Abstract
Pseudomonas cannabina pv. alisalensis (Pcal), which causes bacterial blight of brassicaceous plants, is an economically important pathogen worldwide. Copper fungicide and antibiotics are major strategies to manage the disease caused by Pcal; however, a Pcal strain resistant to these chemicals has already been found, and severe outbreaks of bacterial blight have been reported on cabbage in Japan. Therefore, there is an urgent need to develop new Pcal management strategies. Plant defense activators could be useful not only to protect plants against invading pathogens, but also to reduce the amount of copper fungicides and antibiotics applied. However, the mechanisms by which plant defense activators contribute to controlling diseases remains unclear. In this work, we focused on cabbage and acibenzolar-S-methyl (ASM), a well-known plant defense activator. Expression profiles revealed that ASM induced expression of systemic acquired resistance (SAR) marker genes including PR1, PR2, and PR5 in cabbage plants. We also demonstrated that a soil drench with ASM 2 h before transplanting clearly reduced bacterial blight symptoms and reduced Pcal bacterial populations in cabbage. ASM application was also able to prime cabbage for Pcal resistance by activating stomatal-based defense. Our findings highlight that ASM protects plants from bacterial pathogens by activating stomatal-based defense.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the natural environment, plants are constantly surrounded by numerous microorganisms, including potential pathogens. Although plants have not acquired immune systems like those of animals, they have developed monitoring systems that recognize potential pathogens and activate a wide range of immune responses for self-protection (Hacquard et al. 2017; Jones and Dangl 2006). The first layer of plant immune response against invading pathogens is pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI). Plants recognize conserved molecules such as flagellin and elongation factor Tu (EF-Tu) of an invading bacterial pathogen using plant pattern-recognition receptors (PRRs), such as FLS2 and EFR, respectively (Zipfel 2004, 2008; Zipfel and Felix 2005; Zipfel et al. 2006). Following the recognition of an invading pathogen with PRR, plants then activate immune responses. One of the earliest immune responses in PTI is stomatal-based defense to restrict bacterial pathogen entry through stomata (Melotto et al. 2006, 2008; Underwood et al. 2007). However, bacterial pathogens have successfully acquired multiple virulence factors such as phytotoxins and type III secretion system (TTSS) effectors to overcome stomatal-based defense (Ishiga et al. 2018; Lozano-Durán et al. 2014; Melotto et al. 2017).
Recent outbreaks of plant diseases caused by bacterial pathogens have been reported worldwide. Bacterial blight of plants in the Brassicaceae family, caused by Pseudomonas cannabina pv. alisalensis (Pcal), is becoming an economically important disease (Sarris et al. 2013; Takahashi et al. 2013a; Takikawa and Takahashi 2014). Chemical treatments such as copper fungicides and antibiotics are major strategies to manage Pcal diseases (Horinouchi 2010), but a Pcal strain resistant to these chemicals has already been found (Takahashi et al. 2013b). Therefore, there is an urgent need to develop new Pcal management strategies because severe bacterial blight disease outbreaks have been reported on cabbage in Japan (Takahashi et al. 2013b; Takikawa and Takahashi 2014). Among potential new control strategies against bacterial pathogen diseases, plant defense activators are rising stars. Known to activate the natural immune responses of plants, such as systemic acquired resistance (SAR) against invading pathogens without direct activity against pathogens (Bektas and Eulgem 2015; Zhou and Wang 2018), plant defense activators could be useful not only to protect plants against invading pathogens, but also to reduce the use of copper fungicides and antibiotics against Pcal.
Acibenzolar-S-methyl (ASM), a synthetic salicylic acid (SA) functional analog, can function as a plant defense activator (Kunz et al. 1997; Oostendorp et al. 2001). ASM activates plant resistance against a wide variety of pathogens, including viruses, fungi, and bacterial pathogens (Friedrich et al. 1996; Görlach et al. 1996; Kunz et al. 1997; Lawton et al. 1996). ASM does not act directly on plant pathogens in vitro (Friedrich et al. 1996). Although it does not induce SA biosynthesis, ASM triggers NPR1-dependent SAR (Lawton et al. 1996).
In the present study, we demonstrate that ASM induces PR gene expression in cabbage. We further demonstrate that ASM-treatment suppresses Pcal disease development by activating stomatal-based defense. Thus, our results provide new insights into the mechanisms by which plant defense activators contribute to protecting plants against bacterial pathogens.
