Abstract
The potential antifungal activities of a soy protein fraction containing mainly β-conglycinin and glycinin against the pathogenic fungus Penicillium digitatum, either in vitro or in situ (postharvest orange fruit), were evaluated and compared with the fungicide rhizolax. Traditional microbiological plating was used to evaluate the potential in vitro antifungal activities of the fraction containing β-conglycinin or glycinin. The probable changes in the morphology and cellular compositions of fungal hyphae treated with glycinin and the soy protein fraction containing β-conglycinin were further examined by scanning electron microscopy. The obtained results showed that the soy protein fraction containing β-conglycinin significantly inhibited the in vitro growth of P. digitatum at a wide concentration range (50–3,000 mg/L). It could also completely inhibit the spore germination of P. digitatum at 2,000 mg/L. The MIC and MFC of β-conglycinin against P. digitatum were 50 and 1,900 mg/L, respectively. The soy protein fraction containing β-conglycinin totally inhibited the development of green mould in fruit at 250 mg/L for 7 days after infection with the fungus, and disease incidence and severity remained at minimal levels after 21 days (i.e., 22 and 25 %, respectively). SEM images indicated clear antifungal activity of β-conglycinin against P. digitatum. β-conglycinin caused deformation and death of fungal hyphae and inhibited development of conidiophores and conidia. These results suggest that the soy protein fraction containing β-conglycinin can be used as an effective environmentally friendly fungicidal agent against postharvest fungal infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus fruit has many health benefits, e.g., reducing the risk of cancer and cardiac disease incidence (Attaway 1994) because of its high contents of vitamin C and other bioactive compounds including flavonoids and phenolic acids (Widmer and Montanari 1996). Yet, the spread of fungal infection is a major problem facing the production of citrus fruit all over the world as it causes considerable economic losses after harvest during handling, transportation and storage (Janisiewicz and Korsten 2002). Fungal infection can seriously damage crops, and the quality of postharvest citrus fruit is influenced by physiologic and pathogenic factors. Green mould may cause 60–80 % decay under ambient conditions (Moscoso-Ramírez et al. 2013). Citrus fruit are particularly susceptible to green and blue moulds caused by Penicillium digitatum and Penicillium italicum, respectively (Castillo et al. 2014; Droby et al. 2008). Several authors reported that the causal fungus can be controlled by fungicides such as Imazalil, sodium ortho-phenyl phenate, thiabendazole or octanal (Torres et al. 2007; Tao et al. 2014; Yildiz et al. 2005). However, controlling fungi by conventional fungicides as benzimidazoles and dicarboximides is a critical issue because of the potential resistance arising in the pathogens (Elad et al. 1992). Additionally, a growing public trend has emerged to limit the use of synthetic fungicides to avoid their potentially high and acute residual toxicity in the environment and on food and possible side effects on human health (Askarne et al. 2012; Smilanick et al. 2006; Tripathi and Dubey 2004).
Consequently, the need to explore novel antifungal safe agents to replace current control strategies and reduce our dependence on synthetic fungicides is urgent. Recently, Wickerhamomyces anomalus yeast was reported as effective at reducing weight loss and maintaining firmness of ‘Valencia’ oranges during storage. It reduced the green mould of P. digitatum in inoculated fruit by more than 73 % after 13 days of storage (Aloui et al. 2015). The use of antifungal proteins as a biological control technique has captured the attention of numerous investigators because of the potential economic and health benefits. Glycoproteins of diverse structures such as lectin from Egyptian Pisum sativum seeds may have antifungal activities (Sitohy et al. 2007). Lactoferrin, an iron-binding glycoprotein naturally present in glandular secretions (milk, tears, saliva) has broad-spectrum antimicrobial activity with potent activity against Candida biofilms (Bink et al. 2011).
Soybeans are one of the most important plant protein sources that also have antimicrobial activity (Sitohy et al. 2012; Osman et al. 2013, 2016). One of the main protein components, β-conglycinin, is a glycoprotein that has approximately 5 % carbohydrate, mainly from high-mannose moieties (Kimura et al. 1997). Based on the potential antifungal properties of glycoproteins, the main aim of the current study was to evaluate the potential in vitro antifungal activities of β-conglycinin isolated from soybean and to assess its efficacy in controlling the growth of P. digitatum in postharvest orange fruit. The mode of action of this potential antifungal activity against the growth of P. digitatum was elucidated by scanning electron microscopy (SEM).
