Abstract
Climate change damage induced by growing carbon dioxide (CO2) emissions has rapidly fostered research on capturing, utilizing, and converting CO2 into valuable C1 and C2 chemicals. In particular, electrochemical reduction of CO2 into formic acid and ethylene is a promising way to recycle the wasted CO2 gas, provided that this process is environmental sustainable and economic feasible compared to conventional processes. Here we review electrocatalysts for the production of formic acid and ethylene by electrochemical CO2 reduction. We discuss the optimization of catalysts by structural engineering, construction of metal alloys, development of metal and non-metal composites, defect engineering single-atom catalyst schemes, and metal-functional polymers. We also present life cycle and economic assessments of electrochemical CO2 reduction. Actually, due to the lack of material recycling, and to disadvantages of high electricity consumption, insufficient catalytic performance, and durability, current electrochemical CO2 reduction is still inferior to the conventional process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of industry and the growth of social activities, the emission of CO2 greenhouse gas has led to global warming, sea-level rise, water acidification, and other environmental impacts. Recent studies and data show that the average global concentration of CO2 in the air recorded in April 2022 was 417.11 ppm (Global monitoring laboratory-carbon cycle greenhouse gases, 2021). The global average CO2 concentration has increased by 77.11 ppm from 340 ppm in 1980 and is increasing rapidly at an average rate of 2.58 ppm per year (Fig. 1a, b). At the recent World Economic Forum, carbon capture and utilization technology was identified as one of the world's 10 emerging technologies (Top 10 emerging technologies of 2021, 2021), indicating the overwhelming interest of chemists in the capture, utilization, and transformation of CO2 into economically valuable C1 chemical or C2 or even C2+ chemicals (Ochedi et al. 2020; Zhao and Quan 2021).
a Global monthly average (1980–2021) and b annual mean growth rate of global atmospheric CO2 concentration (1960s–2010s). The concentration of CO2 and average concentration growth rate both show an increasing trend over the past 40 years. Reproduced from: (Global monitoring laboratory-carbon cycle greenhouse gases, 2021)
Environmentally friendly methods of producing C1 and C2 chemicals by reduction of CO2 include photocatalysis, photoelectric chemistry, electrocatalysis, biocatalysis, and thermocatalysis strategies (Foo et al. 2022; Ling et al. 2022). Under the current technological development, the energy barrier of CO2 reduction through thermochemistry is exceptionally high, and high-temperature and high-pressure operations consume a large amount of energy consumption. Reduction of CO2 through photocatalysis is a promising approach. High-performance catalytic materials such as graphitic carbon nitride (g-C3N4) (Ng et al. 2022; Ong et al. 2020; Yu et al. 2021) and MXene (Peng et al. 2019) have been proven to have the potential to solve the problem of high energy consumption and pollution of conventional thermal processes. However, the relatively low stability and efficiency of catalytic materials as well as the unstable sunlight limit the large-scale commercial application of photocatalysis technology (Yang et al. 2020). In contrast, electrochemical CO2 reduction technology, which is central to the carbon cycle, has the advantage of being able to operate under environmental conditions and control the reaction products by controlling the electric potential (Liu et al. 2018). The relatively advanced technology has attracted more and more attention. Notably, the use of renewable sources of electricity such as wind, water, nuclear, and solar energy as electrochemical sources of electricity will contribute to the carbon cycle (Fig. 2) (Zhang et al. 2021a, b, c, d).
Previous work has focused on the review of catalytic technologies for electrochemical reduction of CO2 (Liu et al. 2018; Masood ul Hasan et al. 2020; Zhang et al. 2021a, b, c, d; Zhu et al. 2016), with less emphasis on the discussion of formic acid and ethylene products, and without substantial consideration of environmental sustainability and economic potential. In this work, the catalytic design, performance, and potential of different catalyst materials will be analyzed toward the CO2 electroreduction, and different catalyst schemes will be reviewed in the view of practical application. In the catalyst modification scheme, the theoretical insights and the origin of reaction mechanisms of the production of formic acid and ethylene by electrochemical CO2 reduction will be discussed. Based on the pioneering work of Hori et al. (Ikeda et al. 1987; Kaneco et al. 1998; Kapusta and Hackerman, 1983), this paper discusses the problems existing in single metal catalysts. After that, taking the target product of formic acid and ethylene as the premise, recent modification schemes in structure engineering, alloy strategy, non-metal doping, vacancy control, and non-metal support composite strategy will be presented to establish the structure–activity relationship to decipher the mechanisms of CO2 electroreduction to formic acid and ethylene. Apart from catalytic design, this review also summarizes the life cycle assessment work to discuss the environmental impact of the current electrochemical CO2 reduction process. Moreover, this review elucidates the economic assessment related to the production of formic acid and ethylene by electrochemical CO2 reduction. Finally, insights into the current opportunities and challenges of electrochemical CO2 reduction to produce formic acid and ethylene are prospected. As a whole, this work will cast a favorable future development of electrochemical CO2 reduction technology and provide an understanding of investment value for investors.
Current development of electrochemical CO2 reduction
At present, most commercial production of C1 and C2 products, in particular formic acid and ethylene, is based on a highly energy-consuming and polluting petrochemical process. The production of formic acid comes from CO gas produced by burning fossil fuels (natural gas and coal). The typical process for formic acid production is BASF's methyl formate hydrolysis process, which involves formic acid and a strong alkali (e.g. sodium methoxide) that react under high pressure to produce methyl formate (Fig. 3a), followed by undergoing hydrolysis to produce formic acid (Bulushev and Ross 2018). Fossil fuel combustion, the risk of leakage of strongly alkaline reactants, and the mechanical costs associated with high-pressure operations are all disadvantages of the BASF process that can be further examined. For C2 chemicals, ethylene is used as a synthetic material for a variety of polymers. As a typical high-value C2 chemical, ethylene is produced commercially by naphtha steam cracking, in which a process usually involves high temperature and pressure cracking, oil quenching, compression, acid gas removal, and multistage distillation (Fig. 3b) (Jiang et al. 2020a, b; Karaba et al. 2020). Energy consumption studies on the naphtha steam cracking unit showed that the process accounted for 65% of the total energy consumption, while the total exergy loss was as high as 75% (Ren et al. 2006). The release of acidic gases such as SO2, H2S, and CO2 in the process will further aggravate environmental problems. Therefore, current development requires clean, efficient, low energy consumption, and low carbon emission electrochemical CO2 reduction technology to reduce the environmental damage caused by the production of these chemicals.
The key to producing formic acid and ethylene by electrochemical reduction of CO2 is the design and modification of cathode catalytic materials with different types and classes of nanomaterials. After decades of development, the electrochemical reduction of CO2 technology has gradually become mature. In view of this topic, recent works have been put forward to summarize the state-of-the-art progress (Chatterjee et al. 2021; Liu et al. 2020a, b, c, d; Liu et al. 2018; Masood ul Hasan et al. 2020; Yan et al. 2021; Zhang et al. 2021a, b, c, d; Zhao and Quan 2021; Zhu et al. 2016). For example, Zhu et al. summarized the catalytic properties and principles of transition metals and metal sulfides from the perspective of polyphase inorganic catalysts (Zhu et al. 2016), which demonstrated its for high-efficiency catalysis and longtime stability. Meanwhile, Liu and his colleagues reviewed the effects of metal bases and metal oxide catalysts on the electrochemical reduction properties of CO2 based on a variety of products, such as CO, formic acid, and methane. (Liu et al. 2018). Recent developments in nanotechnology have provided new insights for the design of electrochemical catalysts for CO2 reduction. Hasan and his colleagues have reviewed metal-free carbon-based catalysts. Their work shows that in the absence of metals, carbon-based catalysts still perform well at ambient conditions, but not as well as metal catalysts (Masood ul Hasan et al. 2020). Besides that, Chatterjee et al. expounded the importance of formic acid in future industry and energy in terms of hydrogen storage and summarized the development of homogeneous and heterogeneous electrochemical CO2 reduction in recent years, taking formic acid as the target product (Chatterjee et al. 2021). Kun et al. focused on electrochemical CO2 reduction catalysts toward C2+ oxygenates products (Zhao and Quan 2021). Their work discussed the importance of carbon–carbon coupling processes for the formation of multi-carbon products. It also shows the special potential of carbon-based catalysts in promoting electron transfer and forming C2+ products. Based on the rich technical scheme and summary of predecessors, it will be necessary to understand the specific technological prospect of electrochemical CO2 reduction, its possible environmental impacts as well as the commercial and economic potential of the emerging technology.
Figure 4 presents the development history of the electrochemical reduction CO2 to produce formic acid and ethylene. In the early 1980s, electrochemical CO2 reduction catalyst research focused on finding potentially viable metals. Among them, In (Ikeda et al. 1987; Kapusta and Hackerman 2019), Pb (Todoroki et al. 1995), Sn (Kaneco et al. 1998; Mizuno et al. 1995), and Hg (Paik et al. 1969) metals were shown to be highly active in reducing CO2 to produce C1 chemicals, and formic acid was the main product. In 1985, Cu metal was demonstrated to reduce CO2 to produce C2 chemicals such as ethylene (Tonner et al. 2002). Some representative catalyst modification schemes in recent years are also summarized in Fig. 4. In 2017, Bashir et al. combined Sn oxide with multi-walled carbon nanotubes (MWCNT) to increase the formic acid yield (Bashir et al. 2016). Ma et al. constructed Cu-Pd alloy and studied the influence of different mixing modes (Ma et al. 2017). In 2018, Sun et al. achieved a high formic acid current density (43.8 mA cm−2) by compounding MoP nanoparticles on a porous carbon surface (Sun et al. 2018). The scheme of porous carbon-based support with metal nanoparticles has also been derived from other metals such as Sn, Cu (Gu et al. 2018; Han et al. 2020; Kim et al. 2019). In 2019 and 2020, the Bi metal and its oxide-related catalytic schemes achieved major breakthroughs. Liu's team reported that a combination of bismuth oxide and multiple channel carbon matrix (MCCM) achieved an energy efficiency of 55.3% (Liu et al. 2019), Wu's Bi2O3 and carbon paper composite scheme can achieve a current density of 32.4 mA cm−2 at a low potential (− 0.87 V versus RHE) (Wu et al. 2020a, b). RHE refers to reversible hydrogen electrode, which can act as a reference to electro potential performance. In terms of ethylene production, the morphological modification and crystal structure of CuO nanoparticles to enhance the catalytic activity have become the focus of research (Gao et al. 2020; Kim et al. 2019). In the past three years, some novel schemes such as single-atom catalyst (Lu et al. 2021), metal-free carbon-based scheme (Wang et al. 2017) and metal-functional polymer scheme (Chen et al. 2020a, b, c) have also been found to be environmentally friendly for materials and economical in the preparation process of catalysts.
History of the development of catalysts for electrochemical CO2 reduction. During the 1980s, metal such as Hg, Pb, and Sn were shown to reduce CO2 to formate under ambient conditions (Ikeda et al. 1987; Kaneco et al. 1998; Kapusta and Hackerman 2019; Mizuno et al. 1995; Paik et al. 1969; Todoroki et al. 1995). In 1985, C2+ chemicals reduction product was founded on Cu catalyst (Tonner et al. 2002). In the past five years (2017–2021), nanotechnology has opened up many possibilities for catalyst modification. MWCNT refers to multi-walled carbon nanotube, PC refers to porous carbon, NPs refer to nanoparticles, PTFE refers to polytetrafluoroethylene, Bi2O3NSs@MCCM refers to Bi2O3 nanosheets grown on multi‐channel carbon matrix catalyst, CP refers to carbon paper. Reproduced from reference (Bashir et al. 2016; Gao et al. 2020; Gu et al. 2018; Kim et al. 2019; Liu et al. 2019; Ma et al. 2017; Sun et al. 2018; Wu et al. 2020a, b) with permission from the Elsevier, American Chemical Society, and Wiley
Over the past decade, a series of milestones have been achieved in the field of electrochemical CO2 reduction, with recent statistical results indicating a significant increase in electrochemical CO2 reduction research. As a testament to this, Fig. 5 shows an increasing trend of publications and citations from 2010 to 2021 with the keywords “electroreduction” and “CO2” in the Clarivate Web of Science Core Collection database. A total of 4,241 publications matched the keyword search results. The distribution of publications and citations has shown an upward trend since 2011, 69.42% of which are accounted for in the last three years alone (2019–2021). Besides, the number of citations also showed an exponential growth trend during the survey period, with the number of citations from 2016 to 2021 being 45.55 times that of the previous five years. Given the booming number of publications over the past 5 years and the fact that the community interest in this topic is at an unprecedented level, a systematic review is necessary to review the technical, environmental, and economic feasibility of electrochemical CO2 reduction.
