L, Moro VJ, Bayer C, Zschornack T, Avila LA, Cas Bundt A (2013) Methane efflux in rice paddy field under different irrigation managements. R Bras Ci Solo 37:431–437
Muhr J, Goldberg SD, Borken W, Gebauer G (2008) Repeated drying–rewetting cycles and their effects on the emission of CO2, N2O, NO, and CH4 in a forest soil. Plant Nutr Soil Sci 171:719–728
Nugroho SG, Lumbanraja J, Suprapto H, Ardjasa Sunyoto WS, Haraguchi H, Kimura M (1994) Effect of intermittent irrigation on methane emission from and Indonesian paddy field. Soil Sci Plant Nutr 42:609–615
Panthak H, Prasad S, Bhatia A, Singh S, Kumar S, Singh J, Jain MC (2003) Methane emission from rice-wheat cropping system in the Indo-Gangetic plain in relation to irrigation, farmyard manure and dicyandiamide application. Agric Ecosyst Environ 97(3009):316
Patrick WH Jr, Jugsujinda A (1992) Sequential reduction and oxidation of inorganic nitrogen, manganese, and iron in flooded soil. Soil Sci Soc Am J 56:1071–1073
Pattanaik P, Mishra SR, Bharati K, Mohanty SR, Sethunathan N, Adhya TK (2000) Influence of salinity on methanogenesis and associated microflora in tropical rice soils. Microbiol Res 155:215–220
Peng S, Hou H, Xu J, Mao Z, Abudu S, Luo Y (2011) Nitrous oxide emissions from paddy fields under different water managements in southeast China. Paddy Water Environ 9:403–411
Pennock D, Yates T, Bedard-Haughn A, Phipps K, Farrell R, McDougal R (2010) Landscape controls on N2O and CH4 emissions from freshwater mineral soil wetlands of the Canadian prairie pothole region. Geoderma 155:308–319
Porta J, Lopez-Acevedo M, Poch RM (2008) Introduccion a la Edafologia uso y protección del suelo. Ediciones Mundi-Prensa, Madrid-Barcelona-México
Ryden JC, Lund J, Focht DD (1979) Direct measurement of denitrification loss from soils: I. Laboratory evolution of acetylene inhibition of nitrous oxide reduction. Soil Sci Soc Am J 43:104–110
Saito M, Miyata A, Nagai H, Yamada T (2005) Seasonal variation of carbon dioxide exchange in rice paddy field in Japan. Agric For Meteorol 135:93–109
Scheid D, Stubner S, Conrad R (2003) Effects of nitrate-and sulfate-amendment on the methanogenic populations in rice root incubations. FEMS Microbiol Ecol 43:309–315
Schimel J (2000) Rice, microbes and methane. Nature 403:375–377
Schütz H, Holzapfel PA, Conrad R, Rennenberg H, Seiler W (1989) A 3-year continuous record on the influence of daytime, season and fertilizer treatment on methane emission rates from an Italian rice paddy. J Geophys Res 94:16405–16416
Segers R (1998) Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry 41:23–51
Singh JS, Singh Smita Raghubanshi AS, Singh Saranath Kashyap AK (1996) Methane flux from rice/wheat agroecosystem as affected by crop phenology, fertilization and water level. Plant Soil 183:323–327
Smith CJ, Patrick WH Jr (1983) Nitrous oxide emission as affected by alternate anaerobic and aerobic conditions from soil suspensions enriched with (NH4 +)2SO4. Soil Biol Biochem 15:693–696
Suryavanshi P, Singh YV, Prasanna R, Bhatia A, Shivay YS (2013) Pattern of methane emission and water productivity under different methods of rice crop establishment. Paddy Water Environ 11:321–329
Teira-Esmatges MR, Van Cleemput O, Porta Casanellas J (1998) Fluxes of nitrous oxide and molecular nitrogen from irrigated soils of Catalonia (Spain). J Environ Qual 27:687–697
Thornton FC, Bock BR, Tyler DD (1996) Soil emissions of nitric oxide and nitrous oxide from injected anhydrous ammonium and urea. J Environ Qual 25:1378–1384
Toshiaki I, Sanjit KD, Kharbuja RG (2007) Nitrous oxide emission measurement with acetylene inhibition method in paddy fields under flood conditions. Paddy Water Environ 5:83–91
Wassmann R, Aulakh MS (2000) The role of rice plants in regulating mechanisms of methane emissions. Biol Fertil Soils 31:20–29
Xiao Y, Xie G, Lu C, Ding X, Lu Y (2005) The impact of urea amendments on atmospheric gas regulation services in rice paddy ecosystems and their valuation. Acta Phytoecol Sin 29:577–583
Xiong ZQ, Xing GX, Zhu ZL (2007) Nitrous oxide and methane emissions as affected by water, soil and nitrogen. Pedosphere 17:146–155
Xu YC, Shen QR, Li ML, Dittert K, Sattelmacher B (2004) Effect of soil water status and mulching on N2O and CH4 emission from lowland rice field in China. Biol Fertil Soils 39:215–217
Yagi K, Haruo T, Kanda K, Minami K (1996) Effect of water management on methane emission from a Japanese rice paddy field: automated methane monitoring. Global Biogeochem Cycles 10:255–267
Yan X, Du L, Shi S, Xing G (2000) Nitrous oxide emission from wetland rice soil as affected by the application of controlled availability fertilizers and mid-season aeration. Biol Fertil Soils 32:60–66
Yao Z, Zheng X, Dong H, Wang R, Mei B, Zhu J (2012) A 3-year record of N2O and CH4 emissions from a sandy loam paddy during rice seasons as affected by different nitrogen application rates. Agric Ecosyst Environ 152:1–9
Yoshinari T, Knowles R (1976) Acetylene inhibition and nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun 69:705–710
Zheng XH, Han SH, Huang Y, Wang YS, Wang MX (2004) Re-quantifying the emission factors based on field measurements and estimating the direct N2O emission from Chinese croplands. Glob Biogeochem Cycles 18:1–19
Zheng XH, Zhou ZZ, Wang YS, Zhu JG, Wang YL, Yue J, Shi Y, Kobayashi K, Inubushi K, Huang Y, Han S, Xu Z, Xie B, Butterbach-Bahl K, Yang L (2006) Nitrogen-regulated effects of free-air CO2 enrichment on methane emissions from paddy rice fields. Glob Change Biol 12:1717–1732
Zou J, Huang Y, Jiang J, Zheng X, Sass RL (2005) A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: effects of water regime, crop residue, and fertilizer application. Glob Biogeochem Cycles 19:GB2021. doi:10.1029/2004GB002401
Zou JW, Liu SW, Qin YM, Pan GX, Zhu DW (2009) Sewage irrigation increased methane and nitrous oxide emissions from rice paddies in southeast China. Agric Ecosyst Environ 129:516–522
Acknowledgments
We would like to thank Silvia Porras (University of Lleida) for her assistance during gas sampling as well as the owner of the results, Huntsman International LLC, who gave us permission to use these data for publication. The measurements were performed in the framework of the IRTA-UdL contract “Quantification of ammonia emissions and greenhouse gases (GHGs: CO2, CH4 and N2O) on rice”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maris, S.C., Teira-Esmatges, M.R. & Català, M.M. Influence of irrigation frequency on greenhouse gases emission from a paddy soil. Paddy Water Environ 14, 199–210 (2016). https://doi.org/10.1007/s10333-015-0490-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10333-015-0490-2