Materials and methods
Materials
Rhizolax (tolclofos-methyl) was obtained from a local pesticide company. Soybean (Glycine max L.) seeds were purchased from a local market, Zagazig City, Egypt.
Preparation of protein fractions
Soybean seeds were ground to pass through a 1-mm2 sieve. The resulting powder was defatted for 8 h, using a 3:1 (v/v) chloroform–methanol mixture. The obtained defatted material was combined with water and adjusted to pH 9 with 0.1 N NaOH at room temperature to dissolve protein components. The supernatant was adjusted to pH 4.5 with 1 N HCl (Johnson and Brekke 1983) to precipitate the soy protein fraction, which was subsequently used to isolate glycinin and β-conglycinin according to Nagano et al. (1992) by dissolution at pH 8, then precipitation at pH 6.4 and pH 5, respectively.
Chemical characterization of protein fractions
Ten milligrams of each protein fraction, β-conglycinin and glycinin, was combined with 1000 μL of loading buffer pH 6.8 (1 M Tris–HCl, 50 % glycerol, 10 % SDS, 10 % β-mercaptoethanol, 0.1 % bromophenol blue), heated at 100 °C for 10 min and loaded onto 12 % SDS-PAGE at a volume of 10 µL/well. Protein bands were visualized on the gels by Coomassie brilliant blue R250 (Laemmli 1970). Protein pH-solubility curves were generated for the pH range of 2–10 according to Chobert et al. 1991. The isoelectric points were estimated from the protein pH-solubility curves, being the pH corresponding to the least protein solubility. Carbohydrate content in β-conglycinin was determined according to DuBois et al. 1956 by the dehydration of carbohydrates into furfural derivatives and reacting them with phenol to develop a correlated color measurable at 490 nm. The content of total phenolic compounds was determined in the protein samples by combining 0.1 g of soy protein isolate, β-conglycinin and glycinin with 4 mL 80 % methanol and stirring at room temperature for 24 h. The mixtures were centrifuged at 3,000 rpm for 10 min. The residue was re-extracted twice like before, and the resulting methanolic extracts were combined and used for the determination of total phenolic compounds by the Folin–Ciocalteau assay as described by Singleton and Rossi (1965) with minor modifications. Gallic acid was used as the standard. The results were expressed as milligrams of gallic acid equivalents per gram of dried sample. The isoflavone content in soybeans represents about 72 % of the total phenols (Seo and Morr 1984).
In vitro antifungal activity
Activity on mycelial growth
The effect of 50, 100, 250 and 500 mg/L soybean protein fraction (SPI), soy glycinin (11S), and soy β-conglycinin (7S) on mycelial growth of P. digitatum was compared with the effect of Rhizolax (positive control) using the poisoned food technique (Yahyazadeh et al. 2009). A 6-mm mycelial agar plug from a 7-day-old culture of P. digitatum was placed at the center of each potato dextrose agar (PDA: extract of boiled potatoes, 200 mL; dextrose, 20 g; agar, 20 g and distilled water, 800 mL) plate, and volumes of the tested substances calculated to achieve the various test concentrations were added. This experiment was repeated at a wider concentration range for β-conglycinin (50–3,000 mg/L) under similar conditions. Approximately, 0.05 % (v/v) Tween-80 was then added to the media. Petri dishes were sealed with parafilm and incubated for 7 days at 25 °C. The diameter (mm) of colony zone was measured with a caliper. All of the tests were performed in triplicate. The lowest concentration that inhibits the growth of the fungus after 48 h of incubation at 25 °C was considered as the minimum inhibitory concentration (MIC). The minimum fungicidal concentration (MFC) was regarded as the lowest concentration preventing pathogen growth after 96 h of incubation at 25 °C on a fresh PDA plate, thereby indicating fungicidal activity >99.5 % of the original inoculum (Talibi et al. 2012).
Activity against conidial germination
The activity of β-conglycinin and glycinin on P. digitatum spore germination was determined in potato dextrose broth (PDB) using the dispersed slide technique according to Droby et al. 1997. An aliquot (0.5 mL) of the spore suspension (105 spores/mL) of P. digitatum was combined with 0.5 mL of β-conglycinin or glycinin at different concentrations (50–3,000 mg/L) or sterile distilled water as a control and placed on the cavity of the dispersed slide, which was laid on a U-shaped glass rod in a moistened Petri dish. Petri dishes were incubated at 25 °C for 24 h to allow spore germination to complete. After the incubation period, the germinated spores were counted and compared with the control treatment. The extent of germination reduction (R%) was calculated as R% = [(A − B)/A] × 100, where A is the percentage of germinated conidia in the control and B is the percentage of germinated conidia in the treatment.