Materials and methods
Plant materials and chemical treatment
Cabbage (Brassica oleracea var. capitata) cv. Kinkei 201 plants were used for all experiments. Two-week-old seedlings were grown in 200-hole trays at 24 °C with a light intensity of 200 μE m−2 s−1 and 16 h light/8 h dark. Acibenzolar-S-methyl (ASM, marketed as ACTIGARD®) was supplied courtesy of Syngenta. To evaluate the effect of ASM on plant defense, we treated 2-week-old cabbage seedlings by directly drenching the soil with ASM [as 50% active ingredient (a.i.)] suspended in 500 ml of water (100 mg/l) at the rate of 50 mg a.i./200 plants 2 h before Pcal inoculation. Soils were drenched with water or mock-inoculated with water as controls.
Bacterial strains and growth conditions
Pseudomonas cannabina pv. alisalensis strain KB211 (Pcal), a pathogenic strain, was kindly provided by the Nagano Vegetable and Ornamental Crops Experiment Station, Nagano, Japan. Pcal was grown at 28 °C on mannitol-glutamate (MG; Keane et al. 1970) agar. For inoculum, bacteria were suspended in sterile distilled H2O, and cell densities measured at 600 nm (OD600) using a JASCO V-730 spectrophotometer (JASCO, Tokyo, Japan) just before the inoculation.
Bacterial inoculation
Intact leaves on plants were dipped into a bacterial suspension (OD600 of 0.1) in sterile distilled water containing 0.025% Silwet L-77 (OSI Specialties, Danbury, CT, USA). The plants were then incubated in growth chambers at ∼100% RH for the first 24 h, then at ∼70% RH for the rest of the experiment. At 5 days post-inoculation (dpi), symptoms on the inoculated plants were evaluated.
To quantify bacterial populations in cabbage plants, the internal bacterial population in two harvested leaves was measured at several times. Total mass of each leaf was measured, then the leaves were surface-sterilized with 5% v/v H2O2 for 3 min, then washed three times with sterile distilled water. The plants were then homogenized in sterile distilled water, and these diluted samples were plated onto solid MG agar. The bacterial colony forming units (CFU) were normalized as CFU/g based on the total leaf mass. The bacterial population at 0 dpi was estimated using leaves harvested 1 h post-inoculation (hpi) without surface-sterilization. The bacterial populations were evaluated in three independent experiments.
Real-time quantitative RT-PCR
For expression profiles of cabbage defense genes in response to ASM, cabbage plants were treated by drenching the soil with ASM. After 2, 6, 12, 24, 48, 72, and 168 h, total RNA was extracted from leaves and purified. Total RNA was extracted using RNAiso Plus (Takara Bio, Shiga, Japan) according to the manufacturer’s protocol and used for real-time quantitative RT-PCR (qRT-PCR) as described (Ishiga and Ichinose 2016). Two micrograms of total RNA was treated with gDNA Eraser (Takara Bio) to eliminate genomic DNA, and the DNase-treated RNA was reverse transcribed using the PrimeScript RT reagent Kit (Takara Bio). The cDNA (1:20) was then used for qRT-PCR using the primers shown below with SYBR Premix Ex Taq II (Takara Bio) on a Thermal Cycler Dice Real Time System (Takara Bio). Cabbage UBQ1 gene was used as an internal control. Average CT values, calculated using the 2nd derivative maximum method from triplicate samples, were used to determine the fold expression relative to the controls. Primers used in gene-specific PCR amplification for PR1 (XM_013770002.1) were 5′-GGTCAACGAGAAGGCTAACTATAA-3′ and 5′-GCTTTGCCACATCCAATTCTC-3′; for PR2 (XM_013747042.1), 5′-GAAGAGTGGAACTCCGAGAAAG-3′ and 5′-AGGCTGTTGACTAGGAAGAAAC-3′; for PR5 (XM_013734443.1), 5′-GACGGCTACAACGTCAAGAT-3′ and 5′-CCATGACACGAAGCTCGTTA-3′; and for UBQ1 (XM_013746806.1), 5′-GTCAAGGCCAAGATCCAAGA-3′ and 5′-GGATGTTGTAGTCAGCCAGAG-3′.
