Abstract
A reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay was developed to detect plantago asiatica mosaic virus (PlAMV), one of the most damaging lily-infecting viruses and a member of the genus Potexvirus in the family Alphaflexiviridae. A set of six primers was designed based on the central core region of the coat protein gene of the Li1 isolate of PlAMV, which detected the isolate most efficiently at 65 °C. The RT-LAMP assay specifically detected several PlAMV isolates with a high level of genetic and biological variation, but not potato virus X (another virus species in the same Potexvirus genus). The sensitivity of the RT-LAMP was tenfold higher than that of conventional RT-PCR. Moreover, with a simple method using a toothpick, PlAMV was directly detected from infected lily leaves using the RT-LAMP assay without RNA extraction. This simple and highly sensitive method can be used for rapid surveys for PlAMV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plantago asiatica mosaic virus (PlAMV) is a member of the genus Potexvirus in the family Alphaflexiviridae (Komatsu et al. 2008). PlAMV has a single-stranded RNA genome of positive polarity and is approximately 6100 nucleotides long. The PlAMV genomic RNA contains five open reading frames (ORFs); ORF1 encodes an RNA-dependent RNA polymerase (RdRp), ORF2 to ORF4 encode triple gene block proteins 1–3 (TGBp1–3), and ORF5 encodes a coat protein (CP). Since PlAMV was first isolated from Plantago asiatica in Russia in 1994 (Solovyev et al. 1994), several isolates from different types of plant species have been reported including Li isolates from the monocotyledon lily (Lilium leichtlinii), a Pr isolate from the dicotyledon primrose (Primula sieboldii), and an Na isolate from the woody plant Nandina domestica (Hughes et al. 2005; Komatsu et al. 2008). The genomic RNA of this virus is also highly variable among isolates (Komatsu et al. 2008). This high level of genetic and biological variation makes PlAMV difficult to detect. Because PlAMV causes severe necrosis in lilies in the Netherlands, a rapid and highly sensitive diagnostic tool for this virus is needed (EPPO 2011).
At this time, several diagnostic methods for PlAMV have been developed, including enzyme-linked immunosorbent assay (ELISA), direct tissue blotting, macroarray hybridization and RT-PCR (Chen et al. 2013; Ozeki et al. 2006; Sugiyama et al. 2008). However, these techniques involve time-consuming steps and require specialized laboratory equipment, such as a hybridization oven or a thermal cycler, limiting their use in the field.
Recently, a loop-mediated isothermal amplification (LAMP) assay was developed and has been applied to the diagnosis of many animal and plant pathogens (Mori and Notomi 2009). LAMP assays have also been adapted to rapidly detect plant RNA viruses by adding reverse transcriptase to the reaction, referred to as reverse-transcription LAMP (RT-LAMP) (Fukuta et al. 2003; Nie 2005; Varga and James 2006). LAMP assays are highly specific because the primers recognize at least six independent target regions. In addition, LAMP can isothermally amplify the target sequence in approximately 1 h using a water bath at a constant temperature of 60–65 °C. Moreover, in LAMP assays, the turbidity caused by an insoluble byproduct, white magnesium pyrophosphate precipitate, allows the visualization of target gene amplification. Based on these characteristics, LAMP assays are suitable for on-site diagnostic use. Furthermore, real-time monitoring of the LAMP reaction can be performed using a fluorescent indicator.
In the present study, we developed a LAMP-based detection system for PlAMV. Using a fluorescent indicator, we optimized the assay conditions and evaluated its sensitivity and specificity. Our assay is also capable of detecting PlAMV from infected leaves, which facilitates detection of the virus under practical conditions.
Materials and methods
Plant material and virus inoculation
All viruses used in this study [PlAMV-Li1, PlAMV-Pr, Li1-NaCP and potato virus X (PVX)-OS] were used to inoculate Nicotiana benthamiana using agroinfiltration as described previously (Maejima et al. 2014). Infected plants were maintained in a growth chamber at 25 °C with 16-h light/8-h dark. Lily plants used for direct detection of PlAMV were obtained from a commercial grower.