Mechanism of electrochemical CO2 reduction to HCOOH and C2H4
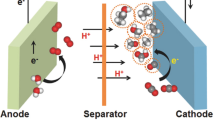
Understanding the principles of producing C1 and C2 chemicals by electrochemical reduction of CO2 is essential for the design and development of catalysts with high selectivity, stability, and efficiency. In the electrochemical reduction reaction, a group of redox reactions takes place at the cathode and anode of the electrolytic tank. The H-type cell is the most commonly used unit for laboratory electrochemical reduction CO2 testing. As shown in Fig. 6, an electrochemical cell consists of a cathode zone and an anode zone separated by an ion-exchange membrane. Under the action of the impressed current, the reduction reaction occurs at the cathode while the oxidation reaction occurs at the anode. The positive charge is transferred from the anode electrolyte to the cathode electrolyte through a cationic exchange membrane to balance the overall charge in the cathode region. CO2 is reduced in the cathode region, and meanwhile, it is competing with the hydrogen evolution reaction (HER). Therefore, the products in the cathode region include a variety of CO2 reduction products, H2, and part of unreacted CO2, whereas the products in the anode region are mainly O2.
Electrochemical CO2 reduction. M+ represents the metal ions in the anode electrolyte, usually K+ or Na+, depending on the type of electrolyte. In the electrochemical reaction, positive charge (+ ve ions) such as H+ migrates from the anode zone to the cathode zone. The electron (e.−) migrates from anode to cathode through a wire
Electrochemical reduction usually takes place at heterogeneous interfaces, where the solid phase is the electrocatalyst, and the liquid phase is an aqueous solution containing a large amount of CO2. These heterogeneous catalytic reactions can generally be summarized into three main steps (Agarwal et al. 2011), which are: (1) the adsorption of CO2 onto the electrocatalyst surface, (2) electron and proton transfer under the action of the catalyst. In this process, the C = O bond of CO2 is broken, and the new C-H forms different reaction intermediates under different permutations and combinations. (3) The intermediate is converted into a different final product and desorbed from the catalyst surface and then diffused into the solution. In the second step of the electrons transfer process, the number of transferred electrons is also different with the influence of the type of electrocatalyst, the type of electrolyte, pressure, temperature, cell configuration, and potential (Garg et al. 2020).
Based on the number of electrons transferred, the electrochemical reduction of CO2 can be divided into 1, 2, 4, 6, and 8-electron pathways in aqueous electrolytes and non-aqueous electrolytes. Possible products include carbon monoxide (CO), formate (HCOO.−) or formic acid (HCOOH) methane (CH4), ethylene (C2H4), ethanol (C2H5OH), methanol (CH3OH), and so forth. Table 1 summarizes the standard redox potentials of common CO2 reduction products (Benson et al. 2009; Lei et al. 2018). To unify the electrochemical reactions of CO2 from different products, Kortlever et al. summarized them as the following reactions: (Kortlever et al. 2015)
P represents the reduction products. Meanwhile, k = 1, n = 2, m = 0 for formate, whereas k = 2, n = 12, m = 4 for C2H4. For the other products k, n, and m have different values. The difference between k, n, and m is due to the different mechanisms that lead to diverse intermediates, resulting in varying products. In the past few decades, many excellent works have discussed the possible intermediates of different reaction processes through experiments and molecular simulation (Alfath and Lee 2020; Back et al. 2016; Baruch et al. 2015; Chaplin and Wragg 2003; Damas et al. 2019; Hori et al. 1997; Mc Murry and Fleming 1974; Schouten et al. 2011; Senanayake et al. 2005).
Reaction pathway for CO2 electroreduction to formic acid or formate
In the past few decades, many researchers have put forward conflicting views on the reaction path of electrochemical reduction of CO2 to formic acid (Alfath and Lee 2020; Back et al. 2016; Baruch et al. 2015; Chaplin and Wragg 2003; Damas et al. 2019). There are two possible reaction pathways for the electrochemical reduction of CO2 to produce formic acid (Chaplin and Wragg 2003). The first possibility is CO2 is dissolved in the electrolyte and inserted into a M-H bond. This insertion mechanism leads to the formation of *COOH intermediates (Fig. 7b). The second possibility is that CO2 obtains an electron and forms *CO2− species. Then *CO2− reacts with *H2O or *H to generate *OCHO intermediate (Fig. 7c) or *COOH intermediate (Fig. 7d). These two kinds of intermediates are bonded to the metal catalyst surface by M-C bonds or M–O bonds, respectively. *COOH intermediates are decomposed into *H and CO2 because of instability; it is also possible to generate CO through its desorption. For the *OCHO intermediate, it can only result in HCOOH. Subsequent experiments in density functional theory computation and electrochemistry have confirmed this theory. The activation barriers for *OCHO, *COOH, and H2 formation were recorded as 1.12, 0.25, and 0.2 eV, respectively, based on the density functional theory calculation computation results by Back et al. (2016). This indicates that the hydrogen evolution reaction is a powerful competitor for the production of formic acid. Therefore, the inhibition of hydrogen evolution should be considered in the design of catalysts to promote the production of formic acid. The author believed that CO2 was first protonated to form *OCHO and then re-protonated to form HCOOH by proton-coupled-electron transfer (PCET).
Electrochemical reduction of CO2 to formic acid and ethylene reaction mechanism. a formation of bicarbonate intermediate. b intermediate of CO2 insertion to M-H, c *OCHO intermedia, d *COOH intermedia. e CO2 Dimerization mechanism, f CO2 insertion mechanism (Alfath and Lee 2020; Hori et al. 1997; Peterson et al. 2010). RDS refers to rate-determining step, subscript ads refer to adsorbed species on the catalyst surface
In recent years, researchers have found a special pathway to reduce CO2 on Sn metals to produce formic acid. Unlike other metals, CO2 is produced by reacting with hydroxide adsorbed on Sn metals. Baruch groups used in situ attenuated total reflectance infrared spectroscopy (ATR-IR) to investigate the mechanism of electroreduction of CO2 in the Sn electrode (Baruch et al. 2015). They inferred that the SnO2 electrode was first transferred to SnII oxyhydroxide, which was a catalytic resting state. The *OH then reacted with CO2 to form *CO3H, which further reacted with an electron and proton to form *COOH and *OH (Fig. 7a). Density functional theory computation results carried out by Damas et al. showed that the electric potential of electrochemical CO2 reduction was about − 1.09 V versus RHE (Damas et al. 2019), implying that it is thermodynamically feasible for CO2 to be inserted into the Sn-OH surface, hence supporting the theory of Baruch.
Based on previous mechanistic studies on the CO2 reduction reaction to formic acid or formate, the reaction pathway can be summarized into four types of processes (Alfath and Lee 2020). These four types are (1) formation of bicarbonate intermediate (Fig. 7a), (2) CO2 insertion to M-H (Fig. 7b), (3) formation of *OCHO intermediate (Fig. 7c), and (4) formation of *COOH intermediate (Fig. 7d).
Reaction pathway for CO2 electroreduction to ethylene
As for the mechanism of CO2 electrochemical reduction to C2 chemicals, the most well-recognized mechanisms are the insertion mechanism and dimerization mechanism (Zheng et al. 2019). In the early studies, Hori et al. used Cu electrode (purity, 99.999%) to study the products of CO2 electrochemical reduction and demonstrated that the electrochemical reduction product of C1 and C2 products were CH4 (Faradaic efficiencies 15.6%), C2H4 (Faradaic efficiencies 4.1%), and C2H5OH (Faradaic efficiencies 0.3%), most of which was hydrogen (Faradaic efficiencies 73.0%) (Hori et al. 1997). Based on this result, Hori et al. proposed a CO-insertion mechanism. In following density functional theory calculation calculations, Andrew and his colleagues proposed that *CHO intermediate is first formed by protonation of CO. Then the absorbed formyl is then transferred by electrons and protons to form C1 chemical CH4, or by dimerization to form C2 chemicals C2H4 (Peterson et al. 2010). They further suggested that C–C bonds in ethylene are formed by non-electrochemical dimerization mechanisms.
In further research, a key dimerization mechanism intermediate *OCCO− has been proposed. The possible response pathways start with the acquisition of electrons for CO2 to become *CO2− species, which was then converted into *CO. Multiple *CO intermediates dimerized with each other to form *OCCO− (Fig. 7e). The C–C bonds became C=C through the McMurry coupling that broke the C-O bonds, which then generated enediol or enediolate and ended up converting to C2H4 (Mc Murry and Fleming 1974; Senanayake et al. 2005). Kendra and his groups used gas chromatography and 1D hydrogen nuclear magnetic resonance to detect reduction products and discovered several products that were not detected before, including acetate, methanol, glycolaldehyde, hydroxyacetone, acetone, and glyoxal (Kuhl et al. 2012). Kendra et al. believed that in the CO2 reduction process, a variety of C1 chemicals formed more complex “enol-like” intermediates through C–C coupling. Although Kendra’s experiments detected a variety of products, ethylene is still the only C2+ alkene observed. The dimerization mechanism is currently the most widely recognized mechanism for the formation of ethylene from CO2 reduction. In the theory of the dimerization mechanism, the energy barrier faced by the formation of ethanol is 0.2 eV higher than that by the formation of ethylene (Calle-Vallejo and Koper 2013). Ethylene is a favorable reduction product, which is consistent with many experiments (Popovic et al. 2020; Vasileff et al. 2020). This theory will serve as a guiding star to the design of electrocatalysts. The key is to facilitate the formation of more dimerizing precursors *CO and McMurry coupling to form *OCCO−. In other words, it is to enhance the adsorption of CO and reduce the energy barrier of the dimerization mechanism to improve the selectivity of the multi-carbon products.
The key to the insertion mechanism lies in the formation of CHO intermediate by CO protonation, which is also the potential limiting step of the insertion mechanism (− 0.74 V) (Peterson et al. 2010). In the subsequent reaction, CHO intermediates undergo further protonation, forming a variety of possible intermediates including *OCHCH2 or *OCHCHO* (Fig. 7f). These intermediates form C2H4 through hydrogenation. The density functional theory calculation calculations by Peterson et al. gave the free energy to form the *OCHCH2 intermediate or to form the *OCHCHO intermediate was − 1.04 eV and − 1.12 eV, respectively (Peterson et al. 2010). The insertion mechanism provides a new direction for the modification of catalysts. For some materials with weak CO binding, large amounts of CO are produced in the reaction, resulting in a low selectivity for ethylene. Thus, improving the selectivity of ethylene by enhancing the binding ability of materials to CHO intermediate will be one of the modification strategies.
Catalyst design for electrochemical CO2 reduction to C1 and C2 products
Through the above discussion on the mechanism of the formation of C1 and C2 compounds by electrochemical reduction of CO2, it is clear that the structure and property of catalysts play a decisive role in the selectivity of the electrochemical reduction products of CO2. The following section will focus on the effect of different catalyst modification options on the efficiency of electrochemical CO2 reduction to formic acid and ethylene from the perspective of practical application.
Electrochemical CO2 reduction to C1 formic acid or formate
Single metal catalysts
To explore the catalysts that enable electrochemical reduction of CO2 to occur at room temperature and atmospheric conditions, some basic investigations on transition metal element catalysts have been carried out (Ikeda et al. 1987; Kaneco et al. 1998; Kapusta and Hackerman, 1983). Hori and his colleagues presented a ground-breaking summary (Table 2) of some of the work done by single metal catalysts on CO2 reduction to produce formic acid and ethylene (Hori 2008). According to the results of Hori et al., In, Pb, and Sn transition metals showed high selectivity to formic acid products. Cu was inclined to form hydrocarbon products, and Pt and Ni had a low activity toward the reduction of CO2 and favored to form H2 products. Studt’s research group studied a variety of metal catalysts via density functional theory and proposed catalyst material requirements based on thermodynamics (Yoo et al. 2016). Calculations show that it is difficult to generate HCOOH by metals or alloys selectively process the *COOH intermediates reaction pathway due to the strong linear relationship between the chemical adsorption of *COOH intermediates and hydrogen adsorption (*H) (Yoo et al. 2016). Therefore, to improve the selectivity of HCOOH generation, the metal catalyst must satisfy CO2 reduction through *OCHO intermediate and weak binding to *H. It can be seen from Fig. 8a that Ag, Pb, Cd, and Sn generated HCOOH at a low potential (− 0.2 to − 0.6 V versus SHE), where SHE refers to standard hydrogen electrode. In particular, Ag and Pb have the lowest potential to generate HCOOH and are closest to the lowest thermodynamic potential required to generate HCOOH.