Stomatal assay
A modified method was used to assess stomatal response as described previously (Chitrakar and Melotto 2010). Briefly, cabbage plants were grown for 2 weeks after germination as described previously. Pcal was grown at 28 °C for 48 h on MG agar, then suspended in sterile distilled water to an OD600 of 0.2. Dip-inoculated cabbage leaves were directly imaged at 1 hpi or 4 hpi using a Leica TCS SP8 confocal microscope equipped with a white light laser (Leica, Wetzlar, Germany). A reflected image of the leaf surface was obtained by illuminating the sample with 561 nm wavelength, and reflected light was detected through a 558–566 nm filter. The aperture width of at least 60 stomata was measured. The average and standard error for the stomatal aperture width were calculated. The stomatal apertures were evaluated in three independent experiments.
Results
ASM induces defense-related gene expression in cabbage
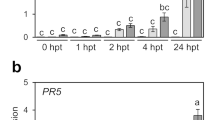
ASM treatments are well known to induce defense-related gene expression including PR proteins. PR1 is one of the most widely used genes to monitor the induction of SAR in plants (Lawton et al. 1996; Tripathi et al. 2010). To evaluate the effect of ASM on defense gene induction in cabbage, we investigated the expression profiles of PR1, PR2, and PR5 in response to ASM. Two-week-old cabbage plants were treated with water as a control (mock), or ASM, and then total RNA was isolated from samples fixed at 2, 6, 12, 24, 48, 72, and 168 h post-treatment (hpt). Soil was drenched with ASM. PR1 expression was induced in leaves within 2 h after ASM treatment and reached a maximum at 48 hpt (Fig. 1a). Similar to the PR1 expression profile, PR2 and PR5 expression was induced by ASM treatment and peaked at 48 hpt (Figs. 1b, c). These results indicate that ASM activates SA-mediated signaling pathways leading to PR protein accumulation.
Gene expression profiles involved in plant defense of 2-week-old cabbage plants in response to ASM. Total RNA was isolated at 2, 6, 12, 24, 48, 72, and 168 h post-treatment (hpt). Expression of aPR1, b PR2, and cPR5 was determined using RT-qPCR with gene-specific primer sets. Expression was normalized using UBQ1. Vertical bars indicate the standard error for three biological replicates
ASM suppresses Pcal disease development
To assess the effect of ASM on bacterial disease development, cabbage was challenged with Pcal 2 h after the soil drench with ASM, and disease symptoms were monitored. Figure 2a shows that control water-treated cabbage plants inoculated with Pcal, had typical bacterial blight symptoms at 5 dpi. However, symptoms were less severe on ASM-treated plants (Fig. 2a). In ASM-treated cabbage leaves, Pcal populations were significantly lower than in the water-treated control (Fig. 2b), suggesting that decreased bacterial multiplication was responsible for the decrease in severity. These results clearly indicate that ASM has a critical effect on suppressing Pcal disease development.
Disease symptoms and bacterial population dynamics in cabbage plants dip-inoculated with a Pcal (5 × 107 CFU/ml) suspension after ASM treatment. Two-week-old cabbage plants were treated with ASM as a soil drench. Two hours after ASM treatment, cabbage plants were dip-inoculated, and bacterial populations were estimated by dilution plating on selective medium as described in the methods. a Necrotic lesions surrounded by chlorotic haloes at 5 days post-inoculation (dpi). b Bacterial populations in leaves at 0, 3, and 5 dpi. Vertical bars indicate the standard error for three independent experiments. Asterisks indicate a significant difference from the water treatment in a t test (** P < 0.01)
ASM activates stomatal-based defense against Pcal
The stomatal-based defense mechanism in plants is responsible for closing stomata in response to the perception of PAMPs of invading pathogens, especially bacterial pathogens (Melotto et al. 2017). Since we demonstrated that a soil drench with ASM 2 h before inoculation can suppress disease development, we next examined cabbage stomatal-based defense after Pcal inoculation with or without ASM. As shown in Fig. 3a, ASM-triggered stomatal closure was not observed on mock-inoculated cabbage leaves at 1 or at 4 hpi. In contrast, stomata on both water- and ASM-treated cabbage leaves inoculated with Pcal were closed at 1 hpi, indicating that PTI, including stomatal-based defense, is induced in cabbage leaves against Pcal. Interestingly, stomata had reopened on water-treated, Pcal-inoculated leaves by 4 hpi, whereas stomata were still closed on Pcal-inoculated leaves treated with the ASM soil drench (Fig. 3a). Consistent with this stomatal-based defense in ASM-treated cabbage leaves, Pcal bacterial populations at 4 hpi were significantly lower than in the water-treated inoculated control (Fig. 3b). These results indicate that ASM activates stomatal-based defense against Pcal.