In situ antifungal activity
Orange fruit preparation
Orange fruit (Citrus sinensis) were purchased from a wholesale distributor located in Zagazig City (Egypt) at commercial maturity and transported to the laboratory in polystyrene boxes to avoid mechanical damage, at ambient conditions of temperature and humidity (18 °C and 75 % RH). Orange fruit were visually selected based on uniform shape, size, color, firmness and absence of mechanical injuries or fungal infection and used within 1 day of purchasing. Selected orange fruit were washed with a solution of sodium hypochlorite (0.1 %) for 1 min, then drained, rinsed with fresh air and air-dried at room temperature (25 °C) before being treated at the same day.
Pathogen and preparation of inoculum
P. digitatum was isolated from an infected orange fruit. The isolate was maintained on PDA at 4 °C, and fresh cultures were grown on PDA plates before use as inoculum.
For the inoculation trials, the P. digitatum isolate was grown on PDA in Petri dishes and incubated at 25 °C for 10 days. Conidia were collected by washing the colonies with sterile distilled water containing 0.05 % (v/v) Tween 80, counted with a hemacytometer and diluted with PDB to 106 spores/mL. The conidial suspension was subsequently dispensed either onto molten soft PDA (0.8 % agar) or into PDB in microwells either for the agar spot assay or the broth microdilution assay.
Fruit inoculation and treatment
Uniform wounds (3-mm deep and 3-mm wide) were made at the equator of each fruit using the tip of a sterile dissecting needle. Twenty microliters of soy protein isolate (SPI), β-conglycinin, glycinin solutions (250 mg/L) or sterilized distilled water was pipetted into each wound in the orange fruit. Two hours later, 15 µL of ~1 × 106 conidia/mL of P. digitatum was added to each wound (Fan et al. 2014). After air-drying, the fruit were sealed in polyethylene-lined 5-L plastic boxes and incubated at 25 °C for 7 days. The form and diameter of any lesions were observed and recorded after 7 days of inoculation to calculate the disease incidence (percentage of wounds with lesions) as Disease incidence = (no. of fruits with lesions)/(total no. of treated fruits) × 100. Disease severity was determined according to the area of the lesion on each fruit using the formula of Masood et al. (2010): Disease severity = (lesion area/Total tissue area) × 100.
The study included three replicate trials of 10 fruit per treatment with a complete randomization in each test, and the test was repeated three times.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) analysis was performed according to Sitohy et al. (2013). Fifty microliters of β-conglycinin, Rhizolax (50 %) or sterile distilled water (control) was added to a 250 mL Erlenmeyer flask containing 50 mL PDB, to which 1 mL of 5 × 107 conidia/mL of P. digitatum was added. After 5 days of incubation at 25 °C on a rotary shaker (50 rpm), the fungal biomass was recovered by centrifugation at 4500×g for 15 min at 4 °C, washed with PBS (pH 7.4) and fixed in 2.5 % glutaraldehyde in PBS. The fixed fungal pellet was then dehydrated in a graded alcohol series, dried and mounted onto stubs using double-sided carbon tape. The fungal specimens were sputter-coated with gold (Polaron sputter coater) and examined with a JEOL scanning electron microscope JXA-840A.
Statistical analyses
The data obtained (means of 3 replicates) for disease incidence, disease severity, linear growth and spore germination were tested for significant differences (P < 0.05 level) using an analysis of variance (ANOVA) in the program SPSS/PC version 12 (IBM Co, Somers, NY, USA). When the differences were statistically significant, Duncan’s multiple range tests were applied to separate the means.
Results
Chemical characterization of protein fractions
Electrophoresis separated the soy protein fraction containing β-conglycinin into three bands (97, 62 and 48 kD) (Fig. 1a, lane 1). Four minor bands were also present, two of which are from the acidic subunit of glycinin, based on the comparison with the bands in the glycinin fraction in lane 2. The identity of the other two contaminant bands are unknown, but their contents seem trivial. The glycinin fraction (lane 2) separated into a band of about 30 kD that corresponds to the acidic subunits and the other at 20 kD that corresponds to the basic subunits. The pH solubility curve (Fig. 1b) of each protein fraction showed the least soluble point at pH 5 and 6.5 for the fraction containing mainly β-conglycinin and glycinin, respectively. The fraction containing β-conglycinin contained 60 mg carbohydrate/g protein, i.e. 6 % (data not shown).
