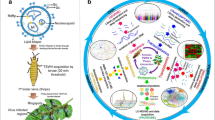
Abstract
Tomato spotted wilt tospovirus (TSWV), a type species of the genus Orthotospovirus, which belongs to the family Tospoviridae, causes necrotic yellow and necrosis diseases of vegetables including tomatoes and green peppers, and to flowers such as chrysanthemum and dahlia, and causes economic damage, yield loss, and loss of quality. TSWV has a spherical enveloped virion that contains three species of singlestrand RNA segments (L, M, and S). The genome encodes fives genes; a putative cell-to-cell movement protein (NSm), a non-structural protein (NSs), a nucleocapsid protein (N), an RNA-dependent RNA polymerase, and a precursor of surface glycoproteins (GN/GC). TSWV is transmitted in a circulative and propagative manner by thrips such as western flower thrips (Frankliniella occidentalis) and onion thrips (Thrips tabaci). TSWV has been controlled using the virus resistance genes of the cultivars and by controlling the vector using insecticides. However, the emergence of TSWV that can no longer be controlled using the resistance gene of the cultivars and the development of resistance to insecticides by thrips is a serious problem worldwide. Hence, the interactions among TSWV, western flower thrips, and Arabisopsis thaliana were analysed to develop a novel way to control TSWV on the basis of the interaction mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most plant viruses are transmitted by insects such as aphids, whiteflies, and thrips. Viruses induce chemical and physical changes in the host to be efficiently transmit from plant to plant (Hurd 2003). Additionally, plant viruses can modify the feeding preferences or feeding behavior of their insect vector to enhance transmission efficiency and spread (Ingwell et al. 2012).
Tomato spotted wilt tospovirus (TSWV), the type species of the genus Orthotospovirus, which belongs to the family Tospoviridae, causes necrotic yellow and necrosis diseases of vegetables such as tomatoes and green peppers and flowers such as chrysanthemum and dahlia, resulting in quality, yield and economic losses. TSWV has a spherical enveloped virion that contains three species of single-strand RNA segments (L, M, and S). The genome encodes fives genes: a putative cell-to-cell movement protein (NSm), a nonstructural protein (NSs), a nucleocapsid protein (N), an RNA-dependent RNA polymerase, and a precursor of surface glycoproteins (GN/GC). TSWV is transmitted in a circulative and propagative manner by thrips such as western flower thrips (Frankliniella occidentalis) and onion thrips (Thrips tabaci). TSWV has been controlled using viral resistance genes of the cultivars and by controlling the vector using insecticides. However, the emergence of TSWV that can no longer be controlled using the resistance gene of the cultivars and the development of resistance to insecticides by thrips is a serious problem worldwide. Hence, the interactions among TSWV, western flower thrips, and Arabidopsis thaliana were analyzed in the present study to develop a novel way to control TSWV on the basis of the interaction mechanism.
Attractiveness of TSWV-infected plants for western flower thrips
Maris et al. (2004) reported that TSWV-infected plants attract their vector thrips more efficiently than uninfected plants. Abe et al. (2008, 2009) reported that jasmonate (JA) plays an important role in a plant’s response and resistance to thrips and that JA-regulated plant defense has a negative impact on the performance and preference of thrips. However, the mechanism of the interaction among TSWV, thrips, and plants was largely unknown. To elucidate the effect of TSWV infection on thrips feeding, we analyzed differences in feeding damage caused by thrips between TSWV-infected plants and mock plants. Feeding damage caused by western flower thrips in TSWV-infected plants was more severe than in mock plants. Additionally, we analyzed the number of adults and larvae to investigate the effect of TSWV infection on the thrips population. We found that adults and larvae were much more abundant on TSWV-infected plants than on mock plants.
To better understand this mechanism, we analyzed the defense response of the host plants. The expression of the salicylic acid (SA)-regulated marker genes, β-1,3-glucanase 2 (BGL2) and pathogenesis-related gene 1 (PR1) was upregulated by TSWV infection. On the contrary, although the expression of the JA-regulated marker genes, lipoxygenase 2 (LOX2) and vegetative storage protein 2 (VSP2) was upregulated by thrips feeding, the expression of these genes after thrips fed on TSWV-infected plant was lower.