Theoretical limit potential energy of production HCOOH by electrochemical CO2 reduction process and volcano plot. a Theoretical limit potential energy for various metals in the electrochemical CO2 reduction process. The horizontal and vertical black dotted lines represent the theoretical potential to generate HCOOH and hydrogen, respectively, whereas the red lines represent the equal theoretical potential to generate HCOOH and hydrogen. The blue region tends to produce HCOOH, and the red region tends to produce H2. Reproduced from reference (Yoo et al. 2016) with permission from Wiley. b Volcano plot of the relationship between partial currents density of different metal catalysts and the binding energy of *OCHO, which is the key intermediate for formic acid formation. Reproduced from reference (Feaster et al. 2017) with permission from American Chemical Society. RHE refers to reversible hydrogen electrode
In addition to Ag and Pb (Fan et al. 2017), the Sn (Zhang et al. 2014a, b) and In (Lai et al. 2017) electrochemical reduction properties have also attracted much attention. Feaster and his colleagues also performed the density functional theory calculation to demonstrate the potential of metal Sn for electrochemical reduction of CO2 production formic acid (Feaster et al. 2017). They plotted the “Volcano” plot through the density functional theory calculation to represent the relationship between the partial current densities of different metals and the binding energies of the *OCHO. From Fig. 8b, it can be seen that Sn is closest to the volcano peak, indicating that Sn has the binding energy closest to the optimal *OCHO intermediate, proving that the Sn electrode is highly selective for formic acid. Formic acid generation by pure In and pure Sn electrodes for CO2 reduction was first proposed by Hackerman and Kapusta (1983). The current efficiency of CO2 was recorded to about 95%, with formic acid as the main reduction product. However, problems such as large overpotential and weak electrode adsorption capacity (coverage rate < 5%) were observed (Kapusta and Hackerman, 1983). Higher overpotential of In, Pb, and Sn in hydrogen evolution reaction could reduce the production of hydrogen and thus promote the production of more formic acid (Ikeda et al. 1987; Kaneco et al. 1998).
As a non-toxic metal, bismuth (Bi) metal has abundant reserves in the strata and relatively low price, making it a cathode metal material with commercial potential (Zhang et al. 2016a, b). Some earlier studies have demonstrated the great potential of Bi metals in the electrochemical CO2 reduction field (Li et al. 2020a, b, c, d, e; Wen et al. 2018; Zhang et al. 2018). For example, density functional theory research has shown that pure polycrystalline Bi metal was at the farthest position from the volcano top among various measured metals (Greeley et al. 2006), indicating the disadvantage of Bi metal for hydrogen adsorption. In other density functional theory studies, Bi metals also showed a similar strong binding force to * OCHO intermediates as Sn, Pb, and In metals (Yoo et al. 2016). Therefore, Bi metal catalysts have the potential to produce formic acid with high selectivity due to their distinct binding capacities for *H intermediates and *OCHO intermediates. However, the polycrystalline Bi metal catalyst also has some unfavorable factors for the electrochemical CO2 reduction reaction. The adsorption stability of the reaction intermediate is a problem. Apart from the transition metals (Cu, Ag, Pb) which have been widely studied, the main group metal Bi (Li et al. 2020a, b, c, d, e), Sn (Li et al. 2017b, a) and In (Li et al. 2020a, b, c, d, e) do not have the corresponding d orbitals in their atomic structures to adsorb the intermediate products. In some breakthrough scheme, adjusting the electronic structure of Bi metal, increasing the energy of p orbital in Bi atom and changing Bi atom to a state with higher energy will be beneficial to enhance the adsorption stability of intermediates and thus enhance the catalytic activity of Bi catalyst (Lei et al. 2018; Zhao et al. 2020a, b). However, the adjustment of electronic structure still needs to be realized through defect engineering, heteroatom doping and other modification methods. In addition, like other single metal catalysts, pure Bi metal also has problems such as high overpotential and insufficient relative surface area, which need to be further optimized (Li et al. 2020a, b, c, d, e).
As described above, Pb, Sn, Ag, and Bi are considered to be the most promising metals for electrochemical reduction of CO2 to generate HCOOH (Li et al. 2019). However, the high cost of Ag as a precious metal renders it economically impractical as an electrocatalyst for industrial-scale CO2 reduction. Although Pb metal has good catalytic ability, its adverse effect on the environment has aroused concern. For other single metal electrocatalysts, the low current density, poor stability, and short service life of the catalysts limit their commercial applications in the electrochemical reduction of CO2. To overcome these limitations, several modification strategies have been proposed, including surface engineering for nanostructures (Gao et al. 2018), alloys scheme (Clark et al. 2017), metal and non-metal composite scheme (Vasileff et al. 2017), and doping engineering (Vasileff et al. 2017).
Structure engineering and morphology optimization
The motivation of material modification through structural engineering is to create a larger catalytic surface area, enhance adsorption capacity, and provide more catalytic active sites (Francke et al. 2018). Li et al. proposed that the porous Sn electrode with an adjustable pore size was prepared by using the acetic acid (bubble stabilizer)-assisted hydrogen bubble dynamic template method (Li et al. 2019). The results showed that the pore structure with a diameter of 50–60 μm (Fig. 9a) was formed on the Sn electrode when 0.15 M acetic acid solution was used. In electrochemical tests, the minimum overpotential for the conversion is 0.473 V, with a current efficiency of up to 95.6% at − 1.6 V versus Ag/AgCl toward HCOOH (Fig. 9b, c) (Li et al. 2019). A similar strategy for preparing porous foam electrodes by electrodeposition has also been applied to Pb electrodes. Wang et al. proposed porous Pb foam electrodes using a similar preparation way (Wang et al. 2016a, b). Scanning electron microscope (SEM) results showed that the modified method formed cellular porous acicular Pb precipitation on Cu substrate, hence providing more low coordination sites. The electrochemical test results exemplified that the proposed scheme achieved a current efficiency of 96.8% at − 1.7 V versus Ag/AgCl. The advantages of this modification scheme through the construction of porous metal materials are also reflected in its larger surface area, more reactive sites, and milder reaction conditions compared to granular catalyst materials. At the same time, it also has the advantages of easy preparation, low-cost, and does not require the removal of the unwanted template. Although three-dimensional dendritic structures can be easily generated in the process of deposition, the structure formed by deposition cannot be controlled artificially due to the disorder of deposition (Shin et al. 2003). The trunk and branches in the porous dendritic structure collapse due to the instability of its structure, resulting in low porosity of the final product and poor catalytic effect (Shin et al. 2003).
Morphology and electrochemical test result of porous Sn. a Scanning electron microscope (SEM) images of top view (row 1 and 2) and cross-sectional (row 3) view of porous Sn foam electrodes. In the red box, the experimental group treated with 0.15 M acetic acid formed the most porous structures. b Current density of different Sn content. Reproduced from reference (Li et al. 2019) with permission from Elsevier. Sn-0.05, Sn-0.1, Sn-0.15, and Sn-0.2 refer to 5%, 10%, 15%, and 20% Sn content, respectively
A similar morphology optimization scheme was also applied to the modification of Bi metal catalyst. Zhang and his colleagues proposed a porous catalyst scheme, in which Cu atoms were introduced into bismuth oxide (Zhang et al. 2021a, b, c, d). In their scheme, the precursor solution containing trivalent Bi ions and divalent Cu ions was mixed with NaNH4 and stirred, followed by lyophilization to obtain the final porous copper-decorated bismuth-based nanofoam (P-Cu-BiNF) catalyst (Fig. 10a). Subsequently, X-ray diffraction (XRD) tests showed that the diffraction peak intensity decreased with the increase of Cu fraction, and that Cu atoms had the effect of reducing the crystallization degree of Bi crystals (Fig. 10b). At the same time, X-ray photoelectron spectroscopy (XPS) test results also show Cu atom has the effect of reducing the binding energy (Fig. 10c). Finally, the Brunauer–Emmett–Teller (BET) surface area of P-Cu-BiNF was 21.4 m2 g−1, 12 times larger compared to the sample without the introduction of Cu. The large surface area and synergistic interaction between Cu and Bi metals led to better electrochemical test results, with Faradaic efficiencies of over 90% for formate in the − 0.78 to − 1.18 V versus RHE potential range (Fig. 10d, e, f). The maximum partial current density for formate was 62.7 mA cm−2 (under the potential of − 1.18 V versus RHE), which was about twice that of the Cu-free group (Zhang et al. 2021a, b, c, d). It can be seen that Cu content is the key factor to regulate the electrocatalytic performance and catalyst morphology. The method of adjusting the morphology of Bi metal catalyst to create structures with a larger surface area is not limited to introducing other metal ions. Recently, Wu et al. proposed a scheme to construct Bi-Sn metal-based aerogel catalysts based on the large specific surface area and interconnected channels of aerogel. Electrochemical test results showed that the maximum current density of Bi-Sn aerogel electrode for formic acid was 9.3 mA cm−2 (under the potential of − 1.0 V versus RHE), and the Faradaic efficiency was 93.9% (Wu et al. 2021a, b, c). Other topographic engineering optimization methods include adjusting the concentration of Bi electrolyte precursors and promoting and regulating the growth of Bi crystals by electrochemical means (Dong et al. 2021); composite Bi atoms with high surface area carbon-based material to improve the relative surface area (Jiang et al. 2020a, b; Wu et al. 2020a, b). Meanwhile, the synergistic effect between Bi and the carrier was used to improve the catalytic performance.
Characterization and electrochemical test results of porous copper-decorated bismuth-based nanofoam (P-Cu-BiNF). a The preparation process for P-Cu-BiNF catalyst scheme. b X-ray diffraction (XRD) patterns for different Cu2+ and Bi.3+ molar ratio. c X-ray photoelectron spectroscopy (XPS) patterns of Cu 2p spectra of P-Cu-BiNF catalyst. Reproduced from reference (Zhang et al. 2021a, b, c, d) with permission from Royal Society of Chemistry. RHE refers to reversible hydrogen electrode
The morphology optimization methods including electrodeposition, introduction of other metal atoms, aerogels, and composites with high porosity carbon-based support materials reviewed in this section highlighted the need of structure engineering to create large specific surface areas and improve adsorption. For example, during the materials drying stage and subsequent electrochemical test, the collapse and accumulation of microstructure under capillary action will lead to the loss of catalytic active sites and the degradation of electrode performance. Therefore, maintaining the stability of nanostructures during preparation and long-term operation is the key to enhance the durability of porous electrocatalysts materials.
Construction of alloy structure
Compared with previous research, the construction of porous nanostructures effectively increases the specific surface area of the electrode and exposed more catalytic active sites. However, the catalytic materials such as Pb and Sn are still prone to deactivation, whereby electrolytic deactivation usually occurs after 1–6 h of electrolytic operation (Innocent et al. 2010; Lai et al. 2017). Therefore, it is a promising method to construct the alloy of Pb or Sn metal with other metals. Based on the performance of single metal electrodes for CO2 reduction reported by Hori et al., In is a potential metal with a current efficiency of close to 95% for HCOO− and only 3.3% for H2 at high potential (Hori 2008). This indicates that In metal has a good selectivity against HCOO− product. To take full advantage of the properties of In metals, Lai et al. proposed a scheme of In-Sn alloy catalyst (Lai et al. 2017). The experimental results show that the addition of Sn into In crystal phase can effectively improve the catalytic activity. When the content of In and Sn-In alloy is 90% and 10%, the highest Faradaic efficiency (92%) is recorded. More importantly, the In0.9Sn0.1 electrode still maintains a relatively high current efficiency after a long electrolytic operation of 22 h (15 mA cm−2), and after replenishing the KHCO3 electrolyte, the efficiency of In-Sn alloy for formate increased back to 90.7% (Lai et al. 2017). The addition of In metal effectively reduces the particle size of In-Sn alloy and provides more catalytic activity sites. At the same time, the biggest advantage of In-Sn alloys over ordinary Sn metals is that they enhance the stability of the catalyst over a long period of time. The reducibility and durability of In-Sn alloy are derived from its unique crystal phase structure. X-ray diffraction showed that the diffraction peak of the alloy In0.9Sn0.1 sample was offset. After alloying with Sn, In has anomalous structure evolution, forming a face-centered tetragonal structure with compression dominated by β phase, which further leads to the increase of the hybridization of 5 s and 5p valence electron bands of In-Sn alloy, and finally enhances the reducibility and durability (Zhang et al. 2016a, b).