SDS-PAGE (a) and pH-solubility curves (b) of soybean protein fractions β-conglycinin (1) and glycinin (2). St standard markers for protein molecular mass, AS and BS refer to the acidic and basic subunits of glycinin. The values are mean ± SE of three replicate. The bands α′, α and β refers to the main subunits of β-conglycinin (with molecular masses of 97, 62 and 48 kD)
The total phenolic compounds contents in the three protein fractions (soy protein isolate, the fraction containing β-conglycinin and glycinin) were 2.4, 1.4 and 1.3 mg gallic acid/g protein. The isoflavonoid contents may be estimated at 1.73, 1.0 and 0.94 mg/g protein based on the fact that isoflavonoids constitute 72 % of the total phenolic compounds (Seo and Morr 1984). After the three different protein fractions were washed with 80 % methanol (40 mL/g protein), the phenolic compounds were totally removed and could not be detected.
In vitro antifungal activity
The in vitro antifungal activities (Fig. 2a, b) of total soybean protein (S), soybean glycinin (A) and the soy protein fraction containing β-conglycinin (B) compared with Rhizolax against P. digitatum were examined at different concentrations (50, 100, 250 and 500 mg/L). Rhizolax inhibited mycelial growth at a wide concentration range (50–500 mg/L), while neither the soybean protein extract nor glycinin inhibited growth under the same conditions. Rhizolax achieved a maximum inhibition at 100 mg/L equivalent to 88 % growth reduction after 5 days of incubation at 25 °C. On the other hand, the fraction containing β-conglycinin showed a concentration-dependent inhibitory action on the fungal growth. The highest inhibitory extent (75 % mycelial growth reduction) was produced after 5 days at 500 mg/L. This difference may be due to the large size of the protein molecule, which translates to higher w/v concentrations. In a similar experiment to examine the potential maximum action of the tested protein (Fig. 2c) using 0–3,000 mg/L of the fraction containing β-conglycinin, fungal growth was completely inhibited by 2,000–3,000 mg/L. Based on the obtained results, the MIC and MFC of the fraction containing β-conglycinin against P. digitatum were estimated as 50 and 1,900 mg/L, respectively. Repeating the same experiment on the washed β-conglycinin fraction (phenolic compounds and isoflavones removed) gave nearly the same inhibitory activity as the product with the phenolic compounds (1.3 mg/g protein equivalent to 0.94 mg isoflavone/g protein) (data not shown).
Linear growth (a) and percentage growth reduction (b) of Penicillium digitatum after 7 days at 25 °C in the presence of soybean protein fraction (S), soy glycinin (A) and β-conglycinin fraction (B) as compared with rhizolax (C) at low concentrations (0, 50, 100, 250 and 500 mg/L) and of the β-conglycinin fraction (c) at a wider range of concentrations (0, 50, 100, 250 and 500, 1,000, 1,500, 2,000 and 3,000 mg/L). Values are mean ± SE of three replicates
In line with these results, high concentrations (2,000–3,000 mg/L) of the fraction containing β-conglycinin completely prevented germination of conidia, but the glycinin subunit did not inhibit germination at all (Fig. 3). Repeating this experiment using the washed glycinin and the washed β-conglycinin fraction gave nearly the same results without any significant differences (data not shown).
In situ antifungal activity
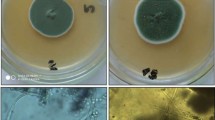
Observations of the treated intact orange fruit (Fig. 4) showed that after 3 days of incubation at room temperature, soy protein fraction did not prevent green mould, and glycinin only slightly limited green mould. After 7 days, mould growth had enormously increased in fruit treated with either soy protein fraction or glycinin. On the other hand, the fraction containing β-conglycinin completely prevented mould after 3 and 7 days of infection under the same conditions. After 3 days of incubation, the rot incidence rate in the fruit treated with SPI or control was 35 %. The rot incidence rates of green mould increased over time. After 7 day of storage, incidence rates in the SPI-treated fruit, glycinin-treated or control were all 100 %, compared with 0 % incidence on fruit treated with the fraction containing β-conglycinin.
Figure 5 indicates that the disease incidence for the positive control (PC) gradually increased during the first 7 days of infection, reaching nearly 100 % by day 7. Disease severity progressed at the same time in the infected fruit, reaching a maximum of 69 % after 21 days. Oranges treated with the fraction containing β-conglycinin (250 mg/L) had minimum disease incidence and severity approaching 0 % after 7 days of incubation. With incubations longer than 7 days, these two indicators progressively increased, reaching 22 and 25 % in the fruit treated with the fraction containing β-conglycinin by 21 days.
