Abstract
Sleep in the primate order remains understudied, with quantitative estimates of sleep duration available for less than 10% of primate species. Even fewer species have had their sleep synchronously quantified with meteorological data, which have been shown to influence sleep–wake regulatory behaviors. We report the first sleep duration estimates in two captive gibbon species, the Javan gibbon (Hylobates moloch) and the pileated gibbon (Hylobates pileatus) (N = 52 nights). We also investigated how wind speed, humidity, temperature, lunar phase, and illumination from moonlight influence sleep–wake regulation, including sleep duration, sleep fragmentation, and sleep efficiency. Gibbons exhibited strict diurnal behavior with little nighttime activity and mean total average sleep duration of 11 h and 53 min for Hylobates moloch and 12 h and 29 min for Hylobates pileatus. Gibbons had notably high sleep efficiency (i.e., time score asleep divided by the time they spent in their sleeping site, mean of 98.3%). We found illumination from moonlight in relation to lunar phase and amount of wind speed to be the strongest predictors of sleep duration and high-quality sleep, with increased moonlight and increased wind causing more fragmentation and less sleep efficiency. We conclude that arousal threshold is sensitive to nighttime illumination and wind speed. Sensitivity to wind speed may reflect adaptations to counter the risk of falling during arboreal sleep.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep architecture captures fundamental aspects of animal behavior and physiology, with great consequence for fitness involving predation risk, mating opportunities, and foraging. Given its importance, it is remarkable that sleep architecture has only been recorded for 7% of primate species, with most of these studies taking place in captive settings (Nunn and Samson 2018). Two factors are leading to increased investigations of sleep: technological innovations that involve infrared videography and actigraphy, and greater appreciation of the importance of sleep in primate and human evolution (Samson et al. 2014,2018,2019; Samson and Shumaker 2015; Samson and Nunn 2015; Nunn 2011; Nunn et al. 2010; Melvin et al. 2019; Kavanau 2005). To advance research in human evolution, sleep studies on the apes are especially important, given that sleep has been quantified only for orangutans (Pongo pygmaeus and Pongo abelii) (Samson 2013) and chimpanzees (Pan troglodytes) (Freemon et al. 1969; 1971; Videan 2006; Videan 2005; Havercamp et al. 2021; Adey et al. 1963). Detailed studies of sleep architecture in small apes (gibbons) have yet to be conducted, although one study looked at correlates of sleep and activity in wild gibbons (Fei et al. 2019).
Sleep is an ancient and a seemingly evolutionarily conserved behavior performed by most animal life. Natural selection has shaped sleep characteristics in relation to benefits, but also in terms of costs. For example, several hypotheses have addressed the function of sleep in relation to the benefits of immunocompetence, cognitive processing, neural development, memory formation, and brain metabolic homeostasis (Rasch and Born 2013; Nunn 2011; McNamara and Auerbach 2010; Preston et al. 2009; Walker 2009; Xie et al. 2013; Marks et al. 1995). Comparative research supports a number of these proposed functions, with many studies highlighting a balance between sleep intensity and sleep duration, with animals optimizing one of these strategies, but not both (Franken et al. 1991; Tobler 2011; Samson et al. 2018). In other words, given an animal’s environment and ecological niche, it may be more advantageous to exhibit lower sleep intensity [measured by looking at the proportion of EEG slow-wave activity within non-rapid eye movement (NREM)] over a period of longer sleep duration, while in other circumstances, it may be favorable to exhibit higher sleep intensity over a period of shorter sleep duration. Costs are also important. Thus, ecological factors such as predation risk and foraging needs help shape this balance between sleep and activity, along with environmental factors such as weather patterns (Lesku et al. 2006; Capellini et al. 2008; Hartse 2011; Lesku et al. 2006; Rattenborg and Amlaner 2002).).
Here, we investigated sleep in gibbons (genus Hylobates). Gibbons are universally classified as diurnal (Ahsan 2001; Gittins 1982; Whitten 1982; Raemaekers 1978; Chivers, 1974,1975; Fan et al. 2009). It has been hypothesized that gibbon activity patterns evolved as a foraging strategy to synchronize with the timing of fruiting trees, where gibbons wake early in the morning to consume a large abundance of fruit to offset low levels of blood sugar from fasting throughout the night (Raemaekers 1978; Gittins, 1982). Gibbons then continue to feed throughout the day, but unlike other primates, tend to retire to their sleeping sites several hours before dusk, forgoing potential foraging as a trade-off for decreased nocturnal predation risk (Fei et al. 2017). Gibbons have been reported to have excellent anti-predation strategies, and along with the foraging constraints of a frugivorous diet, these two ecological pressures in combination likely shaped the evolution of diurnal behavior seen in gibbons (Clarke et al. 2012; Dooley and Judge 2015; Geissmann et al. 2006).
