Abstract
Callitrichids are small Neotropical primates and, due to their cooperative breeding system, infants are of particular interest in research on social dynamics. Although a few studies have investigated the role of helpers in this type of system, there is still a lack of research in field studies seeking to determine whether there is a relationship between the number of helpers (adults) in a social group and the motor development of infants. With that in mind, four groups of wild marmosets (Callithrix jacchus) were observed and the motor behaviors of 1 to 4 month-old infants were recorded. To investigate the influence of the adult:infant ratio on motor diversity, used as an indicator of motor development, we ran a GLMM with a Gaussian distribution and found that: (i) in groups with fewer adults, 2-month-old infants show earlier motor diversity; (ii) motor diversity increases with age regardless of the ratio of adult males per infant; (iii) in groups with more adult females per infant, the motor diversity of 2-month-old infants is significantly lower compared to 3-month-old infants. Although adult callitrichid males play an important role in the care of their offspring, the presence of females appears to be a key factor in motor development at this early stage in the study groups. In a cooperative breeding system, the lack of helpers seems to drive the development of independence in infants, resulting in earlier development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Living in social groups emerged as a key evolutionary response to survival in most primates (Mitani et al. 2012). While social-ecological models aim to identify the advantages and disadvantages of living in a social group with ecological and social factors as determinants (Terborgh and Janson 1996; De la Fuente et al. 2019), only a few investigate the possible effect of group size (Berman et al. 1997; Dunbar et al. 2018) or composition (Lehmann et al. 2007; Jablonski 2021; Markham and Gesquiere 2017) on infants’ development (see also Hinde and Spencer-Booth 1968; Berman et al. 1997).
Callitrichids are small Neotropical primates that exhibit a precocial state in the level of development at birth, that is, they are more developed than newborn infants of altricial species (van Schaik and Isler 2012; see also Isler and van Schaik 2012; Schiel et al. 2010). Despite being considered “precocial”, infants are dependent on adults for a prolonged period, during which motor and cognitive development occur (Schiel et al. 2010; Whiten and Waal 2018). In this respect, these species are of particular interest in research on social dynamics, especially when focused on the development and care of infants (Rapaport 2011; Huang et al. 2020; Saito et al. 2011). Social groups consist of up to ~ 15 individuals (Malukiewicz et al. 2021; Schiel and Souto 2017), which show intense cooperative care of offspring by reproducing and non-reproducing members (the latter are hereafter referred to as “helpers”) (Ford et al. (2009); Rapaport 2011; Barbosa and Silva Mota 2013; Digby et al. 2011). The cooperative care of the offspring is probably due to the peculiar reproductive biology of these animals, which involves high costs such as (i) the birth of twins twice a year; (ii) a high maternal:neonatal weight ratio; and (iii) a postpartum estrus (Tardif et al. 2003, 2008). Thus, it is believed that helpers came to play a key role in the reproductive success of these small primates (Santos and Martins 2000; Rapaport 2011). In their study on captive Callithrix jacchus, Rothe et al. (1993) found that the greater the number of individuals in a group, the greater the benefit of the breeding pair in relieving the burden of caring for the infants. Furthermore, in a field study on C. aurita, Santos and Martins (2000) suggested that a greater number of helpers would be associated with slower development of the infants, presumably due to the easier access to food provided by the adults. Especially when it comes to the effect of the helpers’ sex, males tend to carry the infants more often than females (Yamamoto and Box 1997), and a greater number of male helpers in a group seems to be associated with a higher survival rate of the infants (Koenig 1995; Garber 1997).
Although there have been studies attempting to understand the role of helpers (e.g., Tardif et al. 1995; Rapaport and Brown 2008), these usually focus on the animals that carry the infants (including the sex of the helper) and/or provide the most resources (Santos and Martins 2000; Yamamoto et al. 2008; Rapaport 2011). Moreover, a significant amount of data comes from studies on captive animals, which may represent a limiting factor considering the diverse environmental conditions that free-ranging animals are exposed to (e.g., Hinde and Spencer-Booth, 1968; Santos and Martins 2000; Stoinski et al. 2003). Thus, field studies investigating the relationship between group composition and the associated motor development in infants are still limited, and little is known about motor development in young primates (see Young and Shapiro 2018).