Effect of pretreatment with 250 mg/L of β-conglycinin fraction (B) on green mould incidence (%) and disease severity (%) on orange (Citrus sinensis) fruit 2 h before inoculation with Penicillium digitatum (10 µL of 1 × 105 conidia/mL) as compared with negative and positive controls (NC and PC, respectively). Values are mean ± SE of three replicates
Morphology of the treated fungus
SEM images of P. digitatum treated with the fraction containing β-conglycinin or Rhizolax (100 mg/L) for 4 h at room temperature are shown in Fig. 6. The untreated control fungus had typical hyphae with apparently intact walls. Hyphae in the Rhizolax and the fraction containing β-conglycinin looked deformed and degraded and conidiophores and conidia were under-developed compared with the control. Mycelia were abundant and conidiophores evident in the control samples, but both forms of development were nearly absent after treatments with β-conglycinin or Rhizolax. There were no distinctive differences between the fungus treated with the fraction containing β-conglycinin and that treated with Rhizolax.
Discussion
The electrophoretic patterns of the soy protein fractions are in accordance with previous literature confirming the molecular identity of each one; the fraction containing β-conglycinin had the main three subunits that correspond in size to the α′-, α- and β-subunits of β-conglycinin. The main effect of this fraction may be attributed to β-conglycinin subunit, which are visibly the most constituting of this fraction.
Glycinin has 20 and 30 kD subunits that correspond to the basic and acidic subunits. The isoelectric points of the fraction containing β-conglycinin and glycinin fractions (5 and 6.5) reflect the more acidic nature of the first fraction. The high content of carbohydrate (6 %) confirms that it is a glycoprotein in accordance with Kimura et al. 1997 who reported a content of 5 % carbohydrate.
The in vitro reduction of mycelial growth by the fraction containing β-conglycinin (75 %) at 500 mg/L is in the same range achieved using the antagonistic bacterium Pantoea agglomerans to control citrus green mould caused by P. digitatum at 20 °C (room temperature) and 4 °C (cold storage) (Zamani et al. 2009). However, our results suggested that this control could be increased by increasing the concentration. Indeed, increasing the concentration of the fraction containing β-conglycinin to 2,000 mg/L completely inhibited the fungal growth and conidial germination of P. digitatum. Although this concentration is relatively higher than the concentrations of synthetic fungicides used, use of a natural compound without toxicity hazards (data not published) is preferable. This concentration (2,000 mg/L) is also much lower than that of the crude extract of Eugenia caryophyllata and Curcuma longa (5,000 mg/L) that inhibited hyphal growth of P. digitatum by 100 and 47 %, respectively (Sukorini et al. 2013).
The similarity of the results of antifungal activity on hyphal growth and conidial germination by the washed protein fractions (glycinin and the fraction containing β-conglycinin) with zero content of isoflavone and those containing some levels of them indicate that these activities are exclusively from the specific structure of β-conglycinin, not from isoflavones, as noted by Kramer et al. (1984), which were at a very low level in the applied treatment. For example, the treatment with 500 mg of the fraction containing β-conglycinin (0.5 mg/mL) contained only 0.47 µg isoflvone/mL. This level is very low because the average concentration of isoflavone needed for antifungal activity is around 177 µg/mL (Kramer et al. 1984). The antifungal action of this fraction is mainly due to its components of β-conglycinin, since the other protein contaminants were visibly trivial in the electrophoretic bands. Also, two of these contaminating subunits are from glycinin, which is nearly devoid of antifungal activity.
The fraction containing β-conglycinin at a relatively low concentration (250 mg/L) completely prevented the development of mould on postharvest oranges after 3 and 7 days of inoculation, contrary to the case with the soy protein fraction or soy glycinin. Rot incidence rates increased to 100 % after 7 days in the positive control oranges compared with the fraction containing β-conglycinin (250 mg/L), with minimum disease incidence and severity approaching 0 % after 7 days and 22 and 25 %, respectively, after 21 days. The high antifungal action of the fraction containing β-conglycinin against the incidence and severity of disease is apparently due to the glycoprotein nature of β-conglycinin since glycinin had no antifungal activity. Glycoproteins were reported as active against fungal growth and infection (Deepak et al. 2003). We attribute this antifungal activity to the 5–6 % carbohydrate level, mainly high-mannose moieties as reported by Kimura et al. (1997), which facilitate penetration of the glycoproteins into the fungal cells through certain receptor mechanisms (Brewer et al. 2002). So, the carbohydrate portion in the β-conglycinin (6 %) may partially explain its antifungal activity in line with the reported data for glycoproteins (Sitohy et al. 2007).