Meteorological variables such as wind, temperature, and lunar phase have been reported to influence sleep in animals, including humans and non-human primates (Moffitt 1977; Samson et al. 2018; Lammers and Zurcher 2011; Preuschoft 2002). Lunar phase has been shown to influence sleep across the animal kingdom, including invertebrates such as insects (Povilla adusta) and crabs (Sesarma haematocheir), amphibians such as tungara frogs (Physalaemus pustulosus) and reptiles such as prairie rattlesnakes (Crotalus viridis), birds such as the fiery necked nightjar (Caprimulgus pectoralis) and eagle owls (Bubo bubo), mammals such as the Eurasian badger (Meles meles) and coyotes (Canis latrans), and marine taxa such as white sharks (Carcharodon carcharias) and cape fur seals (Arctocephalus pusillus pusillus) (Kronfeld-Schor et al. 2013; Fallows et al. 2016). In primates, the lunar cycle has been reported as an important regulator of nighttime activity (Pruetz, 2018; Curtis and Rasmussen, 2006; Eppley et al. 2015; Erkert and Kappeler 2004; Kappeler and Erkert 2003; Fernández-Duque et al. 2010; Muñoz-Delgado and Corsi-Cabrera 2010; Maples et al. 1976; Ayers et al. 2020). This is an effect of some lunar phases, such as full moons, producing more illumination than other lunar phases, such as new moons (Pruetz 2018; Ayers et al. 2020). For non-human primates, previous studies report lunar phase as having a strong influence on lemur scotoperiod activity and their documented cathemerality (Curtis and Rasmussen 2006; Eppley et al. 2015; Erkert and Kappeler 2004; Kappeler and Erkert 2003), where scotoperiod refers to the period of darkness, or absence of daylight, experienced by an organism. Chimpanzees (Pan troglodytes verus) (Pruetz 2018) and chacma baboons (Papio ursinus) (Ayers et al. 2020) have been reported to display more activity on nights with increased illumination, which may be interpreted as poorer sleep efficiency on those nights.
Quantitative data on gibbon sleep quotas, including total sleep, NREM, and REM durations, have yet to be recorded. Sleep duration is defined as the total time an individual is asleep, while NREM is defined as a stage of “deep sleep”, and REM is defined as a stage of “light sleep” signified by rapid eye movement (REM) and movement of the diaphragm, and is the period in which dreaming is known to occur in humans (Siegel 2005, 2011). However, previous research on correlates of sleep has been conducted on wild skywalker hoolock gibbons (Hoolock tianxing). This study found that gibbons spent longer periods of time at their sleep sites after dawn on nights where temperatures were colder (Fei et al. 2019). In addition to temperature, windy conditions may influence sleep in arboreal, open-branch-sleeping primates, such as gibbons (Fruth et al. 2018; Stewart et al. 2018). This builds on previous findings showing that wind speed impacts stability for arboreal primates (Lammers and Zurcher 2011; Preuschoft 2002), especially for those who are open-branch sleepers compared to nest sleepers (Reichard 1998; Fruth et al. 2018; Samson and Hunt 2014; Samson and Shumaker 2013; Anderson et al. 2019).
To investigate gibbon sleep and nighttime inactivity, we measured behavioral sleep via actigraphy, concurrently with wind, humidity, temperature, lunar phase, and illumination from moonlight. These data are used to test the hypothesis that lunar and meteorological variables are drivers of sleep–wake regulation in two captive gibbon species, the Javan gibbon (Hylobates moloch) and the pileated gibbon (Hylobates pileatus). Specifically, we predicted that gibbons would display lower sleep efficiency on nights with increased lunar illumination due to increased opportunities for safe arboreal movement when more light is available. We also predicted that gibbon sleep would be sensitive to wind, given that windier nights may make sleeping riskier for this arboreal primate, resulting in lower sleep duration, increased sleep fragmentation, and lower sleep efficiency. Finally, based on observational data in wild gibbons (Fei et al. 2019), we predicted that lower temperatures would influence sleep–wake regulation by reducing sleep duration, increasing sleep fragmentation, and decreasing sleep efficiency.
Methods
Study subjects
We generated actigraphy data from two captive gibbons of different species, one male and one female. We surveyed Reg, a 22-year-old male Javan gibbon, also referred to as the silvery gibbon (Hylobates moloch), and Violet, an 11-year-old female pileated gibbon (Hylobates pileatus). Subjects were housed at the Santa Clarita Gibbon Conservation Center, in California, USA, with complete biographic information available online (https://www.gibboncenter.org/species-at-the-gcc.html). The center’s mission is to promote the conservation of gibbons and to preserve their habitat by partnering with global gibbon rescue organizations, and to care for captive gibbons through public education and scientific research. Gibbons at the center have routine veterinary health examinations in which they are placed under general anesthesia. Given the risks of anesthesia, it is preferable to place collars on an animal that is already scheduled for anesthesia rather than to place an animal under anesthesia solely for the purpose of putting on the collar. Therefore, Violet and Reg were chosen for this study because the timing of their examinations aligned with the starting time of our data collection. Gibbons are housed outside under natural conditions in California, allowing them to experience natural meteorological variables such as wind, daily temperatures, moonlight exposure, and lunar phase exposure. All animal use and methods were approved by the Duke University Institutional Animal Care and Use Committee, protocol number A035-14-02.