Common marmosets (Callithrix jacchus) stand out as a promising model for studies on the effects of social interactions (De la Fuente et al. 2019, 2021), as they are characterized by a cooperative breeding system (Schiel and Souto 2017), associated with a sensitive period of infants’ motor and cognitive development (Schiel and Huber 2006; Schiel et al. 2010; Wang et al. 2014; Young and Shapiro 2018). Factoring in greater ease in obtaining care from adults, we expect that a larger number of adults in the group would lead to delayed motor development in infants (prediction i). Moreover, given that males carry the infants more often, we expect that in groups with more males the infants would show a delayed motor development (prediction ii); and that, on the other hand, the number of females in a group would not affect infants’ motor development (prediction iii).
Methods
Study area
Sampling was carried out in a residential condominium within the Aldeia-Beberibe Environmental Protection Area (31.634 ha; CPRH 2022) featuring fragments of primary and secondary Atlantic Forest, with areas inhabited by humans and areas entirely forested (for a further description of the area, see Souto et al. 2007). The area is in the township of Camaragibe (7º56′97″S, 35º1′23″W), in the state of Pernambuco, northeast Brazil. Several studies have been conducted in the area since 2001 (e.g., Schiel and Huber, 2006; Souto et al. 2007; Schiel et al. 2010; Gunhold et al. 2014).
Subjects
Four groups of wild marmosets totaling 29 individuals were observed (Table 1). The members of each group were identified by sex, size, and their natural markings, such as the size and color of ear tufts, facial scars, hair color, and physical impairments (Schiel et al. 2006). All animals were habituated to the presence of the researchers (Schiel and Huber 2006; Bezerra et al. 2007; Gunhold et al. 2014). Based on the age categories established by Ingram (1977), we classified the animals into adults/sub-adults (≥ 11 months), juveniles (5–10 months), and infants (1–4 months). Table 1 shows the composition of the four observed groups and the individual:infant ratio of these groups (for more details see the data analysis section). During the observations, the composition of the groups remained stable, except in group C, where a new male joined the group immediately after the disappearance of the dominant male. This research study was conducted in compliance with the guidelines for the ethical treatment of animals in behavioral research and teaching (Animal Behaviour 2003; v.65; p 249–255), as well as with the ethical principles for the treatment of non-human primates, established by the American Society of Primatologists.
Procedures
Sampling occurred from October/2001 to April/2002, between 6:00 a.m. and 5:00 p.m., 5 days a week. The observations were carried out using the focal animal method (Altmann 1974; Lehner 1996), with continuous 10-min sessions, for a total of 600 h of observation. All infants were observed for the full 4 months. When an animal was lost from view for a period ≥ 1 min, the session was discarded. All observations were recorded using micro-cassette recorders (Sony M-529 V; Aiwa NFR TP-M330) for later transcription and analysis in Excel spreadsheets.
Importantly, we are using motor diversity as an indicator of motor development, as they are intertwined (Adolph and Robinson 2015; Yamamoto 1993). Thus, we were attentive to the following infants’ behavioral events: prey capture, prey capture attempt, prey stealing, crawling, minor locomotion, major locomotion, traveling, gummivory, weak pounce (with or without prey capture), strong pounce (with or without prey capture), leaf/branch/fruit manipulation with hand/mouth (for the definition of these behaviors see: Schiel and Huber 2006; Schiel et al. 2010; Ngo et al. 2022).