Ren and his colleagues have achieved even better results with Sn alloy, and they have proposed a CuSn-laser-induced graphene (CuSn-LIG) scheme (Fig. 11a) (Ren et al. 2020). In subsequent electrochemical tests, the Faradaic efficiency for formic acid was nearly 99% (Fig. 11b) when the ratio of Cu atoms to Sn atoms in the alloy was close to 1:2, and a partial current density of 26 mA cm−2 for formic acid and a total current density of 30 mA cm−2 were recorded at a moderate overpotential of − 1.0 V versus RHE (Fig. 11c) (Ren et al. 2020). X-ray diffraction indicated that Cu(II) and Sn(IV) were the main crystal structures in CuSn alloy, and their valence states remained unchanged after electrochemical reaction test. In the presence of Sn atoms on Cu (II) surface, the rate-limiting potential through the *OCHO intermediate pathway decreased to − 0.45 V, which was lower than the *COOH intermediate pathway (− 1.01 V), which promoted the highly selective *OCHO pathway for CO2 reduction. The synergistic effect of Cu and Sn effectively reduced the overpotential of *OCHO intermediates and thus enhanced the selectivity of formic acid. This synergistic effect was speculated to be that when the overpotential was lower than the reduction of CO2, the catalyst metal underwent a redox reaction and caused the electron transfer at the catalytic site, resulting in the change of valence state (Ren et al. 2020). This change of valence state promoted the selectivity of CuSn alloy for *OCHO intermediate, and finally excellent selectivity of formic acid was obtained. More recently, Chen et al. also reported a scheme of Cu-Sn alloy, the difference is that they did not use laser-induced graphene carrier as the deposition basis. In their scheme, Sn particles are electrodeposited on the surface of the prepared Cu nanowires (Chen et al. 2021). Similar to the use laser-induced graphene carrier, Cu nanowires also provided porous nanostructure. The subsequent scanning electron microscope (SEM) analysis showed that the reduction of copper foil in alkaline conditions using NH4SO3 reagent formed the fiber forest of nanowires structure. Each nanowire has a very rough surface and porous structure which is a benefit for subsequent deposition of Sn particles (Fig. 12a, b).
Preparation and electrochemical test results of CuSn alloy scheme. a The synthesis and preparation of CuSn-laser-induced graphene (CuSn-LIG) catalyst. b Faradaic efficiency test results of different Cu/Sn atomic ratios experiment groups. c The current density for HCOO.− in the experimental group with different Cu/Sn atomic ratios. CuSn-2 refers to 80:20 of Cu:Sn; 60:40 for CuSn-3; 50:50 for CuSn-4; 30:70 for CuSn-5; 15:85 for CuSn-6; PI refer to phosphoinositides. RHE refers to reversible hydrogen electrode. Reproduced from reference (Ren et al. 2020) with permission from American Chemical Society
Characterization and electrochemical test results of Sn on Cu nanowires (Sn/Nano-Cu). a SEM images of Cu nanowires. b SEM images of prepared Sn/Nano-Cu catalyst. c Linear sweep voltammetry result for Sn/Nano-Cu and control group catalyst under different environments (Scan rate is 20 mV/s, 0.1 M KHCO3 electrolyte). d Faradaic efficiency of the formic acid product. e Current density of Sn/Nano-Cu and control group catalyst. RHE refers to the reversible hydrogen electrode, and SEM refers to scanning electron microscope. Reproduced from reference (Chen et al. 2021) with permission from Elsevier
Similar alloy modification schemes have also been used for Pb metals. Choi et al. proposed a modification scheme of Sn–Pb alloy (Choi et al. 2016). The electrode composed of Sn56,3Pb43.7 was recorded to show the best catalytic efficiency, with Faradaic efficiency up to 79.8% and partial current density recorded to 45.7 mA cm−2 (Choi et al. 2016). Choi et al. studied the surface properties of the metal catalysts using the cyclic voltametric method. It is found that Sn in Sn–Pb alloy can facilitate the formation of SnOx oxide and Pb elemental, which indicates that the addition of Sn metal can inhibit the formation of PbO with low conductivity on the electrode surface, thus reducing the current density and catalytic effect (Choi et al. 2016).
In the subsequent work, Bai et al. optimized the scheme of Sn–Pb alloy by using citric acid as a stabilizer and sodium borohydride as a reducing agent. Pb–Sn alloys were synthesized through wet reduction on an activated carbon substrate. During the synthesis, Pb and Sn contents of the alloy were adjusted by controlling the concentrations of different PbCl2 and SnCl2, and the particle size was controlled by controlling the amount of stabilizer and reducing agent. Density functional theory calculation showed that the energy difference of intermediates formed on Pb-SnO2 was 0.86 eV and that of adsorbate was 0.71 eV, which was higher than other PbOx and SnOx combinations (Bai et al. 2017). This indicates that the Pb-SnO2 structure formed in the alloy was the most conducive to the formation of *OCHO intermediate, leading to formic acid selectivity. At the same time, a very low reaction overpotential (− 0.26 V) and a current efficiency of nearly 100% against formic acid were recorded under this optimal structure (Bai et al. 2017).
Conventional solid alloy catalysts are usually prepared through bottom-up processes such as in situ deposition (Bohlen et al. 2020; Jiménez et al. 2020; Zhang et al. 2020a, b, c, d, e; Zhou et al. 2020), and top-down processes (Liu et al. 2020a, b, c, d; Wang et al. 2020; Zhao et al. 2020a, b). Yuan et al. proposed a scheme to prepare ultra-thin two-dimensional oxides by taking advantage of that liquid metals will automatically form a self-limiting ionic oxide surface layer in air (Yuan et al. 2020a, b) (Fig. 13b). In their scheme, the Sn-Bi alloy is kept as a liquid at 180 °C. When pure oxygen bubbles pass through the liquid alloy layer, the alloy layer forms an oxide skin (Fig. 13a). The characterization results show that there are tin oxide particles and bismuth oxide particles, as well as single atoms and clusters in the nano-oxide layer prepared by this method (Fig. 13c, d). A subsequent electrochemical test showed that the liquid metal Sn-Bi alloy had a Faradaic efficiency of 90.8% (at a potential of − 1.37 V versus RHE) for formic acid. The current density was 45 mA cm−2 (Fig. 13e, f). The merit of this preparation approach is that it avoids the use of complex and expensive precursors as conventional schemes, while the synthesis conditions are relatively mild. In this section, the construction of alloy catalysts based on Pb, Sn, In and Bi elements are reviewed. At present, problems such as uncertain crystal structure, expensive precursor and reagent, and insufficient stability of crystal structure are presented in electrodeposition. Although adopting novel liquid metal preparation method can effectively reduce the complexity of material preparation process, there are also obvious disadvantages, such as the inability to control the particle distribution on the formation of oxide scale, the relatively disordered surface structure and the application only to metals with low melting point.
Characterization and electrochemical test results of 2D SnOx nanoflakes catalyst. a V shape tube used for 2D SnOx nanoflakes catalyst preparation. b 2D SnOx nanoflakes catalyst. c Scanning electron microscope (SEM) image of 2D SnOx nanoflakes catalyst. d Transmission electron microscope (TEM) images of 2D SnOx nanoflakes catalyst. e Current density of 2D SnOx nanoflakes electrode. (f) Normalized Faradaic efficiency of 2D SnOx nanoflakes electrode. RHE refers to reversible hydrogen electrode. Reproduced from reference (Yuan et al. 2020a, b) with permission from American Chemical Society
Construction of carbon support catalysts
Similar electron transfer and coupling synergies effects have been demonstrated between metals and non-metals. Tsujiguchi et al. found that the synergistic effect between Sn and reduced graphene oxide (rGO) composites effectively improves the Faradaic efficiency of CO2 reduction to formic acid (Tsujiguchi et al. 2021). In enhancing CO2 adsorption, the Sn/rGO group treated at 800 ℃ showed the highest CO2 adsorption capacity, which was about 27.5 μmol m−2, almost 4.2 times that of bare Sn (Tsujiguchi et al. 2021) (Fig. 14a). The density functional theory calculation results for the adsorption energy of CO2 showed that the adsorption energy of ketones or carboxyl functional groups on the surface of reduced graphene oxide ranged from − 271 to − 407 meV (Tsujiguchi et al. 2021) (Fig. 14b, c), which significantly improved the adsorption capacity of bare Sn for CO2. In the following scanning electrochemical cell microscopy (SECCM) measurements, three positions were tested respectively, including Sn particle surface, reduced graphene oxide surface, and the interface between Sn particles and reduced graphene oxide surface (Fig. 14e). The results show that the reduction current density at the interface is the largest at − 0.8 V versus RHE potential (Fig. 14f, h, i). This infer that the interface between reduced graphene oxide and Sn particles surface is the real catalytic activity site. The electrochemical test result shows a high formate Faradaic efficiencies at potential of − 0.82 V versus RHE with 98 ± 0.7%. The current density was recorded as − 9.9 mA cm−2 (Fig. 14d, g). This work proposed a different view from the previous CO2 reduction occurred directly on the surface of the catalyst (Lei et al. 2016; Li et al. 2017b, a; Zhang et al. 2014a, b). CO2 was first adsorbed to the surface of reduced graphene oxide, and then due to the synergistic effect between reduced graphene oxide and Sn. After that CO2 molecules migrated to the interface for reduction reaction. Similar synergies effect have been reported between Bi and reduced graphene oxide (Jiang et al. 2020a, b). This new mechanism will facilitate the design of carbon-based composite catalyst materials by enhancing the electron transfer between reduced graphene oxide support and Bi active site.
Characterization and calculation results of Sn/rGO composite scheme. rGO refers to reduced graphene oxide, and GO refers to graphene oxide. RHE refer to the reversible hydrogen electrode. a CO2 adsorption capacity on the surface of Sn/rGO800, Sn/GO800, and bare Sn particles depends on time. CO2 adsorption density functional theory calculation models and the result of rGO with b two ketones functional groups and c one ketones group with one carboxyl group. d Electrochemical test of Sn/rGO800 composite with other reference groups. Tested under conditions of CO2-saturated or Ar-saturated environment and 0.1 M aqueous KHCO3 electrolyte. e Scanning electrochemical cell microscopy measurements process. f Scanning electrochemical cell microscopy measurements result of reduction current on Sn particles surface (position 1), interface (position 2), and reduced graphene oxide surface (position 3). g Overall and partial Faradaic efficiency of Sn/rGO800 group with other reference groups under different potential. h and i Topography and scanning electrochemical cell microscopy measurements current mapping. Reproduced from reference (Tsujiguchi et al. 2021) with permission from American Chemical Society
Other than Sn, and Pb metal, Bi metal has the most potential for commercialization of formic acid production by electrochemical CO2 reduction, which can be further enhanced by combining it with non-metallic carbon-based support (An et al. 2021). Up to now, a number of excellent carbon-based Bi metal composite catalysts have been proposed, all of which have achieved Faradaic efficiencies of more than 90% (Duan et al. 2020; Li et al. 2020a, b, c, d, e; Zeng et al. 2021; Zhang et al. 2021a, b, c, d). The core of these Bi catalyst supported on carbon carriers is to optimize the electronic structure at the interface through the interaction between the Bi metal and the carrier and to improve the efficiency of electron transport by using the better electron transport capability of the carrier, thus improving the catalytic performance. A typical example is the Bi catalysts scheme proposed by Zhang and his colleagues, which is compounded on carbon nanotubes (Zhang et al. 2021a, b, c, d). In their scheme, nano-capillarity and nano-confinement effect of nitrogen-doped carbon nanotubes (NCNTs) promoted the migration efficiency of the material at the active site and avoided the aggregation of Bi atoms (Fig. 15a, b). By scanning electron microscope and high resolution transmission electron microscopy (HRTEM) characterization, it can be seen that Bi nanorods (Bi-NRs) are confined in larger nitrogen-doped carbon nanotubes (Fig. 15c). Electrochemical test results showed that the Faradaic efficiency of Bi-NRs@NCNTs catalyst for formate reached the maximum value of 90.9% at − 0.9 V versus RHE. At this potential, the current density was recorded as 6 mA cm−2 (Fig. 15d, e) (Zhang et al. 2021a, b, c, d). Additionally, the performance of Bi-NRs@NCNTs in the 24 h durability test showed no significant decrease in current density. Analysis showed that the catalytic activity of Bi-NRs@NCNTs catalyst was mainly contributed by the central Bi-NRs, with the NCNTs acting as shells that restricted the combination of the Bi-NRs, allowing the Bi atoms to remain separated. The capillary effect of carbon nanotube shell enhances the mass transfer of reactants in it. After durability testing, Bi was still able to remain at zero valence.