Data collection
Data collection occurred for 2 months from 8 May 2015 to 17 June 2015. Reg and Violet’s daily activity was recorded continuously using MotionWatch 8 (CamNtech) tri-axial accelerometers, generating continuous data at 1-min epochs over the course of a total of 52 days. These actigraphic sensors are lightweight (7 g) and attached to standard nylon pet collars worn around the neck. Veterinary staff placed collars on Reg and Violet during their routine veterinary examinations under general anesthesia, and they were worn for the duration of this study (52 days). Reg and Violet were monitored by staff to ensure no adverse reactions to the collars and smooth acclimation to the collars. The physiological definition of sleep is defined by polysomnography, which involves placing electrode monitors on the scalp and body to record brain waves, breathing, and body movements that correspond to sleep. However, these methods involve physical restriction and/or invasive approaches, and thus are no longer ethically possible with primates. Therefore, we use the convention behavioral sleep as an estimate for physiological sleep used in non-human animal sleep research (Zanghi et al. 2013). Certain behaviors, such as patterned diaphragm breathing, rapid eye movements, and prolonged immobility of the body, have been shown to be strongly correlated with brain phases of sleep (such as REM or NREM). This allows actigraphy, which measures these behavioral activity patterns of sleep, to get at the wake-sleep states without the animal undergoing polysomnography (Zanghi et al. 2013). Housing environment was recorded for each night of sampling, with gibbons selecting sleeping site locations within their outdoor enclosures. Daily recording was indexed by several independent variables: day length (the difference between sunrise and sunset times), moon phase (continuously between 1 = full moon and 0 = new moon), average nighttime temperature (°C) measured hourly from sunset (20:00) to sunrise (05:40), and average nighttime wind speed measured in miles per hour hourly from sunset (20:00) to sunrise (05:40). Independent variables were sourced from the following website https://www.timeanddate.com/moon/phases/usa/santa-clarita that records local meteorological variables. Sun and moon information can be located under the website tabs “Sun & Moon”, “Moonrise & Moonset” and “Sunrise & Sunset”. Weather variables such as humidity, temperature, and wind speed can be located under the “weather” tab, then navigating to “past weather” for the dates of interest.
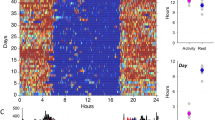
CamNtech’s MotionWare software was used to produce actograms of Reg and Violet’s daily activity levels (Fig. 1), which allows for a comparison of how active each gibbon was during the nighttime period and the daytime period. Dependent variables were generated from MotionWare software (CamNtech) processed activity logs recorded at 1-min epochs. The sensor sampled movement once a second at 50 Hz and accumulated data that assigns an activity value per 1 min epoch. The MotionWatch 8 sensor logs motion data over a user-defined interval, or epoch, using a built-in tri-axial accelerometer. The sensor samples data at 50 Hz and accumulates data over the epoch, ultimately assigning it a numeric value. We collected data on the minute, as continuous 1-min sampling is the most commonly used actigraphic method for measuring sleep–wake activity patterns in both humans (Ancoli-Israel et al. 2003; Johnson et al. 2007) and non-human primates (Sri Kantha and Suzuki 2006). To determine behavioral sleep (i.e., significant periods of scotoperiod inactivity) of gibbons, we used methods similar to those developed for determining human sleep behavior.
24-h average activity plot. Reg’s actogram is in black; Violet’s actogram is in dark grey. Light grey periods indicate nighttime periods. Average sunset time was 20.00 (8:00 pm) and average sunrise time was 5:40 (5:40 am). Black lines show periods of activity; absence of black lines indicates periods of inactivity
Using actigraphy data, we generated an estimate of total average sleep time. Following terminology in previous studies (Andersen et al. 2013; Barrett et al. 2009; Kantha and Suzuki 2006; Zhdanova et al. 2002), we defined sleep in actigraphy as the absence of any force in any direction during the measurement period (1-min epoch) (Campbell and Tobler 1984). The following variables were obtained: sleep duration, central phase measure (CPM) (the midpoint of sleep start and end time), sleep fragmentation, and sleep efficiency. Actigraphy data was paired with nighttime averages of the following meteorological variables: wind (mph), humidity (%), temperature (°C), lunar phase, and illumination from moonlight. Variables were gathered from the website https://www.timeanddate.com/moon/phases/usa/santa-clarita. Gibbon sleep was not artificially manipulated in any way, as the animals slept in their normal enclosures.
Data analysis
Sleep data from the collars were first analyzed using the MotionWare software by CamNtech to produce actograms and summaries of sleep quotas (Table 1). These night-by-night data were analyzed in R version 3.1.3 (R Core Team 2016) using a linear mixed effects model using the MuMin package (Bartoń 2014). We averaged models with ∆AIC < 10 using the MuMIn package, where AIC refers to the Akaike information criterion (Bartoń 2015) and is used to determine the best fit based on predictive accuracy (McElreath 2019). Sleep quotas were set as the response variable (sleep duration, central phase measure, sleep fragmentation, and sleep efficiency), with the following factors: wind speed (mph), humidity (%), temperature (°C), lunar phase, and illumination from moonlight (lux). Individuals were included in the model as random effects, which correspond to species differences in this case. Statistical inferences were made using standardized coefficient estimates with shrinkage, a technique whereby more certain estimates are pooled to inform less certain estimates, and 95% confidence intervals. Pooling, in this instance based on a normal distribution, means that a variable provides information that can be used to improve the estimates for all other variables (McElreath 2019).
Thus, the model testing the aforementioned predictions was the following:
In summary data reported below, sleep duration refers to the total time an individual is asleep, sleep efficiency is the time spent asleep/total time in the sleep site, sleep fragmentation index measures how often sleep was fragmented (periods of being awake throughout the sleeping period) given in a percentage, and central phase measure (CPM) is the midpoint of the sleeping period given in minutes (a negative value is assigned if the midpoint occurs before midnight; a positive value is assigned if the midpoint occurs after midnight).
Results
Descriptive statistical analyses provided averaged total sleep times for Reg and Violet based on the 52 total days of actigraphy. Gibbons showed distinct sleeping patterns which aligned with expected patterns for diurnal primates. Averaging the data for the two gibbon species gives a genus-level sleep duration of 11 h and 41 min (SE 0.38), with a high sleep efficiency of 98.3% (SE 1.00). The sleep fragmentation index on average was relatively low (14.3, SE 1.31), and gibbons had a reported mean CPM of 180.8 min (SE 4.53, Table 1).