Statistical analysis
First, to measure the motor activities of infants based on the observed behaviors, we calculated a motor behavior index (MDI: Motor Diversity Index), which reflects the motor ability of each infant during each month of age (1 to 4 months). To this end, we used the Shannon Index, combining the different types of behavior and their frequency:
\(H=-\sum_{i=1}^{s}\frac{ni}{N}\) ln \(\frac{ni}{N}\)
ni corresponds to the number of each behavior, N is the total number of sampled behaviors, and ni/N is the relative abundance of each behavior. H reaches its maximum when all behaviors occur at equal frequency. The higher the final value, the greater the diversity.
Before testing prediction (i), we examined whether the MDI value was related to the number of sessions sampled for each infant, given that a greater number of sessions could mean a greater chance of observation/sampling a new motor behavior. To accomplish this, we ran a generalized linear mixed model (GLMM) with a Gaussian distribution (normal data), where the MDI value was the response variable, the number of sessions for each age group of each infant was the predictor variable, and the group identity was the random variable (controls the group effect). The results showed that MDI is not affected by the number of sessions (GLMM: F1,19 = 0.30, p = 0.59). Therefore, the MDI was used as the response variable to test prediction (i).
Next, we compared motor diversity across infants’ age groups (1 to 4 months) to examine among which age groups the MDI differs, and to understand which age groups could be included in our analysis to test how group composition affects motor development in infants. This was done by building a GLMM with Gaussian distribution, where the response variable was the MDI, the predictor variable was age (categorical: 1 to 4 months) and the control variable was the group identity. For this model, we allowed heterogeneous variance among different age groups (following Zuur et al. 2009). We found that motor diversity (MDI) varies with age (GLMM: F1,17 = 72,81, p < 0.0001). Tukey’s post hoc test (pairwise comparison) showed that the MDI differs between month 1 and months 2, 3, and 4 (p < 0.0001, Fig. 1), as well as between months 2 and 3 of age (p = 0.021, Fig. 1). Since 1-month-old infants are carried most of the time and do not yet have a good motor development (Yamamoto 1993), this age group was removed from the analysis. The 4-month age group was also removed because motor diversity at this age seems to be already developed (Schiel et al. 2010). Therefore, to test our hypothesis, we used 2- and 3-month-old age groups, where motor diversity varies significantly.
Since the number of infants and adults varies between groups, we calculated the adult:infant ratio in 2- and 3-month-old infants to find the ratio of adults per infant in each group. We then grouped these values into the categories “more” and “less” adults as our corresponding predictor variable for group composition. The same procedure was used to calculate the adult male:infant ratio and the adult females:infant ratio (see Table 1). This ratio was calculated by accounting for all adult individuals in each group, including the reproducers. We assumed that the number of breeding adults among groups remains constant (with each group having one breeding female and one breeding male), so the variation in group composition differs according to the number of non-breeding individuals (helpers). Since we have no genetic information about the identity of the breeding male, we chose to include all adults to avoid possible identification biases. Juveniles were not included in the analyses since they play a minimal role in infant care (Yamamoto and Box 1997).
Finally, to investigate the effect of adult:infant ratio on motor diversity in 2- and 3-month-old infants, we ran a GLMM with Gaussian distribution where the response variable was the MDI, the predictor variables were infants’ age (category: 2 and 3 months) and adult:infant ratio (category: “more” and “less” adults per infant), and the random variable was once more the group identity. To further investigate whether the adult male and adult female ratio have a different effect on the motor diversity of these infants, we built two GLMMs with Gaussian distribution by replacing only the adult:infant ratio variable of the first model with the adult male:infant ratio variable and the adult female:infant ratio variable, respectively.
The statistical software R, version 4.1.1 (R Core Team 2021), was used for all the analyses. All models (GLMMs) were fitted using the “lme” function of the “nlme” statistical package (Pinheiro et al. 2019). We compared the models (null vs. full models) by sequential analysis of the variance using the Anova function from the R Stats package. To conduct pairwise comparisons (Tukey’s test), we used the lsmeans package (Lenth 2016). Before running the comparisons, we examined the models against the assumptions of normality and homogeneity. When the assumption of homogeneity of variance was not met, heterogeneity of variance was incorporated into the model, since it may provide relevant ecological information. The significance level set for all analyses was p ≤ 0.05.