Characterization and electrochemical test results of Bi-NRs@NCNTs catalyst. NCNTs refer to nano-confinement effect of carbon nanotubes, and NRs refer to nanorods, NWs refer to nanowires. RHE refer to the reversible hydrogen electrode. a Capillary phenomenon of Bi-NRs@NCNTs catalyst. b Preparation process of Bi-NRs@NCNTs catalyst. c Scanning electron microscope (SEM) image of Bi-NRs@NCNTs catalyst. d Current density of Bi-NRs@NCNTs electrode. e Nominal Faradaic efficiency of Bi-NRs@NCNTs electrode. Reproduced from reference (Zhang et al. 2021a, b, c, d) with permission from American Chemical Society
Optimizing Bi catalysts by using carbon nanotubes as a carrier has also been proposed by Li et al. In the preparation process, Bi metal is electrodeposited on copper foil with the addition of carboxylated multi-walled carbon nanotube (MWCNT-COOH) (Li et al. 2020a, b, c, d, e). Finally, a composite catalyst contains Bi metal, MWCNT and copper foil. Electrochemical tests result (Fig. 16b, c, d) showed that the catalyst containing MWCNT was able to achieve a Faradaic efficiency of 91.7% against formic acid at − 0.76 V versus RHE. The current density of Bi-MWCNT-COOH/Cu electrode fluctuates between 6 to 7 mA cm−2 during 12 h of operation (Fig. 16e) (Li et al. 2020a, b, c, d, e). In addition, it can also be found in the scanning electron microscope images that the highly porous shape formed after the addition of MWCNT, which was not found in the control group without the addition of MWCNT (Fig. 16a). Therefore, the high stability and good catalytic performance in this catalyst can be attributed to the inhibiting effect of trivalent Bi ions in the catalyst on hydrogen evolution reaction and the increase of the overall surface area and electrical conductivity of MWCNTs. Other than carbon nanotubes, reduced graphene oxide can also provide performance optimization for supported Bi catalysts. Duan and his team proposed a scheme to load ultrafine Bi metal particles on a reduced graphene oxide carrier (Duan et al. 2020). It has been reported that this scheme achieved 98% Faradaic efficiency for formic acid at − 0.8 V versus RHE, while the Bi catalyst group without reduced graphene oxide support achieved 91% Faradaic efficiency for formic acid at − 0.9 V versus RHE (Duan et al. 2020). Electrochemical impedance spectroscopy (EIS) diagram showed that the Bi/rGO group tended to be horizontal. This indicates that the Bi/rGO electrode has a lower resistance than the control group without carrier and with polyvinylpyrrolidone (PVP) as carrier, meaning that electrons migrate faster on Bi/rGO electrode. Therefore, this work successfully took advantage of the high conductivity of reduced graphene oxide to enhance the catalytic performance. In all, loading Bi ions on carbon-based support or carbon-based support with other atom doping is an effective modification method to enhance the stability and electron transfer efficiency. This is because the support materials have a larger specific surface area, better electrical conductivity and protection against oxidation of Bi catalysts.
Characterization and electrochemical test results of Bi-MWCNT-COOH/Cu catalyst. Bi-MWCNT-COOH/Cu refers to Bi metal electrodeposited on copper foil with the addition of carboxylated multi-walled carbon nanotube. a SEM images of Bi-MWCNT-COOH/Cu catalyst b Cyclic voltammograms (CVs) and linear sweep voltammetry (LSV) of Bi-MWCNT-COOH/Cu electrode test under conditions of 0.5 M NaHCO3 solutions, CO2 or N2 saturated environment. c Cyclic voltammograms of the Bi-MWCNT-COOH/Cu with the change of overpotential. d Faradaic efficiency of the Bi-MWCNT-COOH/Cu electrode. e Change of current density of Bi-MWCNT-COOH/Cu electrode under long time operation test. SEM refers to scanning electron microscopy, and MWCNT refers to multi-walled carbon nanotube, RHE refers to reversible hydrogen electrode. Reproduced from reference (Li et al. 2020a, b, c, d, e) with permission from Elsevier
With the development of nanotechnology and related characterization equipment, metal single-atomic catalysts have been favored by more and more researchers because of their high utilization rate, high stability and catalytic activity (Li et al. 2020a, b, c, d, e). For the single-atom catalyst, the most important characteristic is that the catalytic active potential is provided by the metal atoms isolated from each other on the surface, and the electronic structure of the active potential changes under the influence of the carbon-based support, thus improving the catalytic performance (Wang et al. 2018). Compared with conventional catalysts, single-atom catalysts usually have well-defined catalytic centers, which provide unique opportunities for catalyst design. At present, the main form of atomic-dispersed catalysts is metal–nitrogen-doped carbon support (M–N-C) mode (Varela et al. 2018). Among them, the metal parts are mainly Fe (Pan et al. 2020), Cu (Cai et al. 2021; Xu et al. 2020), Ni (Yang et al. 2021; Zhang et al. 2020a, b, c, d, e), and Pd (Bok et al. 2021). The carbon component used in the support is a variety of carbon nanostructures, including carbon nanotubes and graphene (Hou et al. 2020; Zhang et al. 2020a, b, c, d, e) as well as porous carbon (Chen et al. 2020a, b, c; Pan et al. 2020). However, most current electrochemical CO2 reduction catalyst materials with atomically dispersed structures target CO and CH4 production rather than formic acid from the electrochemical CO2 reduction.
In recent years, a number of single-atom catalyst projects have been proposed that target product formic acid. Lu et al. proposed an atomically dispersed In metal on N-doped carbon skeleton (In − N − C) scheme and achieved high turnover frequency (TOF) toward formate product (Lu et al. 2021). In their scheme, In atoms were first mixed to the surface of ZIF-8 to prepare the precursor of In-N–C catalyst. Followed by a process similar to metal replacement reaction, Zn atoms, which were originally located in the dodecahedral coordination center of ZIF-8 was replaced by In atoms. In this work, the construction of the atomic dispersion structure of In atoms is noteworthy. The authors used a process similar to a metal substitution reaction to construct coordination bonds between In and the surrounding N and C atoms. Compared with the method of directly introducing In atom into the center of ZIF-8, the construction of a coordination bond can effectively improve the interaction force between In atom and non-metallic support, which is far greater than the Van der Waals force between molecules. Therefore, in the subsequent HAADF-STEM characterization (Fig. 17a), the atomically dispersed In did not appear agglomeration. Electrochemical test results showed that the current density of In–N–C catalyst was 24.5 mA cm−2 at − 1.1 V versus RHE (Fig. 17b). The maximum Faradaic efficiency for formate is 80% and appeared at − 0.79 V versus RHE (Fig. 17d). Among the electrochemical tests, the most outstanding In–N–C was the turnover frequency test, which reached 26,771 h−1 (Fig. 17c), recording 10 times higher than that of the Sn single-atom (Sn–N–C) catalytic scheme (Lu et al. 2019).
Characterization and electrochemical test results of In-N–C catalyst. a High-angle ring dark field image—Scanning transmission electron microscope (HAADF-STEM) image of In-N–C catalyst. b Current density of In-N–C electrode compared with In deposit group. c Turnover frequency of In-N–C electrode compared with other schemes from literature. RHE refers to reversible hydrogen electrode, SAs refers to single atoms, HNPCS refers to N-doped porous carbon spheres, A-Ni-NSG single-Ni-atom catalysts prepared with the addition of a sulfur precursor (L-cysteine), NC refers to nanocarbon. Reproduced from reference (Lu et al. 2021) with permission from American Chemical Society
A similar single-atom catalyst scheme is used for the Sn metal. Chen and his colleagues proposed a scheme for introducing Sn metal particles onto the surface of porous carbon nanosheets doped with N and S (SnS/Sn-NSC NHs) (Zhao et al. 2021a, b). Unlike Lu et al. who used ZIF-8 as a template to introduce In metal atoms (Lu et al. 2021), Zhao et al. used NaCl as a solid template to mix with the prepared Sn-thiourea precursor and glucose. After subsequent high-temperature treatment, the glucose was converted to graphite, while the NaCl was dissolved and removed by water leaves porous structure, and thiourea was used as the source of sulfur atoms (Fig. 18a). Different from the common single-atomic catalyst, SNS/Sn-NSC NHS is a more duplicated multi-atom co-doping scheme. The final electrochemical test results showed that the SNS/SN-NSC NHS catalyst had more than 80% Faradaic efficiency against formate in the potential range from − 0.6 to − 0.9 V versus RHE (Fig. 18b) (Zhao et al. 2021a, b). The current density of formate at the same potential was − 16 mA cm−2 (Fig. 18b, c). In their further comparison, the SNS/SN-NSC groups were shown better results compared to both the SNS nanoparticle group and the SN-NSC group. XPS studies implied that there was a strong interaction between SnS and Sn-NSC, which promoted the transfer of electrons from SnS to Sn-NSC. As a result, Sn mixed with porous carbon has more negative potential than Sn in SnS, which was easier to transfer electrons to CO2 molecules and reduce them, and finally achieved better catalytic performance. In addition, single-atom catalysts without carbon substrate have also been proposed. For example, Bok et al. use the pores in the metal–organic framework (MOF) to reduce the strong adsorption of *CO intermediate on Pd metal, which was unfavorable in the electrochemical CO2 reduction process, they introduced Au single-atom into the Pd metal. Au single-atom further destroyed the adsorption of *COOH intermediate, and finally enhances the stability of Pd electrocatalyst and its ability to resist CO poisoning (Bok et al. 2021). This is a typical space confinement strategy for the construction of a single-atomic catalyst. Unlike the coordination bond construction strategy, spatial confinement mainly involves confining the metal atoms with porous materials to avoid atomic aggregation due to high free energy. Other than these, defect strategy introduces defects and vacancies in advance and uses defects to capture single atoms (Rong et al. 2020). Sacrificing the precursor template strategy, in which the atoms in the template are replaced with the desired one to take advantage of the porous nature of the original template, has been widely investigated (Yang et al. 2019).
Preparation and electrochemical test result of SnS/Sn-NSC catalyst. SnS/Sn-NSC refers to introducing Sn metal particles onto the surface of porous carbon nanosheets doped with N and S. RHE refer to the reversible hydrogen electrode. a SnS/Sn-NSC catalyst preparation process. b Nominal Faradaic efficiency of SnS/Sn-NSC electrode. c Overall current density of SnS/Sn-NSC electrode. d Formate partial current density of SnS/Sn-NSC electrode. Reproduced from reference (Zhao et al. 2021a, b) with permission from American Chemical Society
All of these are common methods of single-atom anchoring in the construction of single-atom catalysts. It was found that the key to constructing single-atom catalysts is to overcome the large free energy of a single-atom and prevent the aggregation of atoms, hence changing the electronic structure of the catalytic active site by using the synergistic effect between the atom and the carrier and constructing a larger surface area. Nevertheless, current schemes of single-atomic catalysts for the production of formic acid are still limited and they require further research in the future.
From the perspective of environment and sustainability, the impact of metal-based catalysts on the environment in their life cycle cannot be ignored, which is embodied in the mining and metallurgy processes with high energy consumption and high pollution, and the pollution of soil and water by heavy metals in some waste scenarios. The diverse configurations of carbon-based catalysts such as graphene, carbon nitride, and their more sustainable properties have attracted the attention of more and more catalyst researchers (Khan et al. 2019). Recently, some non-metal-doped carbon-based metal-free catalysts were proposed and certain achievements were made in the electrochemical reduction of CO2 to C1 chemicals (Jia et al. 2020; Qin et al. 2021; Wanninayake et al. 2020; Yuan et al. 2020a, b).
Focusing on formic acid in C1 chemicals, Zhang and his colleagues proposed a scheme to use polyethylenimine (PEI) as a co-catalyst with nitrogen-doped carbon nanotubes (NCNT) catalyst (Fig. 19b) (Zhang et al. 2014a, b). In their scheme, CNT film, doping N atom, and polyethylenimine co-catalyst were combined into glassy carbon (GC) by using drop-casting, NH3 plasma, and dip-casting methods. In subsequent electrochemical tests, polyethylenimine-nitrogen-doped carbon nanotubes were recorded as the current density at 9.5 mA cm−2 and Faradaic efficiency against formic acid up to 85%. During the CO2 reduction process, CO2 is first adsorbed to N-dopant sites, including pyridinic N or pyrrolic N, which are then reduced to *CO2− (Fig. 19a) (Zhang et al. 2014a, b). The addition of polyethylenimine co-catalyst and N-doping contribute to the stabilization of *CO2− intermediate. Since *CO2− is usually the rate-determining step (RDS) of CO2 electrochemical reduction, the efficient formation of *CO2− intermediates will effectively improve Faradaic efficiency and overall reaction rate (Koshy et al. 2021; Zhao and Quan 2021). Similarly, Wang and his colleagues proposed a nanoporous carbon nanotube composite membrane (HNCM/CNT), which achieved 81% of the catalytic efficiency for formic acid at − 0.80 V versus RHE (Wang et al. 2017). Chen and his colleagues also proposed nitrogen-doped graphene as a catalyst for electrochemical reduction of CO2, and also recorded a 73% Faradaic efficiency for HCOOH at − 0.84 V versus SHE (Wang et al. 2016a, b). The common feature of these catalyst schemes is that a pyridinic N structure was constructed, and adjacent to pyridinic N, C, was the active site for electrochemical reduction of CO2, indicating that nitrogen dopants stabilized *CO2− and reduced the reduction barrier, thus improving the catalytic efficiency of CO2 reduction.