Stomatal aperture width (μm) and bacterial population dynamics in cabbage plants dip-inoculated with a Pcal suspension (1 × 108 CFU/ml) after ASM treatment. a Aperture width on intact cabbage leaves 1 h and 4 h after Pcal dip-inoculation. Two-week-old cabbage plants were treated with a soil drench of ASM. Two hours after ASM treatment, cabbage leaves were dip-inoculated, then imaged using a Leica TCS SP8 confocal microscope equipped with a white light laser. In all bar graphs, vertical bars indicate the standard error for three biological replicates. Asterisks indicate a significant difference from the water treatment in a t test (**P < 0.01). b Bacterial populations in leaves were estimated by dilution plating on selective media at 4 h post inoculation (hpi). Vertical bars indicate the standard error for three independent experiments. Asterisks indicate a significant difference from the water treatment in a t test (** P < 0.01)
Discussion
In our functional analysis of ASM, a well-known plant defense activator in the plant immune system, we found that a soil drench with ASM led to the accumulation of PR1, PR2, and PR5 mRNAs in cabbage leaves and effectively suppressed Pcal lesion formation on those leaves (Figs. 1 and 2). Surprisingly, ASM induced SAR against Pcal within 2 h after the soil drench, suggesting that mobile signals generated by ASM triggered SAR in untreated leaves. Several mobile SAR signals including methyl SA (MeSA), azelaic acid (AzA), dihydroabetinal (DA), and glycerol-3-phosphate (G3P) have been identified (Chanda et al. 2011; Chaturvedi et al. 2012; Jung et al. 2009; Yu et al. 2013). On the other hand, unlike SA, ASM is highly mobile in tobacco plants (Friedrich et al. 1996). Tripathi et al. (2010) reported that ASM is converted to acibenzolar by SA-binding protein 2 (SABP2), and functional SABP2 is required for ASM-mediated SAR in tobacco plants. Homologs for SABP2 have also been identified in Arabidopsis, and production of these proteins is induced in response to infection with avirulent Pseudomonas syringae, suggesting that cabbage plants also have homologs for SABP2 to catalyze the conversion of ASM to acibenzolar (Vlot et al. 2008). Moreover, ASM might be absorbed by roots and translocated to aboveground parts via transpiration and act directly to induce SAR. Together, these results suggest that ASM or acibenzolar is a highly mobile signal that triggers SAR in cabbage plants.
We also demonstrated that bacterial entry through stomata was more than 100 times greater in water-treated leaves compared with leaves after the ASM soil drench (Fig. 3b), indicating that stomatal-based defense has a crucial role in PTI against invading bacterial pathogens. It is important to note that when virulent P. syringae pv. tomato DC3000 (Pst DC3000) was used to infiltrate the apoplastic space in ASM-treated Arabidopsis leaves, thus bypassing the stomatal-based defense, symptom severity and bacterial populations were both reduced (Lawton et al. 1996), indicating that ASM application contributes not only to stomatal-based defense, but also to other plant immune systems. Therefore, the responses to ASM in various crop plants needs to be further characterized to understand the mechanisms by which plant defense activators contribute to disease control.
Our stomatal response assay demonstrated that stomatal closure was induced in cabbage leaves after the ASM soil drench in response to Pcal infection at 4 hpi (Fig. 3a). Interestingly, the stomata did not close after the ASM treatment unless Pcal was present (Fig. 3a). Stomatal-based defense is characterized by stomatal closure after perception of PAMPs of invading pathogens, especially bacterial pathogens (Melotto et al. 2017). We also showed stomatal closure of cabbage leaves in response to Pcal as a PTI at 1 hpi (Fig. 3a). Successful bacterial pathogens, however, have acquired virulence factors including phytotoxins and type III secretion system (TTSS) effectors, to overcome stomatal-based defense (Lozano-Durán et al. 2014; Melotto et al. 2017). Genome analysis of multiple Pcal strains revealed that Pcal also has these virulence factors (Sarris et al. 2013). Consistent with previous studies on P. syringae, stomata had reopened by 4 hpi on water-treated, inoculated cabbage leaves, but not on ASM-treated inoculated leaves (Fig. 3a), indicating that Pcal can overcome stomatal-based defense. Prodhan et al. (2017) showed the involvement of endogenous SA in PAMP-induced apoplastic ROS production in Arabidopsis thaliana, which resulted in stomatal closure. Furthermore, SA-activated SHAM-sensitive peroxidases generate apoplastic ROS, leading to stomatal closure in A. thaliana (Prodhan et al. 2018; Toum et al. 2016), suggesting that ASM or ASM-related SAR signals can trigger SHAM-sensitive peroxidases associated with ROS production in cabbage. Further characterization of ASM or ASM-related SAR signals in stomatal-based defense is needed to understand the plant immune system, especially PTI.