Results
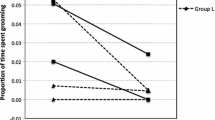
The results show that the interaction between age and the composition of adults in the group (adult:infant ratio) affects infants’ motor diversity (GLMM: F1,8 = 5.49, p = 0.047). More specifically, considering that motor skill is already high by infants with 2 months (see Fig. 1), in groups with fewer adults per infant, motor diversity is similar across the 2- and 3-month age groups, while in groups with more adults per infant, motor diversity of 2-month-old infants is significantly lower than 3-month-old infants (Fig. 2a). This supports our prediction (i) because in groups with fewer adults 2-month-old infants develop earlier than infants in groups with more adults.
When the effect of the adult male:infant ratio on motor diversity was investigated separately on 2- and 3-month-old infants, motor diversity was found to be affected by age only (GLMM: F1,8 = 11.18, p = 0.01), and not by the male:infant ratio or the interaction between the two variables (GLMM: F1,8 = 0.42, p = 0.53). That is to say that infants’ motor diversity increases with age regardless of the ratio of adult males per infant in the group (Fig. 2). When investigating the influence of adult female:infant ratio, we found that it affected infant motor diversity in the same way as the overall adult composition (GLMM: F1,8 = 5.49, p = 0.047). In groups with fewer adult females per infant, motor diversity is similar across the 2- and 3-month-old age groups, while in groups with more adult females per infant, motor diversity of 2-month-old infants is significantly lower than 3-month-old infants (Fig. 2).
Discussion
This study investigated whether group composition affects the motor development (as indicated by motor diversity; Adolph and Robinson 2015) in infants of wild common marmosets, which are primates characterized by a cooperative breeding system. We found that having more adults in the social group delays the motor development of infants, which supports our first prediction. Santos and Martins (2000) suggested that the presence of more helpers in a group could lead to a delay in motor skills in infants, which in turn leads to a delay in the ability to get solid food independently. The same seems to be true in our study. Thus, the lack of helpers would accelerate independence in infants, resulting in earlier development.
On the other hand, in contrast to our prediction, the advanced motor development of infants from larger groups appears to be affected by the ratio of adult females per infant, rather than by the ratio of adult males per infant. Thus, it seems like the presence of more females in a group slows down the motor development of 2-month-old infants. Although adult callitrichid males play an important role in caring (e.g., carrying and sharing food) for their offspring (Santos and Martins 2000; Burkart 2015; Yamamoto 2005; Ziegler et al. 2017), the presence of females seems to have been a key factor in motor development at this early stage in the study groups. While Kostan and Snowdon (2002) observed the adult males of cotton-top tamarins (Saguinus oedipus) to be the attachment figures for infants (especially under stress situations), it should be mentioned that Tardif et al. (1993) found that, although males were the primary carriers, mothers were always preferred as a “contact partner”. Such a preference could be associated with the fact that infants prefer females over males as role models (Schiel and Huber 2006). These two complementary aspects seem to be crucial to explain the longer development time in groups with more female individuals. Our results are comparable to those obtained with rhesus monkeys, in which more prolonged contact with the mother would delay the development of independence in infants when it comes to locomotion on the ground (Maestripieri et al. 2009). Although our study is focused on a species that exhibits cooperative care by group members, the comparison is still useful, since in our case there seems to be a stronger bond with all the females of the group rather than with a specific female, as documented in rhesus monkeys (see also Vochteloo et al. 1993; Maestripieri 2018).