Agroinfectious clones of virus variants for infection of plants
The binary vector of PlAMV-Li1, pLi1, for inoculation was described previously (Ozeki et al. 2006). Binary vectors of PlAMV-Pr and PVX-OS for inoculation were constructed as pLi1; full-length cDNA of each virus, which was controlled by the cauliflower mosaic virus 35S promoter, was cloned into pCAMBIA1301 (Kagiwada et al. 2002; Komatsu et al. 2008). To construct pLi1-NaCP, an artificially synthesized CP gene of the Na isolate of PlAMV (Eurofins MWG Operon, Ebersberg, Germany) was inserted into the corresponding region (nt 5362–5985 of the Li1 isolate) of pLi1 by replacing the original CP gene using the Gibson Assembly Kit (New England Biolabs, Ipswich, MA, USA). All binary vectors for inoculation were used to transform Agrobacterium tumefaciens strain EHA105 and used for agroinfiltration, as described above.
RNA extraction and RT-PCR
Total RNA was extracted from the upper leaves of virus-infected N. benthamiana plants using ISOGEN reagent (Nippon Gene, Tokyo, Japan), according to the manufacturer’s instructions at 10 days after inoculation. After total RNA was dissolved in RNase-free distilled water to make a solution of approximately 500 ng/µL, 1 µL of the total RNA solution was used as a template for RT-LAMP and RT-PCR. RT-PCR was performed with the SuperScript III One-Step RT-PCR System with Platinum Taq (Life Technologies, Carlsbad, CA, USA) using Li-F3 and Li-B3, the PlAMV-specific external primers of the LAMP reaction (see Table 1 for their sequences). RT-PCR was performed in a total volume of 25 µL under the following conditions: RT reaction at 50 °C for 30 min; denaturation at 94 °C for 2 min; 35 cycles of 94 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s; followed by a final extension at 72 °C for 7 min. A total of 2 µL of the RT-PCR products was analyzed using 1 % (w/v) agarose gel electrophoresis.
Primer design
A primer set for the RT-LAMP reaction was designed based on the coat protein gene of the PlAMV Li1 isolate using the PrimerExplorer V4 software (http://primerexplorer.jp/). The set includes two inner primers [FIP (F1c + F2) and BIP (B1c + B2)], two outer primers (F3 and B3), and two loop primers (Loop F and Loop B), whose sequences and positions are shown in Fig. 1. Details of the primers are provided in Table 1.
Alignment of the nucleotide sequences of a 210-nucleotide coat protein-coding region of plantago asiatica mosaic virus isolates (Li1, AB360790; Pr, AB360796; NaMV, AY800279) and potato virus X (PVX, OS isolate, AB056718). Nucleotides identical to Li1 are marked as periods; gaps are indicated by dashes. The sequences used for RT-LAMP primers are boxed and indicated by an arrow line
RT-LAMP
The RT-LAMP reaction was performed as described previously (Sugawara et al. 2012), except that total RNA was used as template instead of DNA and 1.25 U of AMV reverse transcriptase (Nippon Gene) was included in the LAMP reaction mixture (total 25 µL). Unless otherwise stated, the mixture was incubated at 65 °C for 30 min followed by incubation at 80 °C for 5 min to inactivate DNA polymerase. For real-time monitoring of RT-LAMP reactions, fluorescent detection reagent (Eiken Kagaku, Tokyo, Japan) was added to the reaction mix, and fluorescence was detected with the Genie I instrument (OptiGene, Horsham, UK). DNA amplification was also detected based on the fluorescence intensity under a 254-nm ultraviolet light.
Results
RT-LAMP detection of PlAMV and its reaction conditions
To develop a specific, rapid and highly sensitive RT-LAMP method for detecting PlAMV, we first designed five primer sets for the CP gene of the Li1 isolate, which were used for RT-LAMP. In this assay, we used total RNA extracted from Li1-infected N. benthamiana leaves as template. One primer set produced nonspecific amplification, and three other primer sets yielded insufficient amplification. As a result, the primer set depicted in Fig. 1 and Table 1 was considered suitable for PlAMV detection because it resulted in efficient amplification with no nonspecific products (data not shown).
To examine the optimal thermal conditions of the RT-LAMP assay using this primer set, we performed RT-LAMP reactions at temperatures of 59–66 °C for 30 min, with the aforementioned total RNA as a template. As shown in Fig. 2, RT-LAMP detected the Li1 isolate of PlAMV at a wide range of temperatures, while at lower incubation temperatures the increase in fluorescence generally began after a longer incubation. At 65 °C, the final fluorescence intensity was highest, and the fluorescence started to increase as rapidly as at 66 °C (approximately 7 min). Therefore, an incubation temperature of 65 °C was used for all subsequent RT-LAMP assays.