Figure 1 contains the actogram for Reg and Violet as generated by Motionware (CamNtech). With black lines displaying activity, it is observed that the vast majority of activity took place during daylight hours, with little to no activity taking place during the scotoperiod. There were slight differences observed between the two subjects' actograms, with Reg sleeping slightly less than Violet, with a duration of 11 h and 53 min in comparison to her 12 h and 29 min. We assumed group composition would not have a significant effect on sleeping patterns because the gibbons are housed in intragroup pairs (N = 2), but given that intergroup gibbons are within vocalization range of other gibbons, it is possible that enclosure membership could influence nighttime activity.
Reg and Violet could be described to be late risers, with activity not taking place until around 8:00 am, while sunlight is available starting at 5:40 am (on average over the study period). This may be explained by the captive husbandry scheduling of the Gibbon Conservation Center, where breakfast is distributed by keepers to the gibbon population closer to 7:00–8:00 am, instead of at dawn. During the evening, when light becomes absent due to the sun setting at 21:00 (9:00 pm) on average, gibbons may continue to be lightly active for an hour or so, then all activity ceases throughout the entire nighttime sleeping period (Fig. 1).
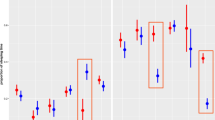
In our statistical model of sleep duration (Table 2), illumination was shown to be the greatest positive driver of sleep duration, with a positive estimate, a confidence interval not crossing zero (Fig. 2) and the highest importance rank. Lunar phase was found to increase sleep duration, but with low importance. Lunar phase was observed to be of importance as a related variable to the amount of illumination produced. Wind speed was reported to be the strongest negative driver of gibbon sleep duration, and the second most important factor overall (after illumination). Figure 2 displays a coefficient plot showing the influence of predictor variables (illumination, lunar phase, wind, humidity, and temperature) on sleep duration.
Bolder line indicates an 89% interval. Sleep Duration: Greater wind (mph) is associated with shorter sleep duration, whereas increased illumination (lux from moonlight) drives longer sleep duration. Humidity and temperature were not of importance, for the coefficient estimates did not exclude zero. Lunar phase was bordering on zero and may be of importance for longer sleep duration
We also investigated other sleep quota variables. Figure 3 shows that sleep fragmentation increased when nighttime average temperatures were warmer and humidity rates were higher. When more illumination was produced by moonlight, sleep fragmentation also increased. Therefore, we conclude that hot, humid nights with increased moonlight reduce the quality of gibbon sleep. In Fig. 4, it is observed that on nights with less illumination from moonlight, gibbons had higher sleep efficiency. Therefore, we conclude that nights with less illumination are conducive to higher-quality gibbon sleep. For sleep efficiency, all other predictor variables had confidence intervals that crossed zero (Fig. 3; Table 2), and therefore were deemed of low importance for predicting sleep efficiency.
Discussion
We found that gibbon sleep is influenced by lunar and meteorological variables. Specifically, we found that increased illumination from moonlight is a driver of sleep–wake regulation, with a greater moonlight resulting in lower sleep efficiency, more fragmented sleep, and longer sleep duration. Wind was the strongest driver of sleep–wake regulation in gibbons, with less wind resulting in higher sleep efficiency and longer sleep duration. We predicted an increase in illumination from moonlight to be a driver of lower sleep efficiency, similar to what has been reported in humans (Turányi et al. 2014). We found support for this prediction. However, we also found increased illumination to be a significant driver of sleep duration and fragmentation. Literature on sleep fragmentation has demonstrated that nights with increased fragmentation are often correlated with longer sleep duration as a compensatory mechanism, including research on humans (Stepanski et al. 1984; Stepanski 2002; Mezick et al. 2009; Bonnet and Arand 2003; Van den Berg et al. 2008), rats (Landis et al. 1988), and three species of macaques (Zhdanova et al. 2002). This may explain why more illumination from moonlight resulted in fragmented sleep, and as a byproduct of this, resulted in longer sleep duration.
We predicted that lunar phase would be a driver of sleep–wake regulation given that previous primate studies have reported differences in nighttime activity on nights with increased illumination (Pruetz 2018). Illumination from moonlight and lunar phase are presumed to be connected, given that the amount of illumination displayed is a product of the lunar phase (with full moons providing more light than new moons). As a result, if one is a driver, it can be assumed the other will be as well. However, this prediction was not supported. This may be due to limitations in the sample size and the number of nights of analysis per individual: a longer study duration that encompasses a full moon cycle may find lunar phase to be a significant driver instead of only trending towards significance. Unlike previous primate studies (Pruetz 2018), the gibbons showed little to no nighttime activity, remaining strictly diurnal even on nights with large amounts of nighttime illumination (Fig. 1).
Nighttime behaviors (including sleep) of many different animal species have been reported to be influenced by lunar phase and the amount of illumination from moonlight produced as a product of that lunar phase and by other meteorological conditions. Examples include song production at dawn by diurnal songbirds (Takeuchi, at el. 2018) and predator–prey interactions of white sharks (Carcharodon carcharias) and cape fur seals (Arctocephalus pusillus pusillus) (Fallows et al. 2016). West African chimpanzees (Pan troglodytes verus) at Fongoli, Senegal, have been reported to forgo longer sleep duration and perform intermitted nocturnal behavior when illumination from moonlight is abundant (Pruetz 2018), although the authors proposed that this behavior may have evolved to cope with heat stress, with temperature being a driver. For wild gibbons, Fei, Thompson, and Fan (2019) argued that colder temperatures influenced wild skywalker hoolock gibbon (Hoolock tianxing) sleep, resulting in longer sleep duration. Building from this, we predicted that lower temperatures would be a driver of sleep–wake regulation and sleep duration in captive gibbons. However, we failed to find support for this prediction, which may reflect differences in captive and wild environments.