Reaction pathway and preparation process of PEI-NCNT catalyst. a Electrochemical reduction of CO2 to produce formate at PEI-NCNT electrode. b Synthesis of PEI-NCNT electrode. PEI-NCNT refers to polyethylenimine as a co-catalyst with nitrogen-doped carbon nanotubes. GC is glassy carbon, and CNT is carbon nanotubes. NCNT refers to N-doping of carbon nanotubes. PEI is polyethylenimine co-catalyst. Reproduced from reference (Zhang et al. 2014a, b) with permission from American Chemical Society
Defect engineering
In recent years, the strategy of doping non-metal S in metals through nanotechnology has been proposed. Sargent and his colleagues suggested that sulfur-dodulated Sn sites (Sn(S)) is a potential solution to enhance the synergistic effect between non-metal atom and metal atom (Zheng et al. 2017). Their density functional theory calculations demonstrate that a S content of 3.8% on the Sn plate can achieve the closest minimum potential required for CO2 production of formic acid via the *OCHO intermediate reaction (Fig. 20) (Zheng et al. 2017). In the electrochemical test, the Sn(S)/Au electrode showed a 93% electrode efficiency at a current density of − 0.75 V versus SHE, with a current density of 55 mA cm−2. The productivity change of the electrode after 40 h of operation was less than 2%, indicating good stability (Zheng et al. 2017). In the scheme proposed by Liu et al., the Sn surface of controllable complex S is the key to achieving efficient electrochemical catalysis. They used atomic layer deposition process to deposit SnS on Au needles, forming sulfur-modulated-tin films. SnSx is converted to Sn(S) by selective reduction. According to field-induced reagents concentration (FIRC), Au needles provide a sharp nanostructure that creates a locally strong negative electric field and attracts positively charged cations, thus enhancing the activity of CO2 reduction (Liu et al. 2016). Similarly, Fan et al. also proposed a dendritic crystal with a nanoscale needle-like tip of Pb electrode (Fan et al. 2017). They synthesized the catalyst structure using the dynamic hydrogen bubble templating (DHBT) method, and showed a Faradaic efficiency of 97% at − 0.99 V versus RHE and an electric current efficiency of 7.5 mA cm−2 (Fan et al. 2017). The presence of dendrite secondary structure promoted the charge concentration and further improved the catalytic efficiency.
Sulfur-modulated tin system density functional theory computation result. a Schematic of different S contents on Sn plate. b Density functional theory calculation result of Gibbs free energy of HCOO* intermediate, *COOH intermediate, and *H at different sulfur content. Reproduced from (Zheng et al. 2017) with permission from Elsevier
For ultra-thin 2D nanomaterials, defects and vacancies can fine-tune the electron valence states and increase exposure of catalytic active sites, so that even a small number of surface defects can bring great potential (Lu et al. 2020). At present, the construction of oxygen vacancy through defect engineering can help to reduce the potential of the SnOx oxide and improve the current density under similar potential. In one study, Li and his colleagues designed that carbon foam (CF)-supported oxygen vacancy-rich SnOx nanosheets (Vo-SnOx/CF) can effectively realize the transfer of electrons and mass, thus achieving better catalytic performance (Li et al. 2020a, b, c, d, e). In their scheme, solvothermal and then plasma treatments are applied to foamed carbon base. During subsequent electrochemical tests, the Vo-SnOx/CF40 group, for which was treated with a 40 V alternating Current power supply (Fig. 21a), was recorded 86% partial Faradaic efficiency toward formic acid. It is noteworthy that the partial current density toward formic acid is 30 mA cm−2 at a potential of − 1.0 V versus RHE (Fig. 21b–d). This value was maintained well during the endurance test of up to 8 h (Fig. 21e) (Li et al. 2020a, b, c, d, e).
Preparation and electrochemical test results of Vo-SnOx/CF scheme. Vo-SnOx/CF refers to carbon foam-supported oxygen vacancy-rich SnOx nanosheets. a Vo-SnOx/CF synthesis process. Electrochemical test result of Vo-SnOx/CF scheme. b Overall current density of each Vo-SnOx/CF composite, which plasma treated by alternating current power of 22 V, 40 V, and 60 V compared with without plasma treatment. c Partial Faradaic efficiency of formate. d Partial current density of formate. e Overall current density of Vo-SnOx/CF60 group in 8 h stability test. The electrochemical CO2 reduction test was carried out under the conditions of CO2-saturated 0.1 M KHCO3 aqueous solution. RHE refers to reversible hydrogen electrode. Reproduced from reference (Li et al. 2020a, b, c, d, e) with permission from Royal Society of Chemistry
Similarly, Liu et al. modified SnO2 from the view of oxygen vacancy defect engineering. In their scheme, Vo-SnO2-60 group, which annealed at hydrogen and Ar for 60 min, was recorded to have a Faradaic efficiency of more than 90% against formate at − 1 V versus RHE (Fig. 22a). At the same potential, the current density was recorded to be around − 7.5 mA cm−2 (Fig. 22b, c) (Liu et al. 2020a, b, c, d). At a lower potential (− 0.7 V versus RHE), the maximum Faradaic efficiency reached 92.4% (Fig. 22a) (Liu et al. 2020a, b, c, d). Similar defect engineering of oxygen vacancy is not limited to Sn oxides. High current density has also been recorded in the modification of Bi metal oxides (Wu et al. 2020a, b). Based on the above examples, the performance improvement caused by oxygen vacancy can be owing to: (1) increased electrochemical surface area, (2) enhanced adsorption activity for CO2, and (3) improved electron and mass transfer capacity. Furthermore, reasonable oxygen vacancy makes the Sn on the catalytic surface more oxygen-friendly, which contributes to the occurrence of *OCHO intermediate reaction pathway, thus promoting high selectivity for formic acid.
Electrochemical test result of SnO2 and VO-SnO2 nanosheets. Vo refers to oxygen vacancy a Overall current density. b Partial current density of formate. c Current density after electrochemical surface areas corrected. d Overall current density after 8 h stability test. The electrochemical CO2 reduction test was carried out under the conditions of CO2-saturated 0.5 M NaHCO3 solution. RHE refers to the reversible hydrogen electrode, ECSA refers to the electrochemical specific surface area, FE refers to Faradaic efficiency. Reproduced from reference (Liu et al. 2020a, b, c, d) with permission from Elsevier
The scheme of Bi catalysts modification by defect engineering and the crystal facet adjustment is the closest solution to the commercialization producing formic acid by electrochemical CO2 reduction process, which can achieve good catalytic performance under low overpotential. In recent years, breakthrough progress has been made in improving the catalytic performance of Bi catalysts by adjusting the electronic structure of Bi catalysts (Garcia de Arquer et al. 2018; Jiang et al. 2021; Lei et al. 2018; Wu et al. 2019). In the last two years of development, atom vacancy schemes for Bi catalysts have been proposed (Wu et al. 2020a, b; Zhao et al. 2020a, b, 2021a). These schemes show good performance in terms of current density, formic acid yield and catalytic performance at low potential.
Zhao and his colleagues reported a scheme for a bismuth oxide catalyst supported on a thin carbon layer containing oxygen atom defects (BiOx@C) (Zhao et al. 2021a, b). The catalyst was synthesized by pyrolysis at the expense of Bi metal–organic framework precursors. The energy-dispersive spectroscope (EDS) detection image showed that the outer layer of the final BiOx@C catalyst contained a certain amount of oxygen atoms and a thin carbon layer (Fig. 23a). XPS spectra recorded that the synthesized catalytic material BiOx@C had a significant peak at the binding energy of 531.7 eV, confirming that oxygen atom vacancies were present on the surface of the catalyst (Fig. 23b, c). In contrast, the peak intensity of the control group (without imidazole) was significantly lower than that of the BiOx@C group. In subsequent electrochemical tests, the BiOx@C catalyst achieved a maximum Faradaic efficiency of 89.3% for formic acid at a potential of − 1.6 V versus SHE (Fig. 23e). At the same time, the maximum current density of 37.8 mA cm−2 was recorded at a potential of − 1.7 V versus SHE. (Fig. 23d, f) The maximum current density of the control group without the addition of imidazole was achieved at − 1.8 V versus SHE and was only 0.357 times that of the BiOx@C group (Zhao et al. 2021a, b). Thus, it can be seen that the addition of imidazole enhances the oxygen vacancy on the BiOx catalyst surface and significantly enhances the performance of the BiOx@C catalyst. In principle, the oxygen vacancy can attract the oxygen atom in the CO2 molecule to bind with the neighboring Bi atom, and the higher adsorption energy also stabilizes the fine adsorption stability of the CO2 molecule on the oxygen vacancy. Due to the combination of oxygen atom and catalyst surface, it is conducive to the subsequent protonation to form the key intermediate *OCHO, thus enhancing the selectivity of formic acid products.
Characterization and electrochemical test results of BiOx@C catalyst. BiOx@C refers to bismuth oxide catalyst supported on a thin carbon layer containing oxygen atom defects a Energy dispersive spectroscope mappings of BiOx@C catalyst. X-ray photoelectron spectroscopy (XPS) of BiOx@C catalyst b 4f region and c 1 s regions. d Linear sweep voltammetry image of BiOx@C electrode. e Faradaic efficiency of 2D BiOx@C electrode. f Partial current density toward formate of BiOx@C electrode. electrochemical CO2 reduction test under conditions of 50 mV/s scan rate, 0.1 M KHCO3 aqueous solution. SCE refers to saturated calomel electrode. Reproduced from reference (Zhao et al. 2021a, b) with permission from Elsevier
Fan et al. also reported a similar scheme to improve the catalytic performance of bismuth oxide metal with oxygen atom defects and achieved a better electrocatalytic effect. In their work, the Bi2O2CO3 nanosheet electrode maintained a Faradaic efficiency of over 90% for formic acid in the potential range of − 0.8 V to − 1.1 V versus RHE. The maximum current density was − 49.7 mA cm−2 at − 1.2 V versus RHE potential (Fan et al. 2021). Different from the previous example, in the scheme proposed by Zhao et al., the surface of the catalyst with higher negative potential was created by introducing Bi atomic vacancy (Zhao et al. 2020a, b). A large number of electrons made the p orbitals in the Bi catalyst move toward the Fermi level, thus enhancing the activity of p orbitals. This scheme makes the Bi atom as the main group have properties similar to the transition metal, which can be hybridized with d orbitals. With the help of the Bi atom vacancy, the more reactive p orbital can be hybridized with the oxygen atom in the CO2 molecule, thus enhancing the binding of the CO2 molecule to the catalytic surface through the oxygen atom. As is well known, stronger oxygen binding means a greater preference for formic acid formation through the *OCHO intermediate pathway. In subsequent electrochemical tests of this work, the maximum Faradaic efficiency of formic acid was 97%, while the maximum Faradaic efficiency of formic acid remained 90% at a low overpotential of 420 mV (Zhao et al. 2020a, b). Therefore, through the summary of previous work, it can be found that the electronic structure of Bi atoms around the defect can be effectively changed through the defects engineering and the catalytic activity of Bi atoms can be enhanced. Meanwhile, the defects facilitate the adsorption of CO2 molecules to the catalyst surface by O atoms, thus guiding the reaction to highly selective formation of formic acid through the *OCHO pathway.
Based on the above discussion on modification schemes such as surface structural engineering, metal–metal alloy strategies, metal–carbon support composite strategies, doping strategies, and defect engineering, it can be concluded that it is technically feasible to reduce CO2 by electrochemical means to generate formic acid. However, despite the sustainability and environmental friendliness of metal-free carbon-based catalysts, their overall efficiency is still lower than that of metal-based catalysts (Pan et al. 2020). Among the Sn and Pb catalysts, Sn catalysts showed better comprehensive properties in the electrochemical reduction of CO2 to produce ethylene and had less environmental impact than Pb metals. In particular, the synergistic effect of SnOx between metals and non-metals improves the current density, which promotes the mass production of formic acid by electrochemical CO2 reduction. Meanwhile, Bi catalyst had better overall catalytic performance, which was mainly due to the inhibition effect of Bi atom on hydrogen evolution reaction (Zhang et al. 2021a, b, c, d). At present, some schemes based on Bi catalyst have achieved a very good catalytic effect, which is very close to the level of commercial application, but the durability of Bi catalyst and the adsorption stability of CO2 molecules still need to be further studied. Table 3 summarizes the recent outstanding modification schemes of catalysts for the electrochemical CO2 reduction to formic acid.