This result confirmed those of previous reports describing antifungal proteins of diverse structures such as the lectin with antifungal activity from Egyptian seeds of Pisum sativum (Sitohy et al. 2007). Lactoferrin, an iron-binding glycoprotein naturally present in glandular secretions (milk, tears, saliva) has broad-spectrum antimicrobial activity and potent activity against Candida biofilms (Bink et al. 2011). The MIC and MFC for β-conglycinin against P. digitatum in the current study (50 and 1,900 mg/L) were lower than those of an essential oil from Eucalyptus globulus against P. digitatum (9,000 and 18,000 mg/L, respectively) (Tyagi and Malik 2011a) and from Mentha piperita (2,250 and 4,500 mg/L, respectively) (Tyagi and Malik 2011b), indicating greater effectiveness of the tested substance. On the contrast, there was no significant difference (P > 0.05) between the effect of SPI or glycinin compared with the control. Rhizolax inhibited the mycelial growth of P. digitatum in a dose-dependent manner (P < 0.05); 100–500 mg/L inhibited nearly all mycelial growth in accordance with previous reports (Tao et al. 2014).
Previously, many glycoproteins were identified for their antifungal activities. An antifungal glycoprotein of 29 kD isolated from bulbs of Urginea indica was identified, but it did not have any sequence similarity with other known antifungal proteins. The glycan part of this protein appears to be involved in antifungal activity (Deepak et al. 2003). Another glycoprotein of 29 kD, isolated from galls of Quercus infectoria, was highly effective in inhibiting the mycelial growth of Rhizoctonia solani and other agronomically important fungal pathogens (Yamunarani et al. 2005). Carbohydrates covalently attached to polypeptide chains can confer many functions to the glycoprotein, for example, resistance to proteolytic degradation, the transduction of information between cells, and ligand–receptor interactions (Deepak et al. 2003). The greater antifungal activity of β-conglycinin compared with glycinin is very different from their antibacterial activity, which is greater for glycinin (being more cationic) than β-conglycinin (Sitohy et al. 2012). The antifungal activity is associated with the glycoprotein nature of β-conglycinin.
The SEM images of P. digitatum treated with the fraction containing β-conglycinin or Rhizolax (100 mg/L) for 4 h at room temperature indicated clearly the treatment with either Rhizolax or the fraction containing β-conglycinin resulted in deformation and degradation of the hyphae and delayed sporulation confirms the fungicidal action of β-conglycinin.
Conclusions
β-conglycinin has three subunits in the molecular sizes of 97, 62 and 48 kD, a high carbohydrate content (6 %), and an isoelectric point at pH 5 that confirm its identity as an acidic glycoprotein.
β-conglycinin can reduce the in vitro growth of P. digitatum by 75 % at 500 mg/L and 100 % at 2,000 mg/L, representing higher antifungal activity than other biological antagonists and crude extracts of some plants. At 2,000 mg/L, the conidial germination of P. digitatum can be totally inhibited. Although this concentration is relatively higher than concentrations of synthetic fungicides used, the use of a natural compound without toxicity hazards is preferred. The MIC (50 mg/L) and MFC (1,900 mg/L) for β-conglycinin against P. digitatum obtained in the current study are lower than for the essential oil from Eucalyptus globulus against P. digitatum (9,000 and 18,000 mg/L, respectively) and from Mentha piperita (2,250 and 4,500 mg/L, respectively) indicating greater efficacy.
The carbohydrate portion in the β-conglycinin (6 %) may partially explain its antifungal activity, through facilitating penetration of protein molecules into the fungal cells.
β-conglycinin at a relatively low concentration (250 mg/L) can completely prevent mould growth on postharvest orange fruit for 3–7 days after inoculation with P. digitatum, in contrast to the soy protein fraction or soy glycinin. Pretreating orange fruit with 250 mg/L β-conglycinin protected fruit, keeping the incidence and severity of green mould disease to a minimum (0 % after 7 days, 22 % incidence and 25 % severity after 21 days). SEM images of the fungal cells treated with either the test substance or Rhizolax further showed the fungicidal effects of β-conglycinin.