Optimization of RT-LAMP reaction temperature for plantago asiatica mosaic virus. RT-LAMP amplification curves with the primer set listed in Table 1, using 100 ng of total RNA isolated from a Li1-infected Nicotiana benthamiana plant as a template, are shown. RT-LAMP reactions were run at incubation temperatures from 59 to 66 °C for 30 min. Similar results were obtained in two independent experiments. Time interval between each symbol is 45 s
Specificity of PlAMV detection by RT-LAMP
Because the primer set for RT-LAMP in this study was designed based on the Li1 isolate of PlAMV (Fig. 1), it remains unknown whether this primer set can be used for the detection of other PlAMV isolates, including Pr and Na. However, the Na isolate of PlAMV is not available. Thus, we constructed chimeric virus variants of Li1, which contains the CP gene of the Na isolate of PlAMV, and determined whether they could be detected using the RT-LAMP developed in this study. This strategy, in which a chimeric virus with the CP gene of unavailable virus isolates was constructed to express the CP of interest in plants, was successfully used to determine whether the plum pox virus (PPV) detection kit based on immunochromatography could detect minor PPV strains (Maejima et al. 2014).
When N. benthamiana was agroinfiltrated with a chimeric Li1 variant, Li1-NaCP, that possesses the CP gene of Na isolate of PlAMV, the chimeric virus systemically infected the host and caused weak necrosis on the upper leaves (data not shown). We also confirmed by RT-PCR and direct sequencing of the amplified products that Li1-NaCP retained the chimeric sequence in the upper leaves. We obtained similar results with two other chimeric clones, which confirmed stability of the chimeric virus.
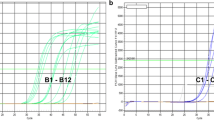
We extracted total RNA from the upper leaves of N. benthamiana plants infected with Li1, Pr, Li1-NaCP, and PVX (isolate OS) (Kagiwada et al. 2002), and used these total RNA samples for RT-LAMP reactions. Among these four viruses, our RT-LAMP assay could detect three PlAMV isolates, but not PVX (Fig. 3). Consistent with the nucleotide sequence identities of the CP genes with Li1, which are 93, 78, and 37 % for Pr, Na and PVX, respectively, the fluorescence started to increase at later times with decreasing CP nucleotide sequence identities with Li1. These results demonstrated that the RT-LAMP assay developed in this study could detect different PlAMV isolates.
Specificity of the RT-LAMP primer set for detecting plantago asiatica mosaic virus using total RNA from a plant of Nicotiana benthamiana infected with Li1, Pr, Li1-NaCP or potato virus X (PVX) as the template. RT-LAMP reaction was run for 30 min at 65 °C; shown is a representative experiment of two independent experiments with similar results. Time interval between each symbol is 45 s
Sensitivity of PlAMV detection using RT-LAMP compared with RT-PCR
To compare the sensitivity of our RT-LAMP assay with that of RT-PCR, total RNA extracted from a Li1-infected N. benthamiana leaf was diluted serially up to 109-fold, and each sample was subjected to RT-LAMP and RT-PCR simultaneously. RT-LAMP successfully detected PlAMV from as little as 10 fg of total RNA, whereas RT-PCR could amplify the CP gene of PlAMV from as little as 100 fg of total RNA (Fig. 4a, c). Note that even when we used primers specifically designed for RT-PCR in the previous report (CP-gene-specific primers CP-F [5′-ATG GCA CTC AAC CAA GCT CCG AC-3′] and CP-R [5′-GTC GGA GGG GGA GGG GAG GAA TT-3′]; Ozeki et al. 2006) instead of the external primers for our RT-LAMP, the sensitivity of these primers was not as high as that of the primers used in Fig. 4c (data not shown). Amplification of the PlAMV-CP gene by RT-LAMP was also confirmed based on visual observation of the reaction tubes under UV light (Fig. 4b). These results suggest that, to detect PlAMV, RT-LAMP was about tenfold more sensitive than RT-PCR, with a detection limit of 10 fg of total RNA extracted from virus-infected leaves.