It might be tempting to evaluate the earlier motor fitness of marmoset infants in larger groups as positive and assume it to be true for smaller groups as well. However, caution is needed when evaluating earlier independence as positive or negative without considering some important aspects. For example, it is well known that dependence on caregivers at a stage of biological and/or psychosocial immaturity means protection from starvation and predators (e.g., Fragaszy and Bard 1997). Thus, large groups of marmosets would provide greater protection, in turn, allowing the infant to explore the environment more safely than when in a small group. This possibility to explore more would presumably be a facilitator for the motor development of infants, as observed in our study. It is also worth noting that in humans, except in extreme cases such as abandonment, situations characterized by both greater and lesser dependence have positive aspects for the motor development of children. Thus, the “attachment theory” has shown the positive psychological implications of close and harmonious social relationships, which can produce an increase in the child’s self-confidence, with benefits when it comes to the exploration of the environment (e.g., Holmes 2014; van Londen et al. 2007). However, as previously mentioned, motor skills such as teeth-brushing or bathing alone were facilitated in groups whose mothers needed to go to work in the fields (Wulan and Kurniawati 2020). Therefore, as long as there is no premature independence or excessive care (something hard to imagine in non-human primates in the wild), both strategies would have survival benefits, depending on ecological, biological, and/or psychosocial aspects.
Overall, our results showed that more helpers lead to delayed development, and females seem to have a greater effect on this process. While most studies address the identity and/or the role of the helper, little is known about how these variables impact the motor development of infants. In addition, environmental and mental factors, such as personality (see Šlipogor et al. 2021) may affect infant care. Thus, while data is still scarce and more studies are needed to better understand motor development in primate infants, we hope our study will encourage further investigations on this fascinating subject.
References
Adolph, KE, Robinson SR (2015). Motor development. In: Lerner RM, Liben LS, Mueller U (ed.) Handbook of child psychology and developmental science. Vol 2, Cognitive Processes, 7th edition. Hoboken, NJ: John Wiley & Sons, Inc. p. 113 – 157.
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267. https://doi.org/10.1163/156853974x00534
Animal Behaviour (2003) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 62:249–255. https://doi.org/10.1016/j.anbehav.2011.10.031
Barbosa MN, Silva Mota MT (2013) Alloparental responsiveness to newborns by nonreproductive, adult male, common marmosets (Callithrix jacchus). Am J Primatol 75:145–152. https://doi.org/10.1002/ajp.22092
Berman CM, Rasmussen KLR, Suomi SJ (1997) Group size, infant development and social networks in free-ranging rhesus monkeys. Anim Behav 53:405–421. https://doi.org/10.1006/anbe.1996.0321
Bezerra BM, Souto AS, Schiel N (2007) Infanticide and cannibalism in a free-ranging plurally breeding group of common marmosets (Callithrix jacchus). Am J Primatol 69:945–952. https://doi.org/10.1002/ajp.20394
Burkart JM (2015) Opposite effects of male and female helpers on social tolerance and proactive prosociality in callitrichid family groups. Sci Rep 5:9622. https://doi.org/10.1038/srep09622
CPRH 2022. http://www2.cprh.pe.gov.br/uc/apa-aldeia-beberibe/. Accesses in April 24th 2022.
De La Fuente MF, Sueur C, Garber PA, Bicca-Marques JC, Souto A, Schiel N (2021) Foraging networks and social tolerance in a cooperatively breeding primate (Callithrix jacchus). J Anim Ecol 1:1–17. https://doi.org/10.1111/1365-2656.13609
Digby LJ, Ferrari SF, Saltzman W. (2011) Callitrichines: The role of competition in cooperatively breeding species. In: Campbell, et al., (eds.) Primates in Perspective (2nd edition). New York: Oxford University Press, pp. 91–107.