Electrochemical CO2 reduction to C2 ethylene
Single metal catalyst
Compared with the electrochemical reduction of CO2 to produce C1 chemicals, the production of C2 or C2+ chemicals has more economic value and research potential (Kas et al. 2020). Among the catalyst metals for the electrochemical reduction of CO2 to produce C2 chemicals, Cu shows unique properties for the production of alkene, aldehydes, and alcohols. Through the results of Hori and his colleagues, Cu is a unique electrochemical catalyst that can reduce CO2 to ethylene and C2+ alcohol or aldehydes. Meanwhile, in Hori's experiment, due to the specific current density (− 5 mA cm−2), reaching this current density requires a certain height of overpotential on the metal catalyst. Compared with the overpotential of metals whose main reduction products are formate, such as Cd (− 1.63 V), Pb (− 1.63 V), Sn (− 1.48 V), In (− 1.55 V), and Bi (− 1.56 V), the overpotential of Cu (− 1.44 V) is relatively small (Nitopi et al. 2019). Cu is the only metal that can reduce CO2 into various hydrocarbons under the condition of moderate potential. Although the overpotential changes with pH value, electrolyte composition, and system operation time, the results of Hori et al. reveal the uniqueness of Cu in the electrochemical reduction of CO2.
Bagger et al. divided the metal catalysts into four groups based on the different binding energies of *CO and *H (Bagger et al. 2017). These four groups are (1) Fe, Ni, Pd, Pt, Ga and Ti, whose reduction products are mainly H2; (2) Zn, Ag, and Au, whose reduction products are mainly CO; (3) Cd, In, Sn, Hg, Tl, and Pb, whose reduction products are mainly HCOOH; (4) Cu, whose reduction product beyond *CO (Bagger et al. 2017). As can be seen from Fig. 24b, the binding energies of catalyst metals in H2 group are both negative for *CO and *H, while the binding energies of catalyst metals in CO group and HCOOH group are both positive for *CO and *H. The difference is that the HCOOH group has greater binding energies for *H. Two years later, Bagger and others have carried on the further research of this classification method, where a series of Cu facets (Fig. 24a) was tested (Bagger et al. 2019). The results (Fig. 24d) showed that all Cu facets can realize combining with *CO without appearing *H underpotential deposited (Bagger et al. 2019). However, pure Cu metal catalysts also have many disadvantages, including the decline of reactivity and selectivity with operation time, relatively low current density under similar overpotential. Nitopi et al. proposed that Cu catalysts might have surface properties similar to Pt (Nitopi et al. 2019); the surface crystal structure recombined and changed in crystal structure when a reduction reaction occurs (Climent and Feliu 2011; Gómez-Marín and Feliu 2012). Hence, to optimize Cu catalysts for CO2 reduction to produce ethylene, some excellent works have been proposed (Clark et al. 2017; Feng et al. 2018; Gao et al. 2019; Hoang et al. 2018; Liu et al. 2016; Mistry et al. 2016; Ning et al. 2019; Reller et al. 2017; Wu et al. 2018; Yang et al. 2018; Zhang et al. 2020a, b, c, d, e).
Cu facets and binding energy of reduction intermediates. a Schematic of different Cu facets. b Binding energy of *CO and *H for four types of metal catalysts. Black horizontal line indicates \({\text{CO}}\left( {\text{g}} \right) \leftrightarrow *CO\); while vertical black line indicates \({\text{H}}^{ + } + {\text{e}}^{ - } { } \leftrightarrow {\text{ *H at }}0{\text{ V versus RHE}}\) c Binding energy of *CO and *H at different Cu facets. Color bar indicates the C2+ products Faradaic efficiency for ethylene, ethanol, propionaldehyde, alcohol, and propanol. Reproduced from reference (Bagger et al. 2017, 2019) with permission from American Chemical Society
Among the potential metal elements, Cu is the only metallic element currently capable of catalyzing CO2 to produce C2+ chemicals due to its unique role in the dimerization mechanism. However, the overall efficiency of the system is hindered by the decline of reactivity and selectivity over time. Despite this, the underlying principles of electrochemical ethylene production garnered through these studies will serve as a basis for subsequent discussion in the following sections.
Structure engineering and morphology optimization
Nanostructured copper has been widely used in modification strategies for Cu catalysts (Nitopi et al. 2019). Compared with ordinary foil type electrodes, the nanostructure can improve the catalytic performance of Cu catalyst by optimizing the adsorption performance of reactants, changing the local pH value, creating preferential faceting, and other ways (Francke et al. 2018; Nitopi et al. 2019; Zhang et al. 2021a, b, c, d). Gao et al. reported a Cu nanostructure scheme for halide adsorption to create rough surfaces (Gao et al. 2019). In their scheme, they compared three halogen-modified Cu electrodes, Cl, Br, and I. The morphology of the three samples before and after the electrochemical test can be seen from scanning electron microscope images (Fig. 25a). All the three halogen-modified Cu produced rough surfaces, in which Cu-I and Cu-Br samples showed polyhedral crystal morphology and Cu-Cl samples from nanocubes. In subsequent electrochemical tests, all the halogen-bound Cu samples showed a higher current density than traditional Cu foil at the same potential, in which the total current density of Cu-I was recorded as the maximum (around − 75 mA cm−2) (Fig. 25c)), and the Cu-Br sample showed a partial current density for ethylene similar to that of the Cu-I sample under the condition of − 1.0 V versus RHE (Fig. 25b) (Gao et al. 2019). It can be seen that the rough surface structure significantly improved the catalytic activity of Cu catalysts. A greater Faradaic efficiency (80%) was also recorded for C2+ compounds in the Cu-I sample than in the Cu-Br sample (Fig. 25d) (Gao et al. 2019). In view of this phenomenon, Cu-Br samples formed a large flat rough structure, and Cu-I samples formed a unique nanoscale acicular structure. As Liu et al. proposed a morphological strategy of the concentration of the field-induced reagent, which states that nanostructures with high curvatures, such as sharp spicules, can cause more negative local electric fields, attract more positive charges, and promote CO2 reduction reaction (Liu et al. 2016). Thus, the acicular structure of Cu-I sample results in greater current density and higher catalytic efficiency.
Morphology and electrochemical test result of nanostructure copper catalyst. a Scanning electron microscopy (SEM) result of (A) Cu-Cl sample, (B) Cu-Br sample, and (C) Cu-I sample before and after 1 h of electrochemical reaction. b Partial current density toward ethylene product of different copper halogen precursor. c Total current density of different copper halogen precursor. b Partial Faradaic efficiency toward C2 chemicals product and total Faradaic efficiency of different copper halogen precursor. Electrochemical test carried out under -1.0 V versus RHE condition by using 0.1 M KHCO3 as electrolyte. RHE refers to reversible hydrogen electrode, EC refers to electrochemical test, and FE refers to Faradaic efficiency. Reproduced from reference (Gao et al. 2019) with permission from Wiley
Reller et al. have prepared an in situ deposited nanodendrites cooper (Reller et al. 2017). In their experiments, the modification of halogens also led to the optimization of Cu electrocatalytic activity. After excluding the effect of halogen substances, they formed branchlike nanostructures by electrodeposition on the carbon fiber gas diffusion layer (GDL), and the branchlike structures were observed to be mainly composed of Cu and Cu2O. The electrochemical test recorded a 57% ethylene Faradaic efficiency at high current density (170 mA cm−2) and an acidic environment (pH = 2.5), as well as a decrease in ethylene Faradaic efficiency after 50 min (Reller et al. 2017). Peng and his colleagues have also reported nanoscale Cu electrodes for CO2 reduction in aqueous solutions, but unlike Reller et al. 's electrodeposition (bottom-up) strategy, Feng et al. used the dealloying process (top-down) to construct the porous nanostructured Cu structure (Feng et al. 2018). In Peng's scheme, they formed a nanopore structure by heating the Cu–Zn alloy under inert gas protection and removing the Zn from the alloy. The results showed that samples with a 120 nm aperture achieved a 35% ethylene current efficiency and a current density of about 21 mA cm−2 at − 1.3 V versus RHE (Feng et al. 2018). As a whole, halogen modification of Cu in Gao et al. and Reller et al.’s solution helped to construct a rougher catalytic surface and improve catalytic efficiency, but Reller et al.’s solution was able to achieve a breakthrough in ethylene production at commercial current density. The scheme of dealloying to construct porous nanostructures proposed by Peng et al. provides a different approach for the synthesis of Cu catalysts. However, these modification schemes have the problems of insufficient current density or insufficient selectivity of ethylene, as well as the obvious degradation of ethylene Faradaic efficiency due to the toxicity of catalytic active sites under long-term use.
Similar results have been obtained for other dimensions of Cu catalyst morphology engineering. Recently, Lyu et al. reported that controlled surface oxidation of Cu nanowires can effectively improve the selectivity and stability of C2 chemicals products (Lyu et al. 2021). In their scheme, the surface of the prepared Cu nanowires is oxidized by either O2 or H2O2 solutions. The transmission electron microscope (TEM) image shows that the CuXO layer on the surface of the copper nanowires oxidized by O2 in air is thin and rough (Fig. 26a), while the CuXO layer on the surface of the copper nanowires is oxidized by H2O2 in the liquid phase is thick and relatively smooth (Fig. 26b). In subsequent electrochemical tests, the copper nanowire group oxidized in the liquid phase performed poorly. Faradaic efficiencies of 57.8% (A-CuNWs) and 48% (H-CuNWs) for ethylene were achieved in the two groups of copper nanowire samples at a potential of − 1.0 V versus RHE (Fig. 26b, c), respectively. However, the current density for H-CuNWs toward ethylene is − 12.2 mA cm−2, which is larger than that of A-CuNWs (− 9.5 mA cm−2) (Fig. 26e, f). The air-oxidized copper nanowire sets have a rougher surface, thus having a larger specific surface area and more exposed active sites, and having better catalytic performance. However, the rough surface also leads to surface morphology degradation, which leads to the current density decrease faster in A-CuNWs group under long-term operation, and the stability is not as good as that in H-CuNWs group (Fig. 26d, g).
Morphology and electrochemical test result of Cu nanowires catalyst (CuNWs). a TEM image of A-CuNWs catalyst (oxidized in the air by O2) and b H-CuNWs catalyst (oxidized in the aqueous solution by H2O2). b Faradaic efficiency of A-CuNWs catalyst and c H-CuNWs catalyst. Current density changes of the d A-CuNWs catalyst, and g H-CuNWs catalyst under 20 h operation. e Total current density of A-CuNWs catalyst and H-CuNWs catalyst. f Partial current density toward ethylene of A-CuNWs catalyst and H-CuNWs catalyst. RHE refers to reversible hydrogen electrode, and TEM refers to transmission electron microscope. Reproduced from reference (Lyu et al. 2021) with permission from Wiley
In general, morphology engineering is characterized by the construction of a rougher surface that enhances the adhesion of Cu particles. At the same time, the rougher surface is also conducive to the formation of defects, which will enhance the adsorption capacity of Cu for *CO intermediates (Liu et al. 2020a, b, c, d). For single-atom catalysts, morphological engineering can effectively enhance the binding strength of catalytic active atoms on the surface of the support, reduce the polymerization, and then improve the catalytic performance (Jia et al. 2019).