Relative sensitivity of RT-LAMP and one-step RT-PCR to detect plantago asiatica mosaic virus. a RT-LAMP amplification curves using serial tenfold dilutions of total RNA (100 ng to 1 fg) from a Li1-infected plant of Nicotiana benthamiana as the template. RT-LAMP reaction was run for 40 min at 65 °C. The time interval between each symbol is 45 s. b Visual detection of the RT-LAMP using ultraviolet light. Tubes 1–9 100 ng to 1 fg total RNA preperations from a Li1-infected plant of N. benthamiana. Tube 10 indicates the nontemplate control. c Agarose gel electrophoresis of one-step RT-PCR using the same dilutions of total RNA as in a with primers PlAMV-F3 and –B3. Lane 1 is the 100-bp DNA ladder (New England Biolabs); lanes 2–9 are amplified products using 100 ng to 1 fg of total RNA from a Li1-infected plant as template. Lane 10 indicates the nontemplate control. Similar results were obtained in two independent experiments using total RNA extracted from two different Li1-infected plants as template
Direct detection of PlAMV from infected lily leaves
To examine whether our RT-LAMP assay could detect PlAMV from infected leaves without extracting total RNA, we employed a simple method using a toothpick. After pricking the infected leaf with a toothpick several times, we dipped the toothpick into the LAMP reaction mix prepared in a PCR tube and performed RT-LAMP reactions. When we pricked Li1-infected leaves of N. benthamiana and dipped the toothpick into a reaction tube, a rapid increase in fluorescence was observed (Fig. 5; sample no. 8). In the same experiment, PlAMV was successfully detected from rust-coloured and necrotic lily leaves from five independent plants (Fig. 5b; sample nos. 1–5), although real-time monitoring of the LAMP reaction revealed that the reaction time needed for sufficient fluorescence differed depending on samples (Fig. 5a; approximately 25–65 min). In contrast, no virus was detected from healthy, symptomless lily leaves after a RT-LAMP reaction of 70 min (Fig. 5). RNA extraction and subsequent conventional RT-PCR and sequencing of these lily leaves confirmed that the lily leaves with rust-coloured symptoms used in Fig. 5a, b were infected with PlAMV, while symptomless lily leaves were not infected (Fig. 5c). Therefore, the toothpick method was applicable for the direct detection of PlAMV from infected lily leaves.
Direct detection of plantago asiatica mosaic virus (PlAMV) from infected leaves using a toothpick dipped into the RT-LAMP reaction mixtures without templates before the reaction. The toothpick had been used to prick PlAMV-infected lily leaves from five independent plants (nos. 1–5), healthy lily leaves (nos. 6, 7), or a PlAMV-infected leaf of Nicotiana benthamiana (no. 8). RT-LAMP reaction was run for 70 min at 65 °C. a RT-LAMP amplification curves using the three toothpick-pricked leaf samples as templates. Time interval between each symbol is 60 s. b Visual detection of the RT-LAMP using ultraviolet light. Samples are numbered as in a. c Agarose gel electrophoresis of PlAMV-specific, one-step RT-PCR using total RNA extracted from the lily leaves used in RT-LAMP as a template. Samples are numbered as in a. Primer set used (different than in Fig. 4): the reverse primer polydT-ClaNot (5′-AAA GCG GCC GCA TCG ATT TTT TTT TTT TTT TTT TTT TTT TTT TTT TTV-3′) and the forward primer ru-preTGBp2-F (5′-GTC GGA CTC TAC ATC AGC CT-3′)
Discussion
Lily is one of the most popular and important ornamental plants in the world. Because most lilies are propagated vegetatively by bulbs, they are susceptible to virus infection and accumulate viruses during propagation. Several viruses, including cucumber mosaic virus, lily symptomless virus, lily mottle virus, and PlAMV are known to infect lily plants (Kwon et al. 2013). Among these, PlAMV is one of the most damaging threats to lily production because it can cause necrosis in the whole lily plant, reducing the yield and quality of cut flowers (EPPO 2011). Considering the growing global trade of ornamental flowers, including lilies, rapid and highly sensitive diagnostic tools are critical for controlling outbreaks of PlAMV.