References
Aloui H, Licciardello F, Khwaldia K, Hamdi M, Restuccia C (2015) Physical properties and antifungal activity of bioactive films containing Wickerhamomyces anomalus killer yeast and their application for preservation of oranges and control of postharvest green mould caused by Penicilliumdigitatum. Int J Food Microbiol 200:22–30
Askarne L, Talibi I, Boubaker H, Boudyach EH, Msanda F, Saadi B, Serghini MA, Ait Ben Aoumar A (2012) In vitro and in vivo antifungal activity of several Moroccan plants against Penicillium italicum, the causal agent of citrus blue mould. Crop Prot 40:53–58
Attaway JA (1994) Citrus juice flavonoids with anticarcinogenic and antitumor properties. Food phytochemicals for cancer prevention I. Maple, York, pp 240–248
Bink A, Pellens K, Cammue BPA, Thevissen K (2011) Anti-biofilm strategies: how to eradicate Candida biofilms? Open Mycol J 5:29–38
Brewer CF, Miceli MC, Baum LG (2002) Clusters, bundles, arrays and lattices: novel mechanisms for lectin–saccharide-mediated cellular interactions. Current Opin Struct Biol 12:616–623
Castillo S, Pérez-Alfonso CO, Martínez-Romero D, Guillén F, Serrano M, Valero D (2014) The essential oils thymol and carvacrol applied in the packing lines avoid lemon spoilage and maintain quality during storage. Food Control 35:132–136
Chobert JM, Touati A, Bertrand-Harb C, Dalgalarrondo M, Nicolas MG, Haertlé T (1991) In vitro proteolysis and functional properties reductively alkylated β-casein derivatives. J Dairy Res 58:285–298
Deepak AV, Thippeswamy G, Shivakameshwari MN, limath BP (2003) Isolation and characterization of a 29-kDa glycoprotein with antifungal activity from bulbs of Urginea indica. Biochem Biophys Res Commun 311:735–742
Droby S, Wisniewski ME, Cohen L, Weiss B, Touitou D, Eilam Y, Chalutz E (1997) Influence of CaCl2 on Penicillium digitatum grapefruit peel tissue and biocontrol activity of Pichia guilliermondii. Phytopathol 87:310–315
Droby S, Eick A, Macarisin D, Cohen L, Rafaela G, Stange R, Mccolum G, Dudai N, Nasser A, Wisiniewski M, Shapira R (2008) Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol Technol 49(3):386–396
DuBois M, Gilles K, Hamilton J, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Elad Y, Yunis H, Katan T (1992) Multiple fungicide resistance to benzimidazoles, dicarboximides and diethofencarb in field isolates of Botrytis cinerea in Israel. Plant Pathol 41:41–46
Fan F, Tao NG, Jia L, He XL (2014) Use of citral incorporated in postharvest wax of citrus fruit as a botanical fungicide against Penicillium digitatum. Postharvest Biol Technol 90:52–55
Janisiewicz WJ, Korsten L (2002) Biological control of postharvest diseases of fruit. Annu Rev Phytopathol 40:411–441
Johnson EA, Brekke J (1983) Functional properties of acylated pea protein isolates. J Food Sci 48:722–725
Kimura Y, Ohno A, Takagi S (1997) Structural analysis of N-glycans of storage glycoproteins in soybean (Glycine max. L) seed. Biosci Biotechnol Biochem 61(11):1866–1871
Kramer RP, Hindorf H, Jha HC, Kallage J, Zilliken F (1984) Antifungal activity of soybean and chickpea isoflavones and their reduced derivatives. Phytochem 3l:2203–2205
Laemmli UK (1970) Cleavage of structural proteins during properties of acidic subunits of soyabean 11S globulin. Agric Biol Chem 39:945–951
Masood A, Saeed S, Iqbal N (2010) Methodology for the evaluation of symptoms severity of mango sudden death syndrome in Pakistan. Pak J Bot 42:1289–1299
Moscoso-Ramírez PA, Montesinos-Herrero C, Palou L (2013) Control of citrus postharvest Penicillium moulds with sodium ethylparaben. Crop Prot 46:44–51
Nagano T, Hirotsuka M, Mori H, Kohyama K, Nishinari K (1992) Dynamic viscoelastic study on the gelation of 7S globulin from soybeans. J Agric Food Chem 40:941–944
Osman A, Mahgoub S, Sitohy M (2013) Preservative action of 11S (glycinin) and 7S (β-conglycinin) soy globulin on bovine raw milk stored either at 4 or 25 °C. J Dairy Res 80:174–183
Osman A, El-Didamony G, Sitohy M, Khalifa M, Enan G (2016) Soybean glycinin basic subunit inhibits methicillin resistant vancomycin intermediate Staphylococcus aureus (MRSA-VISA) in vitro. Int J Appl Res Nat Product 9:17–26