We also analyzed the effect of TSWV infection on the thrips. We performed a thrips choice assay for mock plants and TSWV-infected plants. In the assay using wild-type A. thaliana plants (Colombia), the thrips were attracted to the TSWV-infected plants. Conversely, when the coi1-1 (coronatine insensitive 1–1) mutant was used for the choice assay, the thrips were not attracted to TSWV-infected plants. Therefore, our findings revealed that crosstalk between SA and JA plays an important role in the attractiveness of TSWV-infected plants for western flower thrips (Abe et al. 2012).
Attractiveness of thrips-nontransmissible TSWV-infected plants for western flower thrips
For investigating the relationship between vector preference for TSWV-infected plants and the vector transmissibility of TSWV, we analyzed whether thrips were attracted to A. thaliana plants that were infected with thrips-nontransmissible TSWV.
Thrips-nontransmissible TSWV isolate (TSWV-Mo) was obtained from a wild-type TSWV (TSWV-wt) isolate via single local-lesion isolation using Petunia × hybrida after several passages in Nicotiana rustica plants. In the transmission test, although TSWV-wt was transmitted by western flower thrips and onion thrips (transmission ratio: western flower thrips, 25%; onion thrips, 10%), TSWV-Mo was never transmitted by the thrips. Feeding damage caused by western flower thrips in A. thaliana plants was more severe in TSWV-wt-infected plants than in TSWV-Mo-infected plants, despite comparable preferences.
The expression analyses of the marker genes associated with plant defenses showed that SA-regulated marker genes BGL2 and PR1 were upregulated 3- to 6-fold by both TSWV-wt and TSWV-Mo infection. Conversely, the expression of JA-regulated marker genes LOX2 and VSP2 was not affected by the infections.
Pull assays for thrips were performed for analyzing the potential of thrips-nontransmissible TSWV-infected plants as attractants to control the behavior of western flower thrips. The results showed that western flower thrips preferred adjacent TSWV-Mo-infected plants rather than uninfected plants, suggesting that TSWV-Mo-infected plants can be used as attractants for behavior control of thrips. In conclusion, the findings of this study showed that the transmissibility of TSWV by thrips is not associated with the preference of vector thrips. In other words, the interaction between TSWV and plants plays an important role in the attractiveness of TSWV-infected plants for western flower thrips (Tomitaka et al. 2015).
References
Abe H, Ohnishi J, Narusaka M, Seo S, Narusaka Y, Tsuda S, Kobayashi M (2008) Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol 49:68–80
Abe H, Shimoda T, Ohnishi J, Kugimiya S, Narusaka M, Seo S, Narusaka Y, Tsuda S (2009) Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biol 9:97
Abe H, Tomitaka Y, Shimoda T, Seo S, Sakurai T, Kugimiya S, Tsuda S, Kobayashi M (2012) Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol 53:204–212
Hurd H (2003) Manipulation of medically important insect vectors by their parasites. Annu Rev Entomol 48:141–161
Ingwell LL, Eigenbrode SD, Bosque-Pérez NA (2012) Plant viruses alter insect behavior to enhance their spread. Sci Rep 2:578
Maris PC, Joosten NN, Goldbach RW, Peters D (2004) Tomato spotted wilt virus infection improves host suitability for its vector Frankliniella occidentalis. Phytopathology 94:706–711
Tomitaka Y, Abe H, Sakurai T, Shiny T (2015) Preference of the vector thrips Frankliniella occidentalis for plants infected with thrips-non-transmissible Tomato spotted wilt virus. J Appl Entomol 139:250–259
Acknowledgements
I express my heartfelt gratitude to Dr. Shinya Tsuda, Professor, Hosei University, for his valuable guidance and advice throughout this research. I also express my sincere gratitude to Dr. Masatomo Kobayashi and Dr. Hiroshi Abe of RIKEN BioResource Center, Dr. Tamito Sakurai of the National Agricultural Research Center of NARO, and researchers and technical assistants at the RIKEN Bioresource Center and the NARO for their advice and support in conducting our research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflicts of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tomitaka, Y. Studies on the interaction between tomato spotted wilt tospovirus and thrips. J Gen Plant Pathol 85, 465–467 (2019). https://doi.org/10.1007/s10327-019-00879-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-019-00879-1