PR1, PR2, and PR5 transcripts began to accumulate in cabbage within 2–6 h after the ASM soil drench (Fig. 1), in agreement with the expression of PR1, PR2, and PR5 in A. thaliana after ASM application and during SAR (Lawton et al. 1996; Uknes et al. 1992). ASM also induced PR1 and PR5 expression in maize leaves (Morris et al. 1998). These results suggest that PR1, PR2, and PR5 can serve as SAR marker genes in cabbage.
In this study, we first demonstrated that an ASM soil drench protects cabbage plants against Pcal, a causal agent of bacterial blight disease, by enhancing their inherent disease resistance mechanisms, such as stomatal-based defense. Because numerous foliar bacterial pathogens target stomata as an entry site, we believe that ASM and/or ASM-related SAR signals will provide an additional disease management tool to prevent crop disease losses against bacterial pathogens.
References
Bektas Y, Eulgem T (2015) Synthetic plant defense elicitors. Front Plant Sci 5:804
Chanda B, Xia Y, Mandal MK, Yu K, Sekine KT, Gao QM, Selote D, Hu Y, Stromberg A, Navarre D, Kachroo A, Kachroo P (2011) Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat Genet 43:421–427
Chaturvedi R, Venables B, Petros RA, Nalam V, Li M, Wang X, Takemoto LJ, Shah J (2012) An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J 71:161–172
Chitrakar R, Melotto M (2010) Assessing stomatal response to live bacterial cells using whole leaf imaging. J Vis Exp 44:e2185
Friedrich L, Lawton K, Ruess W, Masner P, Specker N, Rella MG, Meier B, Dincher S, Staub T, Uknes S, Métraux JP, Kessmann H, Ryals J (1996) A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J 10:61–70
Görlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, Kogel KH, Oostendorp M, Staub T, Ward E, Kessmann H, Ryals J (1996) Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 8l:629–643.
Hacquard S, Spaepen S, Garrido-Oter R, Schulze-Lefert P (2017) Interplay between innate immunity and the plant microbiota. Annu Rev Phytopathol 55:565–589
Horinouchi H (2010) Occurrence and control of root browning symptom of Japanese radish caused by Pseudomonas syringae pv. maculicola (in Japanese). Plant Prot (Shokubutsu Boueki) 64:220–223
Ishiga Y, Ichinose Y (2016) Pseudomonas syringae pv. tomato OxyR is required for virulence in tomato and Arabidopsis. Mol Plant Microbe Interact 29:119–131
Ishiga T, Ishiga Y, Betsuyaku S, Nomura N (2018) AlgU contributes to the virulence of Pseudomonas syringae pv. tomato DC3000 by regulating production of the phytotoxin coronatine. J Gen Plant Pathol 84:189–201
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT (2009) Priming in systemic plant immunity. Science 324:89–91
Keane PJ, Kerr A, New PB (1970) Crown gall of stone fruit II. Identification and nomenclature of Agrobacterium isolates. Aust J Biol Sci 23:585–595
Kunz W, Schurter R, Maetzke T (1997) The chemistry of benzothiadiazole plant activators. Pestic Sci 50:275–282
Lawton KA, Hunt Friedrich L, M, Weymann K, Delaney T, Kessmann H, Staub T, Ryals J, (1996) Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J 10:71–82
Lozano-Durán R, Bourdais G, He SY, Robatzek S (2014) The bacterial effector HopM1 suppresses PAMP-triggered oxidative burst and stomatal immunity. New Phytol 202:259–269
Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126:969–980
Melotto M, Underwood W, He SY (2008) Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol 46:101–122
Melotto M, Zhang L, Oblessuc PR, He SY (2017) Stomatal defense a decade later. Plant Physiol 174:561–571
Morris SW, Vernooij B, Titatarn S, Starrett M, Thomas S, Wiltse CC, Frederiksen RA, Bhandhufalck A, Hulbert S, Uknes S, (1998) Induced resistance responses in maize. Mol Plant Microbe Interact 11:643–658