We found wind speed to be the strongest driver of sleep–wake regulation and sleep duration. Our results indicated that when wind speed was lower, Reg and Violet had higher-quality sleep (longer duration, less fragmentation, higher sleep efficiency). Gibbons are strictly arboreal primates. Unlike other apes, who construct sleeping platforms (i.e. nests) at night, gibbons sleep on open branches (Reichard 1998). Gibbons appear to be particular when selecting a sleeping tree. Trees that are chosen for sleep sites are typically emergent full-grown trees that are durable, sturdy, and have plentiful foliage. Several researchers interpret sleeping site location to be an anti-predation tactic (Reichard 1998; Fei et al. 2012; Cheyne et al. 2013). However, it may be that sleeping high up in trees causes gibbons to be more exposed to meteorological elements, with strongly blowing wind representing a significant threat to arboreal sleeping primates. Wind has been reported to impact stability during arboreal location in many different animal species including primates (Lammers and Zurcher 2011; Preuschoft 2002; Casteren et al. 2013; Fei et al. 2012). Primates have been reported to fall from tree canopies during the nighttime sleeping period due to increased wind (Fruth et al. 2018), with open-branch sleepers such as gibbons being more susceptible to lethal falls compared to platform sleepers such as the great apes (Fruth et al. 2018), who create a platform nest structure of tree branches woven together to create a stable base (Samson and Hunt 2014; Samson and Shumaker 2013; Anderson et al. 2019). However, even with platform sleepers, wind still has an impact on sleep. For example, West African chimpanzees at Fongoli used larger support branches and increased the depth of their nests on nights with windier conditions (Stewart et al. 2018). In addition, increased wind speed in the exposed canopy may explain why female chimpanzees build more complex sleeping platforms higher in trees (Samson 2012; Samson and Hunt 2012). Given that open-branch sleepers are more at risk for falling than platform sleepers, this selective pressure may drive gibbons to adapt heightened sensitivity to wind, explaining why wind was found to be our strongest driver of gibbon sleep.
Limitations
The use of actigraphy and algorithmic determination of sleep–wake states in non-human primates has some limitations worth considering. While polysomnography is the hallmark method of sleep research, this invasive technology cannot be used in gibbons in either wild or captive contexts. Thus, we used behavioral sleep as an estimate for physiological sleep (Zanghi et al. 2013). In primate species that display more restless behaviors throughout the night, infrared videography may be used to confirm the individual is within their sleeping site location and appears at rest (sustained inactive species-specific posture). However, given the close phylogenetic relatedness to humans and the strict behavioral quiescence uniquely characteristic of diurnal gibbons, this was not necessary within the current preliminary study. We note that actigraphy data should be viewed with caution because it can overestimate sleep given the lack of sensitivity for identifying sleep–wake differentiation (Kawada 2013). Additionally, sleep–wake algorithms have been developed in humans, but none exist for non-human primates. However, relative to other primates previously studied using this methodology (Bray et al. 2017; Melvin et al. 2019; Samson et al. a, b, c; Samson et al. 2018), gibbons uniquely exhibited strict diurnal behavior (with a near absence of nighttime activity), resulting in clearly differentiated sleep–wake periods of activity.
Another limitation to this study is the small sample of individuals from which sleep quotas were generated. Although the sample recorded in this study may not be statistically representative of Hylobates, it does reveal a capability of the species (Healey 2009). Specifically, due to the sample size and the fact that individual ID was collinear with species and sex, we did not analyze either factor as a predictor in the statistical model. Thus, our interpretation of the results remains at the genus level. Future work, with greater statistical representation for each species balanced by sex, can expand upon these preliminary findings.
Conclusions
With only a handful of primates having their sleep analyzed, additional reports of primate sleep quotas are urgently needed to test evolutionary hypotheses. A greater understanding of ape sleep will yield critical insights into how modern human sleep evolved. Given that humans are diurnal, studying sleep in diurnal apes is of particular interest. Gibbon sleep aligned with predicted patterns of diurnal arboreal primates, exhibiting little to no nighttime activity. Gibbons were reported to have overall higher-quality sleep on nights when the wind speed was low and less illumination was present. A future direction is to replicate our study with wild gibbons to see whether temperature is a driver of sleep under natural ecological conditions, and to increase sample size to capture possible sleep variation within gibbon species. The current study contributes sleep variables to a growing primate sleep database aimed at testing evolutionary hypotheses targeting the function of sleep in primates.
References
Adams PM, Barratt ES (1974) Nocturnal sleep in squirrel monkeys. Electroencephalogr Clin Neurophysiol 36:201–204
Adey WR, Kado RT, Rhodes JM (1963) Sleep: cortical and subcortical recordings in the chimpanzee. Science 141(3584):932–933
Ahsan MF (2001) Socio-ecology of the hoolock gibbon (Hylobates hoolock) in two forests of Bangladesh. In: The apes: challenges for 21st century. Brookfield Zoo, 286–299
Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP (2003) The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26(3):342–392
Andersen ML, Diaz MP, Murnane KS, Howell LL (2013) Effects of methamphetamine self-administration on actigraphy-based sleep parameters in rhesus monkeys. Psychopharmacology 227:101–107
Anderson JR (1998) Sleep, sleeping sites, and sleep-related activities: awakening to their significance. Am J Primatol 46(1):63–75
Anderson JR, McGrew WC (1984) Guinea baboons (Papio papio) at a sleeping site. Am J Primatol 6(1):1–14
Anderson JR, Ang MY, Lock LC, Weiche I (2019) Nesting, sleeping, and nighttime behaviors in wild and captive great apes. Primates 60(4):321–323
Ayers AM, Allan ATL, Howlett C, Tordiffe ASW, Williams KS, Williams ST, Hill RA (2020) Illuminating movement? Nocturnal activity patterns in chacma baboons. J Zool 310(4):287–297
Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE (2009) Early adverse rearing experiences alter sleep–wake patterns and plasma cortisol levels in juvenile rhesus monkeys. Psycho Neuroendocrinology 34:1029–1040
Bartoń K (2014) MuMIn: Multi-model inference. Available from: http://CRAN.R-project.org/package=MuMIn.