Seo A, Morr CV (1984) Improved high performance liquid chromatographic analysis of phenolic acids and isoflavonoids from soybean protein products. J Agric Food Chem 32:530–533
Singleton VL, Rossi JAJ (1965) Colorimetric of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Sitohy M, Doheim M, Badr H (2007) Isolation and characterization of a lectin with antifungal activity from Egyptian Pisum sativum seeds. Food Chem 104:971–979
Sitohy M, Mahgoub S, Osman A (2012) In vitro and in situ antimicrobial action and mechanism of glycinin and its basic subunit. Int J Food Microbiol 154:19–29
Sitohy M, Mahgoub S, Osman A, El-Masry R, Al-Gaby A (2013) Extent and mode of action of cationic legume proteins against Listeria monocytogenes and Salmonella enteritidis. Probiotics Antimicrob Proteins 5:195–205
Smilanick JL, Mansour MF, Gabler FM, Goodwine WR (2006) The effectiveness of pyrimethanil to inhibit germination of Penicillium digitatum and to control citrus green mould after harvest. Postharvest Biol Technol 42(1):75–85
Sukorini H, Sangchote S, Khewkhom N (2013) Control of postharvest green mold of citrus fruit with yeasts, medicinal plants, and their combination. Postharvest Biol Technol 79:24–31
Talibi I, Askarne L, Boubaker H, Boudyach EH, Msanda F, Saadi B, Ait Ben Oumar A (2012) Antifungal activity of some Moroccan plants against Geotrichum candidum, the causal agent of postharvest citrus sour rot. Crop Protec 35:41–46
Tao N, Fan F, Jia L, Zhang M (2014) Octanal incorporated in postharvest wax of Satsuma mandarin fruit as a botanical fungicide against Penicillium digitatum. Food Control 45:56–61
Torres RC, Nunes JS, Garcia M, Abadias I, Vinas T, Manso M, Olmo Usall J (2007) Application of Pantoea agglomerans CPA-2 in combination with heated sodium bicarbonate solutions to control the major postharvest diseases affecting citrus fruit at several Mediterranean locations. Eur J Plant Pathol 118:73–83
Tripathi P, Dubey NK (2004) Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol Technol 32(3):235–245
Tyagi AK, Malik A (2011a) Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapor phase against food spoilage microorganisms. Food Chem 126(1):228–235
Tyagi AK, Malik A (2011b) Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapor phase against food spoiling microorganisms. Food Control 22(11):1707–1714
Widmer WW, Montanari AM (1996) The potential for citrus phytochemicals in hypernutritious foods. In: Finley JW, Armstrong DJ, Nagy S, Robinson SF (eds) Hypernutritious foods. Agscience, Auburndale, pp 75–89
Yahyazadeh M, Zare R, Omidbaigi R, Faghih-Nasiri M, Abbasi M (2009) Control of Penicillium decay on citrus fruit using essential oil vapors of thyme or clove inside polyethylene and nano-clay polyethylene films. J Hortic Sci Biotechnol 84(4):403–409
Yamunarani K, Jaganathan R, Bhaskaran R, Govindaraju P, Velazhahan R (2005) In vitro antifungal activity of a 29-kDa glycoprotein purified from the galls of Quercus infectoria. Acta Phytopathol Entomol Hungarica 40:43–54
Yildiz F, Kinay P, Yildiz M, Sen F, Karacali I (2005) Effects of preharvest applications of CaCl2, 2,4-D and benomyl and postharvest hot water, yeast and fungicide treatments on development of decay on Satsuma mandarins. J Phytopathol 153:94–98
Zamani M, Sharifi Tehrani A, Ahmadzadeh M, Hosseininaveh V, Mostofy Y (2009) Control of Penicillium digitatum on orange fruit combining Pantoea agglomerans with hot sodium bicarbonate dipping. J Plant Pathol 91(2):437–442
Acknowledgments
The authors thank Zagazig University for financially supporting this research within its ordinary research budget.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osman, A., Abbas, E., Mahgoub, S. et al. Inhibition of Penicillium digitatum in vitro and in postharvest orange fruit by a soy protein fraction containing mainly β-conglycinin. J Gen Plant Pathol 82, 293–301 (2016). https://doi.org/10.1007/s10327-016-0686-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-016-0686-3