Currently available techniques for PlAMV detection include ELISA and RT-PCR, but these are time-consuming and require specialized equipment, which is impractical for field use. In contrast, the RT-LAMP assay developed in this study takes only 30 min to detect PlAMV isolates from total RNA extracted from infected plants. Despite the effect of incubation temperature on such assays, our RT-LAMP assay successfully detected PlAMV at a wide range of temperatures (59–66 °C), which renders it appropriate for field diagnostics because the reaction can easily be performed in a water bath. Moreover, we found that the RT-LAMP exhibits tenfold higher sensitivity compared with RT-PCR in accordance with other studies showing that RT-LAMP is more sensitive than conventional RT-PCR (Fukuta et al. 2013). In addition, our RT-LAMP assay can directly detect PlAMV using a toothpick without extracting total RNA from infected lily and N. benthamiana plants. This feature allows for efficient diagnosis of field samples.
However, in our direct RT-LAMP assay using a toothpick, the time when fluorescence starts to increase (approximately 21–50 min) appears to be later than that needed to increase the limit of detection of total RNA, which was demonstrated in Fig. 4 (approximately 18 min for the last amplified dilution). Although we cannot exclude the possibility that our primer set did not perfectly anneal to PlAMV isolates from lilies, which contributes to a delay in amplification, the delay is most likely due to an inhibitory effect of lily sap in RT-LAMP reaction mixture. Indeed, when we pricked healthy lily leaves using a toothpick and dipped it into the LAMP reaction mixture with total RNA extracted from Li1-infected N. benthamiana leaves as a template, amplification time was delayed (Fig. S1). In the future, studies to determine the exact detection limit of PlAMV using a toothpick and direct RT-LAMP assay should be done to increase the confidence in the direct RT-LAMP method for PlAMV.
To our knowledge, RT-LAMP has been developed to detect only two viruses in the genus Potexvirus; namely, Cymbidium mosaic virus, which causes severe damage to orchids, and Pepino mosaic virus (PepMV), an emerging virus that infects tomato plants worldwide (Hasiów-Jaroszewska and Borodynko 2013; Lee et al. 2011; Ling et al. 2013). Recently, for the detection of PepMV (which has a high level of within-species genetic diversity), a genotype-specific RT-LAMP was developed (Ling et al. 2013). This system is useful and allows tomato growers to determine the genetic diversity of the virus in their own fields. However, we cannot adapt this genotype-specific detection system to PlAMV, a genetically heterogeneous potexvirus species similar to PepMV, because insufficient sequence information is available for genotype classification of this virus. Instead, in the case of PlAMV, it is important to develop a system that can detect a wide variety of isolates. To this end, we designed the RT-LAMP primers for PlAMV targeting the central core region of the CP gene. Because this primer target region is relatively conserved within the genus Potexvirus, our RT-LAMP assay can detect PlAMV isolates with high genomic sequence diversity. In contrast, our assay cannot detect PVX, which is a less closely related species of the same genus. This relatively broad spectrum of the RT-LAMP developed in this study can be adapted to detect unreported PlAMV isolates and can be used for the early detection of emerging viruses that infect lilies and affect their quality. Future studies should determine the detection range of the RT-LAMP and the lower limit of potexvirus nucleotide sequence identity that can be detected. Based on our results, whether other PlAMV isolates are detected or not using our RT-LAMP assay remains unclear.
References
Chen CC, Jhang YL, Lin BY, Chiang FL, Cheng YH, Deng TC (2013) Serological reagent preparation and improvement of serological method for the detection of Plantago asiatica mosaic virus in lily. J Taiwan Agric Res 62:268–279
EPPO (2011) New pest records in EPPO member countries: Plantago asiatica mosaic virus (Potexvirus, PlAMV) found on Lilium spp. in the Netherlands. EPPO Reporting Service 2011/082. http://www.eppo.int/PUBLICATIONS/reporting/reporting_service.htm. Accessed 09 Aug 2014
Fukuta S, Kato S, Yoshida K, Mizukami Y, Ishida A, Ueda J, Kanbe M, Ishimoto Y (2003) Detection of tomato yellow leaf curl virus by loop-mediated isothermal amplification reaction. J Virol Methods 112:35–40