Bartoń K (2015) Version 1.15.6 ed, https://cran.r-project.org/web/packages/MuMIn.
Bonnet MH, Arand DL (2003) Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev 7(4):297–310
Bray J, Samson DR, Nunn CL (2017) Activity patterns in seven captive lemur species: Evidence of cathemerality in Varecia and Lemur catta? Am J Primatol 79(6):e22648
Campbell SS, Tobler I (1984) Animal sleep: A review of sleep duration across phylogeny. Neurosci Biobehav Rev 8:269–300
Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL (2008) Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolut: Int J Org Evolut 62(7):1764–1776
Cheyne SM, Höing A, Rinear J, Sheeran LK (2013) Sleeping site selection by agile gibbons: the influence of tree stability, fruit availability, and predation risk. Folia Primatol 89(36):299311. https://doi.org/10.1159/000342145
Chivers DJ (1974) The siamang in Malaya: a field study of a primate in a tropical forest. Contr Primatol 4:1–335
Chivers DJ (1975) Daily patterns of ranging and feeding in siamang. In: Kondo S, Kawai M, Ehara A (eds) Contemporary primatology, proceedings of the 5th international congress of primatology. Karger, Basel, pp 362–372
Clarke E, Reichard UH, Zuberbühler K (2012) The anti-predator behaviour of wild white-handed gibbons (Hylobates lar). Behav Ecol Sociobiol 66(1):85–96
Curtis DJ, Rasmussen MA (2006) The evolution of cathemerality in primates and other mammals: a comparative and chronoecological approach. Folia Primatol 77(1–2):178–193
Dooley HM, Judge DS (2015) Kloss gibbon (Hylobates klossii) behavior facilitates the avoidance of human predation in the Peleonan forest, Siberut Island, Indonesia. Am J Primatol 77(3):296–308
Eppley TM, Ganzhorn JU, Donati G (2015) Cathemerality in a small, folivorous primate: proximate control of diel activity in Hapalemur meridionalis. Behav Ecol Sociobiol 69(6):991–1002
Erkert HG, Kappeler PM (2004) Arrived in the light: diel and seasonal activity patterns in wild Verreaux’s sifakas (Propithecus v. verreauxi; Primates: Indriidae). Behav Ecol Sociobiol 57(2):174–186
Fallows C, Fallows M, Hammerschlag N (2016) Effects of lunar phase on predator-prey interactions between white shark (Carcharodon carcharias) and Cape fur seals (Arctocephalus pusillus pusillus). Environ Biol Fishes 99(11):805–812
Fan P, Ni Q, Sun G, Huang B, Jiang X (2009) Gibbons under seasonal stress: the diet of the black crested gibbon (Nomascus concolor) on Mt. Wuliang, Central Yunnan, China. Primates 50(1):37
Fei HL, Scott MB, Zhang W, Ma CY, Xiang ZF, Fan PF (2012) Sleeping tree selection of cao vit gibbon (Nomascus nasutus) living in degraded Karst Forest in Bangliang, Jingxi, China. Am J Primatol 74(11):998–1005
Fei HL, Zhang D, Yuan SD, Zhang L, Fan PF (2017) Antipredation sleeping behavior of skywalker hoolock gibbons (Hoolock tianxing) in Mt. Gaoligong, Yunnan, China. Int J Primatol 38(4):629–641
Fei HL, Thompson C, Fan PF (2019) Effects of cold weather on the sleeping behavior of Skywalker hoolock gibbons (Hoolock tianxing) in seasonal montane forest. Am J Primatol 81(9):e23049
Fernández-Duque E, De La Iglesia H, Erkert HG (2010) Moonstruck primates: owl monkeys (Aotus) need moonlight for nocturnal activity in their natural environment. PLoS ONE 5(9):e12572
Franken P, Dijk D, Tobler I, Borbely AA (1991) Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol-Regul 261:0198–0208
Freemon FR, Walter RD (1970) Electrical activity of human limbic system during sleep. Compr Psychiatry 11(6):544–551
Freemon FR, McNew JJ, Adey WR (1969) Sleep of unrestrained chimpanzee: cortical and subcortical recordings. Exp Neurol 25(1):129–137
Freemon FR, McNew JJ, Adey WR (1971) Chimpanzee sleep stages. Electroencephalogr Clin Neurophysiol 31(5):485–489
Fruth B, Tagg N, Stewart F (2018) Sleep and nesting behavior in primates: a review. Am J Phys Anthropol 166(3):499–509
Geissmann T, Nijman V, Dallmann R (2006) The fate of diurnal primates in southern Sumatra. Gibbon Journal 2:18–24
Gittins SP (1982) Feeding and ranging in the agile gibbon. Folia Primatol 38(1–2):39–71
Hartse K (2011) The phylogeny of sleep. In: Montagna P, Chokroverty S (eds) Handbook of clinical neurology. Elsevier B.V, New York, pp 97–109
Havercamp K, Morimura N, Hirata S (2021) Sleep patterns of aging Chimpanzees (Pan troglodytes). Intern J Primatol 42(1):89–104
Healey JF (2009) Statistics: a tool for social research. Thomson/Wadsworth, Belmont, CA
Johnson NL, Kirchner HL, Rosen CL, Storfer-Isser A, Cartar LN, Ancoli-Israel S, Redline S (2007) Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes. Sleep 30(7):899–905
Kantha SS, Suzuki J (2006) Sleep quantitation in common marmoset, cotton top tamarin and squirrel monkey by non-invasive actigraphy. Comp Biochem Physiol A: Mol Integr Physiol 144(2):203–210
Kappeler PM, Erkert HG (2003) On the move around the clock: correlates and determinants of cathemeral activity in wild redfronted lemurs (Eulemur fulvus rufus). Behav Ecol Sociobiol 54(4):359–369
Kavanau JL (2005) Evolutionary approaches to understanding sleep. Sleep Med Rev 9(2):141–152
Kawada T (2013) Sleep parameters in rhesus monkeys by using actigraphy. Psychopharmacol 228:509–509
Kronfeld-Schor N, Dominoni D, De la Iglesia H, Levy O, Herzog ED, Dayan T, Helfrich-Forster C (2013) Chronobiology by moonlight. Proc Royal Soc B: Biol Sci 280(1765):20123088
Lammers AR, Zurcher U (2011) Stability during arboreal locomotion. Theoretical biomechanics 319–334.