Dunbar RIM, Carron PM, Shultz S (2018) Primate social group sizes exhibit a regular scaling pattern with natural attractors. Biol Lett 14:20170490. https://doi.org/10.1098/rsbl.2017.0490
Ford SM, Porter LM, Davis LC. (eds). (2009). The smallest Anthropoids. Developments in Primatology: Progress and Prospects. Boston: Springer
Fragaszy DM, Bard K (1997) Comparison of development and life history in Pan and Cebus. Int J Primatol 18:683–701. https://doi.org/10.1023/A:1026339712071
De La Fuente MF, Schiel N, Bicca-Marques JC, Caselli CB, Souto A, Garber PA (2019) Balancing contest competition, scramble competition, and social tolerance at feeding sites in wild common marmosets (Callithrix jacchus). Am J Primatol e22964. https://doi.org/10.1002/ajp.22964
Garber PA (1997) One for all and breeding for one: cooperation and competition as a tamarin reproductive strategy. Evol Anthropol 5:187–199. https://doi.org/10.1002/(sici)1520-6505(1997)5:6%3c187::aid-evan1%3e3.0.co;2-a
Gunhold T, Massen JJM, Schiel N, Souto A, Bugnyar T (2014) Memory, transmission and persistence of alternative foraging techniques in wild common marmosets. Anim Behav 91:79–91. https://doi.org/10.1016/j.anbehav.2014.02.023
Hinde RA, Spencer-Booth Y (1968) Review lecture—the study of mother-infant interaction in captive group-living rhesus monkeys. Proc R Soc B: Biol Sci 169:177–201. https://doi.org/10.1098/rspb.1968.0005
Holmes J (2014) John Bowlby and attachment theory (Second edition). Routledge, Taylor & Francis Group, p 272
Huang J, Cheng X, Zhang S, Chang L, Li X, Liang Z, Gong N (2020) Having infants in the family group promotes altruistic behavior of marmoset monkeys. Curr Biol 30:4047–4055. https://doi.org/10.1016/j.cub.2020.07.045
Ingram JC (1977) Interactions between parents and infants, and the development of independence in the common marmosets (Callithrix jacchus). Anim Behav 25:811–827. https://doi.org/10.1016/0003-3472(77)90035-5
Isler K, van Schaik CP (2012) Allomaternal care, life history and brain size evolution in mammals. J Hum Evol 63:52–63. https://doi.org/10.1016/j.jhevol.2012.03.009
Jablonski NG (2021) Social and affective touch in primates and its role in the evolution of social cohesion. Neuroscience 464:117–125. https://doi.org/10.1016/j.neuroscience.2020.11.024
Koenig A (1995) Group Size, Composition, and Reproductive Success in Wild Common Marmosets (Callithrix jacchus). Am J Primatol 35:311–317. https://doi.org/10.1002/ajp.1350350407
Kostan KM, Snowdon CT (2002) Attachment and social preferences in cooperatively-reared cotton-top tamarins. Am J Primatol 57:131–139. https://doi.org/10.1002/ajp.10040
Lehmann J, Korstjens AH, Dunbar RIM (2007) Group size, grooming and social cohesion in primates. Anim Behav 74:1617–1629. https://doi.org/10.1016/j.anbehav.2006.10.025
Lehner PN (1996) Handbook of ethological methods. Cambridge University Press, Cambridge
Lenth R (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33. https://doi.org/10.18637/jss.v069.i01
van Londen WM, Juffer F, van IJzendoorn MH (2007) Attachment, Cognitive, and Motor Development in Adopted Children: Short-term Outcomes after International Adoption. J Pediatr Psychol 32:1249–1258. https://doi.org/10.1093/jpepsy/jsm062
Maestripieri D (2018) Maternal influences on primate social development. Behav Ecol Sociob 72:1–12
Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD (2009) Mother–infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol Behav 96:613–619. https://doi.org/10.1016/j.physbeh.2008.12.016
Malukiewicz J, Boere v, Oliveira MAB, D’Arc M, Ferreira JVA, French J, Housman G, Souza CI, Jerusalinsky L, Melo FR, Valença-Montenegro MM, Moreira SB, Silva IO, Pacheco FS, Rogers J, Pissinatti A, del Rosario RCH, Ross C, Ruiz-Miranda CR, Pereira LCM, Schiel N, Silva FFR, Souto A, Šlipogor V, Tardif S (2020) An introduction to the Callithrix genus an overview of recent advances in marmoset research. ILAR J 61: 110–138. https://doi.org/10.1093/ilar/ilab027