Fukuta S, Tamura M, Maejima H, Takahashi R, Kuwayama S, Tsuji T, Yoshida T, Itoh K, Hashizume H, Nakajima Y, Uehara Y, Shirako Y (2013) Differential detection of Wheat yellow mosaic virus, Japanese soil-borne wheat mosaic virus and Chinese wheat mosaic virus by reverse transcription loop-mediated isothermal amplification reaction. J Virol Methods 189:348–354
Hasiów-Jaroszewska B, Borodynko N (2013) Detection of Pepino mosaic virus isolates from tomato by one-step reverse transcription loop-mediated isothermal amplification. Arch Virol 158:2153–2156
Hughes PL, Harper F, Zimmerman MT, Scott SW (2005) Nandina mosaic virus is an isolate of Plantago asiatica mosaic virus. Eur J Plant Pathol 113:309–313
Kagiwada S, Yamaji Y, Nakabayashi H, Ugaki M, Namba S (2002) The complete nucleotide sequence of Potato virus X strain OS: the first complete sequence of a Japanese isolate. J Gen Plant Pathol 68:94–98
Komatsu K, Yamaji Y, Ozeki J, Hashimoto M, Kagiwada S, Takahashi S, Namba S (2008) Nucleotide sequence analysis of seven Japanese isolates of Plantago asiatica mosaic virus (PlAMV): a unique potexvirus with significantly high genomic and biological variability within the species. Arch Virol 153:193–198
Kwon JY, Ryu KH, Choi SH (2013) Reverse transcription polymerase chain reaction-based system for simultaneous detection of multiple lily-infecting viruses. Plant Pathol J 29:338–343
Lee MS, Yang MJ, Hseu YC, Lai GH, Chang WT, Hsu YH, Lin MK (2011) One-step reverse transcription loop-mediated isothermal amplification assay for rapid detection of Cymbidium mosaic virus. J Virol Methods 173:43–48
Ling KS, Li R, Bledsoe M (2013) Pepino mosaic virus genotype shift in North America and development of a loop-mediated isothermal amplification for rapid genotype identification. Virol J 10:117
Maejima K, Himeno M, Netsu O, Ishikawa K, Yoshida T, Fujita N, Hashimoto M, Komatsu K, Yamaji Y, Namba S (2014) Development of an on-site plum pox virus detection kit based on immunochromatography. J Gen Plant Pathol 80:176–183
Mori Y, Notomi T (2009) Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother 15:62–69
Nie X (2005) Reverse transcription loop-mediated isothermal amplification of DNA for detection of Potato virus Y. Plant Dis 89:605–610
Ozeki J, Takahashi S, Komatsu K, Kagiwada S, Yamashita K, Mori T, Hirata H, Yamaji Y, Ugaki M, Namba S (2006) A single amino acid in the RNA-dependent RNA polymerase of Plantago asiatica mosaic virus contributes to systemic necrosis. Arch Virol 151:2067–2075
Solovyev AG, Novikov VK, Merits A, Savenkov EI, Zelenina DA, Tyulkina LG, Morozov SYu (1994) Genome characterization and taxonomy of Plantago asiatica mosaic potexvirus. J Gen Virol 75:259–267
Sugawara K, Himeno M, Keima T, Kitazawa Y, Maejima K, Oshima K, Namba S (2012) Rapid and reliable detection of phytoplasma by loop-mediated isothermal amplification targeting a housekeeping gene. J Gen Plant Pathol 78:389–397
Sugiyama S, Masuta C, Sekiguchi H, Uehara T, Shimura H, Maruta Y (2008) A simple, sensitive, specific detection of mixed infection of multiple plant viruses using macroarray and microtube hybridization. J Virol Methods 153:241–244
Varga A, James D (2006) Use of reverse transcription loop-mediated isothermal amplification for the detection of Plum pox virus. J Virol Methods 138:184–190
Acknowledgments
We thank Mr. T. Moriyama for providing lily plants. This work was supported in part by Grants-in-Aid for Young Scientists (B) (26850231) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10327_2015_599_MOESM1_ESM.pptx
Effect of healthy lily sap on RT-LAMP reaction. RT-LAMP amplification curves using total RNA (1 pg or 100 fg) isolated from a Li1-infected plant of Nicotiana benthamiana as the template are shown. In the sample marked “+ healthy lily sap”, a toothpick which had pricked healthy lily leaf (Fig. 5, healthy lily2) was dipped into the LAMP reaction mixture. RT-LAMP reaction was run for 50 min at 65 °C. (PPTX 49 kb)
Rights and permissions
About this article
Cite this article
Komatsu, K., Maejima, K., Fujita, N. et al. A detection method based on reverse transcription loop-mediated isothermal amplification for a genetically heterogeneous plantago asiatica mosaic virus. J Gen Plant Pathol 81, 297–303 (2015). https://doi.org/10.1007/s10327-015-0599-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-015-0599-6