Landis CA, Robinson CR, Levine JD (1988) Sleep fragmentation in the arthritic rat. Pain 34(1):93–99
Lesku JA, Roth TC, Amlaner CJ, Lima SL (2006) A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. Am Nat 168:441–453
Maples WR, Maples MK, Greenhood WF, Walek ML (1976) Adaptations of crop-raiding baboons in Kenya. Am J Phys Anthropol 45(2):309–315
Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP (1995) A functional role for REM sleep in brain maturation. Behav Brain Res 69(1–2):1–11
McElreath R (2019) Statistical rethinking: a Bayesian course with examples in R and Stan. Chapman and Hall/CRC
McNamara P, Auerbach S (2010) Evolutionary medicine of sleep disorders: toward a science of sleep duration. In: McNamara P, Barton RA, Nunn CL (eds) Evolution of sleep: phylogenetic and functional perspectives. Cambridge University Press, Cambridge, pp 107–122
Melvin E, Samson D, Nunn CL (2019) Eulerian videography technology improves classification of sleep architecture in primates. Primates 60(5):467–475
Mezick EJ, Matthews KA, Hall M, Kamarck TW, Buysse DJ, Owens JF, Reis SE (2009) Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology 34(9):1346–1354
Moffitt JD (1977) The effects of meteorological variables on certain sleep characteristics. In: Doctoral dissertation, University of the Pacific
Muñoz-Delgado J, Corsi-Cabrera M. (2010) Chronoecology of neotropical primates: the spider monkey Ateles geoffroyi. Biological Clocks: Effects on Behavior, Health And Outlook, 139.
Nunn CL (2011) The comparative approach in evolutionary anthropology and biology. The University of Chicago Press, Chicago and London
Nunn CL, Samson DR (2018) Sleep in a comparative context: investigating how human sleep differs from sleep in other primates. Am J Phys Anthropol 166(3):601–612
Nunn CL, McNamara P, Capellini I, Preston BT, Barton RA (2010) Primate sleep in phylogenetic perspective. Evolution of sleep: Phylogenetic and functional perspectives, 123–145.
Preston BT, Capellini I, McNamara P, Barton RA, Nunn CL (2009) Parasite resistance and the adaptive significance of sleep. BMC Evol Biol 9:7
Preuschoft H (2002) What does “arboreal locomotion” mean exactly and what are the relationships between “climbing”, environment and morphology? Zeitschrift Für Morphol Anthropol 83:171–188
Pruetz JD (2018) Nocturnal behavior by a diurnal ape, the West African chimpanzee (Pan troglodytes verus), in a savanna environment at Fongoli, Senegal. Am J Phys Anthropol 166(3):541–548
Raemaekers J (1978) Changes through the day in the food choice of wild gibbons. Folia Primatol 30:194–205
Rasch B, Born J (2013) About sleep’s role in memory. Physiol Rev 93:681–766
Rattenborg NC, Amlaner CJ (2002) Phylogeny of sleep. Sleep medicine, Hanley and Belfus Philadelphia, pp 7–22
R Core Team (2016) R: A language and environment for statistical computing. Vienna, Austria. Retrieved from https://www.R-project.org/
Reichard U (1998) Sleeping sites, sleeping places, and presleep behavior of gibbons (Hylobates lar). Am J Primatol 46(1):35–62
Samson DR (2012) The chimpanzee nest quantified: morphology and ecology of arboreal sleeping platforms within the dry habitat site of Toro-Semliki Wildlife Reserve, Uganda. Primates 53:357–364
Samson DR (2013) Orangutan (Pongo pygmaeus) sleep architecture: testing the cognitive function of sleep and sleeping platforms in the hominidae (Doctoral dissertation, Indiana University).