Markham AC, Gesquiere LR (2017) Costs and benefits of group living in primates: an energetic perspective. Philos Trans R Soc B 372:20160239. https://doi.org/10.1098/rstb.2016.0239
Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB (2012) The evolution of primate societies. The University of Chicago Press, Chicago and London, p 730
Ngo V, Gorman JC, De La Fuente MF, Souto A, Schiel N, Miller CT (2022) Active vision during prey capture in wild marmoset monkeys. Curr Biol 32:1–6. https://doi.org/10.1016/j.cub.2022.06.028
Pinheiro J, Bates D, DebRoy S, Sarkar, D e R Core Team (2019) nlme: Linear and nonlinear mixed effects models. R package version 3.1‐137. Retrieved from: https://CRAN.R‐project.org/package=nlme
R Core Team (2021) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Retrieved from: https://www.R‐project.org/
Rapaport LG (2011) Progressive parenting behavior in wild golden lion tamarins. Behav Ecol 22:745–754. https://doi.org/10.1093/beheco/arr055
Rapaport LG, Brown GR (2008) Social influences on foraging behavior in young nonhuman primates: learning what, where, and how to eat. Evol Anthropol 17:189–201. https://doi.org/10.1002/evan.20180
Rothe H, Darms K, Koenig A, Radespiel U, Juenemann B (1993) Long-term study of infant-carrying behavior in captive common marmosets (Callithrix jacchus): Effect of nonreproductive helpers on the parents’ carrying performance. Int J Primatol 14:79–93. https://doi.org/10.1007/BF02196504
Saito A, Izumi A, Nakamura K (2011) Development of infant common marmosets’ (Callithrix jacchus) preference for their parents over adults from another group. Primates 52:43–50. https://doi.org/10.1007/s10329-010-0205-7
Santos CV, Martins MM (2000) Parental care in the buffy-tufted-ear marmoset (Callithrix aurita) in wild and captive groups. Rev Bras Biol 60:667–672. https://doi.org/10.1590/S0034-71082000000400018
van Schaik CP, Isler K (2012) Life-history evolution in primates. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB. The evolution of primate societies. Chicago and London: The University of Chicago Press, p. 220–244. https://doi.org/10.5167/uzh-71056
Schiel N, Huber L (2006) Social influences on the development of foraging behavior in free-living common marmosets (Callithrix jacchus). Am J Primatol 68:1150–1160. https://doi.org/10.1002/ajp.20284
Schiel N, Souto A (2017) The common marmoset: an overview of its natural history, ecology and behavior. Dev Neurobiol 77:244–262. https://doi.org/10.1002/dneu.22458
Schiel N, Souto A, Huber L, Bezerra BM (2010) Hunting strategies in wild common marmosets are prey and age dependent. Am J Primatol 72:1039–1046. https://doi.org/10.1002/ajp.20860
Šlipogor V, Massen JJM, Schiel N, Souto A, Bugnyar T (2021) Temporal consistency and ecological validity of personality structure in common marmosets (Callithrix jacchus): a unifying field and laboratory approach. Am J Primatol 83:e23229. https://doi.org/10.1002/ajp.23229
Souto A, Bezerra BM, Schiel N, Huber L (2007) Saltatory search in free-living Callithrix jacchus: environmental and age influences. Int J Primatol 28:881–893. https://doi.org/10.1007/s10764-007-9165-1
Stoinski TS, Beck B, Bloomsmith MA, Maple TL (2003). A behavioral comparison of captive-born, reintroduced golden lion tamarins and their wild-born offspring. Anim Behav 140:137–160. https://doi.org/10.1163/156853903321671479
Tardif SD, Harrison ML, Simek MA (1993) Communal infant care in marmosets and tamarins: relation to energetics, ecology, and social organization. In: Rylands AB (ed) Marmosets and tamarins: systematics, behaviour, and ecology. Oxford University Press, New York, pp 220–234
Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME (2003) Reproduction in captive common marmosets (Callithrix jacchus). Comp Med 53:364–368
Tardif SD, Araújo A, Arruda MF, French JA, Sousa MBC, Yamamoto ME (2008) Reproduction and aging in marmosets and tamarins. Interdisc Top Gerontol 36:29–48. https://doi.org/10.1159/isbn.978-3-8055-8523-1
Terborgh J, Janson CH (1996) The socioecology of primate groups. Annu Rev Ecol Evol Syst 17:111–136. https://doi.org/10.1146/annurev.es.17.110186.000551
Vochteloo JD, Timmermans PJA, Duijghuisen JAH, Vossen JMH (1993) Effects of reducing the mother's radius of action on the development of mother–infant relationships in longtailed macaques. Anim Behav 45:603–12
Wang Y, Fang Q, Gong N (2014) Motor assessment of developing common marmosets. Neurosci Bull 30:387–393. https://doi.org/10.1007/s12264-013-1395-y
Whiten A, van de Waal E (2018) The pervasive role of social learning in primate lifetime development. Behav Ecol Sociobiol 72:80. https://doi.org/10.1007/s00265-018-2489-3
Yamamoto ME, Albuquerque FS, Lopes NA, Ferreira ES (2008) Differential infant carrying in captive and wild common marmosets (Callithrix jacchus). Acta Ethol 11:95–99. https://doi.org/10.1007/s10211-008-0046-1
Yamamoto ME, Box HO (1997) The role of non-reproductive helpers in infant care in captive Callithrix jacchus. Ethol 103:760–771. https://doi.org/10.1111/j.1439-0310.1997.tb00184.x
Yamamoto ME (1993) From dependence to sexual maturity: the behavioural ontogeny of Callitrichidae. In: Rylands AB (ed.) Marmosets and tamarins: systematics, behaviour, and ecology. New York: Oxford University Press. p 235–254. https://doi.org/10.1007/BF02735808
Yamamoto ME (2005) Infant care in Callitrichids: cooperation and competition. Annu Rev Biomed Sci 7:149-160. 10.5016/48
Young JW, Shapiro LJ (2018) Developments in development: What have we learned from primate locomotor ontogeny? Am J Phys Anthropol 165:37–71. https://doi.org/10.1002/ajpa.23388
Ziegler TE, Sosa ME, Colman RJ (2017) Fathering style influences health outcome in common marmoset (Callithrix jacchus) offspring. PLoS ONE 12:e0185695. https://doi.org/10.1371/journal.pone.0185695
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer. https://doi.org/10.1007/978-0-387-87458-6
Acknowledgements
We are very thankful to Bruna M. Bezerra for her assistance in data collection. This study was funded by CAPES (Coordination for the Improvement of Higher Education Personnel) through a PhD scholarship granted to Nicola Schiel and a master’s scholarship for Alexandre Malta.
Funding
The study was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES), through a scholarship granted to NS and AM.
Author information
Authors and Affiliations
Contributions
Conception: AS, CC, MFDF, NS; design of the study: CC, MFDF, NS; sampling: NS; data analysis: CC and MFDF; writing of the article: all authors. All authors have read and approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest to declare.
Ethical approval
The present research study was conducted in compliance with the guidelines for the ethical treatment of animals in behavioral research and teaching (Animal Behaviour, 2003; v.65; p 249–255), as well as with the ethical principles for the treatment of non-human primates, established by the American Society of Primatologists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Malta, A., Caselli, C., Souto, A. et al. Number of adult females in a group affects infant motor development of a cooperative breeding primate (Callithrix jacchus). Primates 63, 683–689 (2022). https://doi.org/10.1007/s10329-022-01016-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-022-01016-x