Oostendorp M, Kunz W, Dietrich B, Staub T (2001) Induced disease resistance in plants by chemicals. Eur J Plant Pathol 107:19–28
Prodhan MY, Issak M, Nakamura T, Munemasa S, Nakamura Y, Murata Y (2017) Chitosan signaling in guard cells requires endogenous salicylic acid. Biosci Biotechnol Biochem 81:1536–1541
Prodhan MY, Munemasa S, Nahar MN, Nakamura Y, Murata Y (2018) Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol 178:441–450
Sarris PF, Trantas EA, Baltrus DA, Bull CT, Wechter WP, Yan S, Ververidis F, Almeida NF, Jones CD, Dangl JL, Panopoulos NJ, Vinatzer BA, Goumas DE (2013) Comparative genomics of multiple strains of Pseudomonas cannabina pv. alisalensis, a potential model pathogen of both monocots and dicots. PLoS ONE 8:59366
Takahashi F, Ochiai M, Ikeda K, Takikawa Y (2013a) Streptomycin and copper resistance in Pseudomonas cannabina pv. alisalensis (abstract in Japanese). Jpn J Phytopathol 79:35
Takahashi F, Ogiso H, Fujinaga M, Ishiyama Y, Inoue Y, Shirakawa T, Takikawa Y (2013b) First report of bacterial blight of crucifers caused by Pseudomonas cannabina pv. alisalensis in Japan. J Gen Plant Pathol 79:260–269
Takikawa Y, Takahashi F (2014) Bacterial leaf spot and blight of crucifer plants (Brassicaceae) caused by Pseudomonas syringae pv. maculicola and P. cannabina pv. alisalensis. J Gen Plant Pathol 80:466–474
Toum L, Torres PS, Gallego SM, Benavídes MP, Vojnov AA, Gudesblat GE (2016) Coronatine inhibits stomatal closure through guard cell-specific inhibition of NADPH oxidase-dependent ROS production. Front Plant Sci 7:1851
Tripathi D, Jiang YL, Kumar D (2010) SABP2, a methyl salicylate esterase is required for the systemic acquired resistance induced by acibenzolar-S-methyl in plants. FEBS Lett 584:3458–3463
Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J (1992) Acquired resistance in Arabidopsis. Plant Cell 4:645–656
Underwood W, Melotto M, He SY (2007) Role of plant stomata in bacterial invasion. Cell Microbiol 9:1621–1629
Vlot AC, Liu PP, Cameron RK, Park SW, Yang Y, Kumar D, Zhou F, Padukkavidana T, Gustafsson C, Pichersky E, Klessig DF (2008) Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J 56:445–456
Yu K, Soares J, Mandal MK, Wang C, Chanda B, Gifford AN, Fowler JS, Navarre D, Kachroo A, Kachroo P (2013) A feedback regulatory loop between G3P and lipid transfer proteins DIR1 and AZI1 mediates azelaic-acid-induced systemic immunity. Cell Reports 3:1266–1278
Zhou M, Wang W (2018) Recent advances in synthetic chemical inducers of plant immunity. Front Plant Sci 9:1613
Zipfel C (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20:10–16
Zipfel C, Felix G (2005) Plants and animals: a different taste for microbes? Curr Opin Plant Biol 8:353–360
Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428:764–767
Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125:749–760
Acknowledgements
We thank Dr. Christina Baker for editing the manuscript. Pcal was kindly provided by the Nagano Vegetable and Ornamental Crops Experiment Station, Nagano, Japan. This work was supported in part by the JST ERATO NOMURA Microbial Community Control Project, JST, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human participants or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishiga, T., Iida, Y., Sakata, N. et al. Acibenzolar-S-methyl activates stomatal-based defense against Pseudomonas cannabina pv. alisalensis in cabbage. J Gen Plant Pathol 86, 48–54 (2020). https://doi.org/10.1007/s10327-019-00883-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-019-00883-5