Samson DR, Hunt KD (2012) A thermodynamic comparison of arboreal and terrestrial sleeping sites for dry-habitat chimpanzees (Pan troglodytes schweinfurthii) at the Toro-Semliki Wildlife Reserve, Uganda. Am J Primatol 74:811–818
Samson DR, Hunt KD (2014) Chimpanzees preferentially select sleeping platform construction tree species with biomechanical properties that yield stable, firm, but compliant nests. PLoS ONE 9(4):e95361
Samson DR, Nunn CL (2015) Sleep intensity and the evolution of human cognition. Evol Anthropol 24(6):225–237
Samson DR, Shumaker RW (2013) Documenting orang-utan sleep architecture: sleeping platform complexity increases sleep quality in captive Pongo. Behaviour 150(8):845–861
Samson DR, Shumaker RW (2015) Orangutans (Pongo spp.) have deeper, more efficient sleep than baboons (Papio papio) in captivity. Am J Phys Anthropol 157(3):421–427
Samson DR, Hurst D, Shumaker RW (2014) Orangutan night-time long call behavior: sleep quality costs associated with vocalizations in Captive Pongo. Adv Zool 2014
Samson DR, Bray J, Nunn CL (2018) The cost of deep sleep: environmental influences on sleep regulation are greater for diurnal lemurs. Am J Phys Anthropol 166(3):578–589
Samson DR, Crittenden AN, Mabulla AI, Mabulla AZP, Nunn CL (2017a) Hadza sleep biology: evidence for flexible sleep-wake patterns in hunter-gatherers. Am J Phys Anthropol 162(3):573–582
Samson DR, Crittenden AN, Mabulla IA, Mabulla AZ, Nunn CL (2017b) Chronotype variation drives night-time sentinel-like behaviour in hunter–gatherers. Proc Royal Soc B: Biol Sci 284(1858):20170967
Samson DR, Manus MB, Krystal AD, Fakir E, Yu JJ, Nunn CL (2017c) Segmented sleep in a nonelectric, small-scale agricultural society in Madagascar. Am J Human Biol 29(4):1–13, e22979
Samson DR, Bray J, Nunn CL (2018) The cost of deep sleep: environmental influences on sleep regulation are greater for diurnal lemurs. Am J Phys Anthropol 166(3):578–589
Samson DR, Crittenden AN, Mabulla IA, Mabulla AZP, Nunn CL (2018) Does the moon influence sleep in small-scale societies. Sleep Health 4(6):509–514
Samson DR, Vining A, Nunn CL (2019) Sleep influences cognitive performance in lemurs. Anim Cogn 22(5):697–706
Siegel JM (2005) REM sleep. Princ Pract Sleep Med 4:120–135
Siegel JM (2011) Sleep in animals: a state of adaptive inactivity. Princ Pract Sleep Med 5:126–138
Sri Kantha S, Suzuki J (2006) Sleep quantitation in common marmoset, cotton top tamarin and squirrel monkey by non-invasive actigraphy. Comp Biochem Physiol-A Mol Integr Physiol 144(2):203–210
Stepanski EJ (2002) The effect of sleep fragmentation on daytime function. Sleep 25(3):268–276
Stepanski E, Lamphere J, Badia P, Zorick F, Roth T (1984) Sleep fragmentation and daytime sleepiness. Sleep 7(1):18–26
Stewart FA, Piel AK, Azkarate JC, Pruetz JD (2018) Savanna chimpanzees adjust sleeping nest architecture in response to local weather conditions. Am J Phys Anthropol 166(3):549–562
Tobler I (2011) Phylogeny of sleep regulation. In: Kryger MH, Roth T, Dement WC (eds) The principles and practice of sleep medicine, 5th edn. Elsevier Saunders, St. Louis, MI
Turányi CZ, Rónai KZ, Zoller R, Véber O, Czira ME, Újszászi Á, Szőcs JL (2014) Association between lunar phase and sleep characteristics. Sleep Med 15(11):1411–1416
Van Casteren A, Sellers WI, Thorpe SK, Coward S, Crompton RH, Ennos AR (2013) Factors affecting the compliance and sway properties of tree branches used by the Sumatran orangutan (Pongo abelii). PLoS ONE 8(7):e67877
Van den Berg JF, Neven AK, Tulen JHM, Hofman A, Witteman JCM, Miedema HME, Tiemeier H (2008) Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam study. Int J Obes 32(7):1083–1090
Videan EN (2005) Sleep and sleep-related behaviors in chimpanzee (Pan troglodytes) (Doctoral dissertation, Miami University).
Videan EN (2006) Sleep in captive chimpanzee (Pan troglodytes): the effects of individual and environmental factors on sleep duration and quality. Behav Brain Res 169(2):187–192
Walker MP (2009) The role of sleep in cognition and emotion. Ann NY Acad Sci 1156:168–197
Whitten AJ (1982) Diet and feeding behaviour of Kloss gibbons on Siberut Island, Indonesia. Folia Primatol 37:177–208
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, Iliff JJ (2013) Sleep drives metabolite clearance from the adult brain. Science (new York, N.y.) 342:373–377
Zanghi BM, Kerr W, Gierer J, de Rivera C, Araujo JA, Milgram NW (2013) Characterizing behavioral sleep using actigraphy in adult dogs of various ages fed once or twice daily. J Vet Behav 8(4):195–203
Zhdanova IV, Geiger DA, Schwagerl AL, Leclair OU, Killiany R, Taylor JA, Madras BK (2002) Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol Behav 75:523–529
Acknowledgements
University of Toronto and Duke University are acknowledged for providing funding for this research. Gratitude is extended to Gabi Skollar and the Gibbon Conservation Center of Santa Clarita, California, USA, for allowing our research to take place, and thanks is extended to Reg and Violet for their participation in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Reyes, K.R., Patel, U.A., Nunn, C.L. et al. Gibbon sleep quantified: the influence of lunar phase and meteorological variables on activity in Hylobates moloch and Hylobates pileatus. Primates 62, 749–759 (2021). https://doi.org/10.1007/s10329-021-00920-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-021-00920-y