Construction of alloy structures
Cu modification is an effective strategy to improve the selectivity of multi-carbon compounds by building alloy materials with other metal materials to inhibit hydrogen evolution reaction. According to the results of Hori et al., Ag metals show strong selectivity against CO products (Table 2), which promoting CO production will facilitate the C–C coupling process in the ethylene dipolymerization mechanism, thereby facilitating ethylene production. Based on this idea, Hoang et al. tested a variety of CuAg alloy samples with different mixing ratios and proposed that 6% Ag and Cu formed an alloy film with the best selectivity for producing ethylene by electrochemical reduction of CO2 (Hoang et al. 2018). Electrochemical test results showed that at potential (− 0.7 V versus RHE), the CuAg alloy treated with 3,5-diamino-1,2,4-triazole (DAT) reached an ethylene partial current density of about − 175 mA cm−2, with a Faradaic efficiency for ethylene of 60%. A small amount of Faradaic efficiency was also recorded for CO and more than 25% C2H5OH (Hoang et al. 2018). The X-ray diffraction test showed that the CuAg-wire sample processed by 3,5-diamino-1,2,4-triazole has a small peak at 2θ = 36.95º (Fig. 27a), and X-ray photoelectron microscopy can also show a series of smaller peaks (Fig. 27b), which manifested that the existence of Ag promotes the formation of Cu2O on the Cu surface, thereby improving the selectivity for ethylene. For CuAg alloy catalyst, Clark et al. hypothesized that the compressively strained effect is the key to improving the CO2 reduction for carbon–oxygen products (Clark et al. 2017). For silver-rich CuAg electrode, Cu in Ag phase was enriched to the surface due to the good adsorption of Ag to CO during the electrochemical reduction of CO2. Compared with the pure Cu electrode, the change in the surface distribution area of Cu led to the inhibition of hydrogen evolution reaction (Clark et al. 2017). The selectivity of this product was also improved due to the decrease of oxygen affinity of Cu, which caused the decrease of C-O bond dissociation rate, thus promoting the formation of ethylene. Clark et al. recorded a Faradaic efficiency increase (around 7%) for ethylene products in the 20% Cu sample compared with the pure Cu sample (Clark et al. 2017). Therefore, it can be seen from the results of Hoang and Clark that Cu formed alloys with Ag shown enhanced CO2 reduction capacity under low Ag content (Ag around 6%) (Hoang et al. 2018) and high Ag content (Ag > 20%) (Clark et al. 2017), among which, C2H4 products are more likely to be formed under low Ag content and CO is more likely to be synthesized under high Ag content. A similar alloy strategy was not limited to Ag and interestingly, Feng et al. reported that, by laser ablation in liquid, they prepared a homogeneous CuZn alloy, with an electrochemical test indicating a Faradaic efficiency of 33.3% for ethane at the medium point (− 1.1 V versus RHE) (Feng et al. 2018). The Zn in the alloy also showed the promotion of the dimerization mechanism. In conclusion, the key to ameliorating the selectivity of Cu to ethylene by constructing alloys is to inhibit the hydrogen evolution reaction of Cu and promote the adsorption of CO, thus promoting the reduction of CO2 to produce ethylene through the dimerization mechanism.
a X-ray diffraction patterns and b X-ray photoelectron spectroscopy patterns for CuAg-poly (6% Ag) sample proceed without 3,5-diamino-1,2,4-triazole, Cu-wire (0% Ag) sample proceed with 3,5-diamino-1,2,4-triazole, and CuAg-wire (6% Ag) sample proceed with 3,5-diamino-1,2,4-triazole. Reproduced from reference (Hoang et al. 2018) with permission from American Chemical Society
Construction of carbon support catalysts
In addition to the alloy scheme, the combination of copper and non-metallic carbon-based support such as g-C3N4 (CN) also achieves good catalytic results. Fu et al. proposed a scheme of Cu monatomic catalyst supported on g-C3N4 support and studied the reaction mechanism (Fu et al. 2021). In their work, Cu atoms are embedded in the cavity of g-C3N4, where Cu atoms, along with carbon and nitrogen in g-C3N4, are considered to be catalytic active sites. In subsequent density functional theory studies, it was found that the C–C coupling process, a key step in the reaction pathway for the production of C2 chemicals, showed lower free energy at the Cu and carbon sites. Therefore, it can be considered that in Cu-g-C3N4, Cu single-atom forms a better synergistic effect with carbon in g-C3N4, which effectively reduces the adsorption energy of *CO intermediate. Further studies showed that the combination of g-C3N4 support and Cu metal could provide additional active sites for the electrochemical CO2 reduction process.
Recently, Zhang and his colleagues reported a g-C3N4 (CN) loaded Cu2O. In their scheme, g-C3N4 was prepared by conventional urea calcination and mixed with Cu2O nanocrystals to produce a Cu2O/CN catalyst (Fu et al. 2021). In terms of relative surface area, the Brunauer–Emmett–Teller surface area of Cu2O catalyst with the addition of CN is 20.6 m2 g−1, which is 2.75 times that of Cu2O nanocrystal without CN (7.5 m2 g−1). Electrochemical tests showed that the Cu2O/CN catalyst had the highest ethylene Faradaic efficiency (32.2%) and the current density is − 4.3 mA cm−2 recorded at − 1.1 V versus RHE. The analysis suggested that at the beginning of the reaction, CO2 is first adsorbed to CN and produces *CO intermediate. Then, the synergistic effect between CN and Cu2O makes *CO migrate to Cu2O site and further C–C coupling reaction takes place, and finally forms ethylene. A similar scheme for improving catalytic performance with synergistic effect is proposed by Zhuang et al., in which Cu was compounded on a thin nitrogen-doped carbon layer (Cu@NxC). Electrochemical test results show that the overall Faradaic efficiency of C2 product is close to 80% at − 1.1 V versus RHE potential, but the Faradaic efficiency for ethylene is still limited (Zhang et al. 2021a, b, c, d). The use of a nitrogen-mixed carbon-based carrier can effectively form a synergistic effect with Cu, adsorb CO2 molecules on the carrier, and promote the migration of *CO intermediates to the metal site. However, the performance level of the carbon-based carrier scheme is generally still lower than that of other modification schemes.
The multi-component three-dimensional co-catalyst scheme has the potential to produce C2 chemicals by the electrochemical CO2 reduction process. For example, the 3D hierarchical metal/polymer-carbon paper (M/Polymer-CP) scheme has been known to achieve remarkable results (Jia et al. 2021a, b). In one study, Han and his colleagues report a method for designing metal/polymer hybrids. In their scheme, three synthetic methods including in situ electro-polymerization, conventional electrodeposition and thermal synthesis were compared (Fig. 28a) (Jia et al. 2021a, b). The results showed that a network of polyaniline (PANI) layer was formed on the surface of the catalyst prepared by the electro-polymerization method, while the catalyst prepared by electrodeposition and drop coating showed a relatively disordered surface structure (Fig. 28b–d). A subsequent electrochemical test showed that the Cu/PANI-CP catalyst synthesized by electro-polymerization had the highest ethylene Faradaic efficiency (59.4%) and current density (30.2 mA cm−2) at a potential of − 1.2 V versus RHE (Fig. 28e, f) (Jia et al. 2021a, b). Analysis suggests that the high catalytic performance stems from the electro-polymerization approach, which makes the metal layer, polymer layer and carbon layer have better contact. Apart from that, electro-polymerization is conducive to the formation of a thinner nanometer copper layer. The polymer layer is also beneficial to the dispersion of copper nanoparticles, reducing the agglomeration, thus enhancing the catalytic performance.
Electrochemical test results of M/polymer-CP catalyst. M refers to different metal loading including Cu, Pd, Zn, Sn. a Three kinds of M/polymer-CP synthesis routes as well as M-CP control group preparation process. b Scanning electron microscope image of M/polymer-CP I synthesized by electro-polymerization; c Scanning electron microscope image of M/polymer-CP II synthesized by drop coating; d Scanning electron microscope image of M/polymer-CP III synthesized by conventional thermal synthesis method. e Charging current density against scan rate over different electrodes. f Faradaic efficiency and total current density of different electrodes. Reproduced from reference (Jia et al. 2021a, b) with permission from Wiley
Similarly, the copper catalyst of three-component copolymer was also reported by Wang et al. The phenylpyridinium layer in their scheme is the key to improving the stability of the catalytic material (Wang et al. 2021). In addition, the polyamine incorporated Cu electrodes scheme even achieved better results for ethylene production. Electrochemical tests showed that the Faradaic efficiency of ethylene was 72% at a potential of − 0.97 V versus RHE, with a partial current density of 312 mA cm−2 for ethylene (Wang et al. 2021). This current density is even higher than the 200 mA cm−2 commercial target recommended by previous work in technical and economic analysis (Bushuyev et al. 2018; Jouny et al. 2018). What is more, depending on the properties of the polymer layer, the resulting multilayer catalyst can also show specificity in other ways. Daasbjerg and his colleagues added the hydrophobic polymers polyvinylidene difluoride (PVDF) and polyethylene (PE) to the Cu catalyst (Liang et al. 2021). These hydrophobic polymers make the surface of Cu catalyst hydrophobic, the proton source provided by water is greatly weakened, and the pH of the catalytic surface is increased, thus inhibiting hydrogen evolution reaction and increasing ethylene production.
In summary, the introduction of additional polymer into the Cu catalyst can effectively improve the electrocatalytic performance. The specific effect is reflected in the formation of thin nano-copper layers on polymer layers that aid in the well-dispersion of Cu particles. In contrast to modification schemes that optimize the characteristics of the catalyst itself, the addition of functional polymers directly acts on the environment around the electrode, inhibiting hydrogen evolution reaction by increasing the pH of the aqueous solution and thus improving the selectivity of ethylene.
Defect engineering
It is well known that the activity of catalysts depends on the charge distribution on the surface of the catalyst material, while the adsorption behavior relies on the surface structure of the reactive zone (Kas et al. 2020; Yuan et al. 2020a, b). Defects are widely found in materials and can be classified into point defects, linear defects and plane defects according to their different forms (Zhou et al. 2018). Adjusting the structure of the catalyst through proper preparation methods and defect engineering will be beneficial to improving the performance of the catalyst (Daiyan et al. 2020; Kas et al. 2020). Zhang and his colleagues proposed a strategy to improve the catalytic performance of Cu by constructing vacancy defect structures at the nanoscale (Fig. 29f) (Zhang et al. 2020a, b, c, d, e). Using K2SO4 electrolyte, they prepared nanodefective Cu nanosheets by electrochemical method on the pre-formed Cu nanosheet. The Cu nanosheet that was prepared was characterized by the high-angle darkened field microscopy (HAADF-STEM). Pits with a diameter of 2–14 nm were observed on the surface of the Cu nanosheets (Fig. 29a–e), and the number of atomic defects increased from the pit edge to the center (Zhang et al. 2020a, b, c, d, e). Such a defective structure brings the improvement of catalytic efficiency. Electrochemical experiments showed that the maximum ethylene Faradaic efficiency (83.2%) was achieved at − 1.18 V versus RHE at moderate potential (Fig. 29g), and the ethylene Faradaic efficiency was higher than 60% from low potential (− 0.88 V versus RHE) to high potential (− 1.48 V versus RHE) (Zhang et al. 2020a, b, c, d, e). In the subsequent density functional theory study, the Cu(110) interface with defective structure effectively reduces the absorption energy of *CO dimerization to generate *OCCO intermediates from 0.7 eV to 0.59 eV (Fig. 29h) compared with the non-defective structure. Therefore, the advantage of defect structure lies in reducing the energy barrier required by the dimerization mechanism so as to promote the electrochemical dimerization mechanism of CO2 and thus improve the selectivity of ethylene.
High-angle ring dark field image-scanning transmission electron microscope (HAADF-STEM) images and density functional theory calculation result of Cu nanosheet. a Morphology of Cu nanosheets with a lateral size of 1 μm. b Enlargement of (a), c Enlargement of (b) and size distribution of the nanodefects on Cu nanosheet. d Cu nanosheet images under fast Fourier transform patterns. e Enlargement of square area in (d). f Profile of intensity along the line shown in (e). g Absorption energy for key intermediate of *CO dimerization, Cu (111) with defect has lower absorption energy. h Energy diagrams of *CO dimerization. Reproduced from reference (Zhang et al. 2020a, b, c, d, e) with permission from American Chemical Society
Besides the strategy of vacancy, doping is also widely used in the defective modification of catalysts (Zhi et al. 2021). Doping usually refers to the addition of a small amount of a foreign component or atom into the whole to improve the catalytic performance (Zhang et al. 2021a, b, c, d). Wu et al. proposed the inclusion of cuprous ions in the ceria lattice to improve the selectivity of the material toward ethylene (Wu et al. 2018). Si is usually used as a substrate because of its strong stability (Si et al. 2012). Meanwhile, they also observed that ceria substrate has a stabilizing effect on cuprous ions in the experiment, which can extend the electrochemical activity of Cu+ under long-term operation. Electrochemical tests showed that ceria mixed with cuprite was recorded a selectivity of 47.6% for ethylene at a potential of − 1.1 V versus RHE, and the current density remained at − 3.2 mA cm−2 after 6 h of operation, which is higher than the oxidation-derived Cu nanoparticles in the same test condition (Wu et al. 2018). Unlike the defective Cu nanosheet, the CumCeOx catalyst proposed by Wu et al. did not support the dimerization mechanism of *CO due to the long distance between the two active sites (around 3.65 Å). In the density functional theory calculation, the energy barrier for *CH2-*CH2 coupling recorded for the CumCeOx catalyst was 0.58 eV, lower than that for *CH2 protonation (1.04 eV). Therefore, for the CumCeOx catalyst, it is more inclined to produce ethylene through insertion mechanism (Fig. 29h). In the process of ethylene generation through the insertion mechanism, it is easy to cause the generation of *CH2 by protonation into CH4. Although Wu et al. proposed the tendency of CumCeOx catalyst toward ethylene, it was still recorded that part of methane was formed, which limited the performance of this catalyst. This section has reviewed two mainstream defect engineering modification methods, including vacancy construction and doping modification. Due to the particularity of the dimerization mechanism of Cu, the modification of crystal structure and electronic structure through defects and doping heteroatoms should focus on exposing more catalytic crystal faces of Cu(110), enhancing the adsorption of *CO intermediates, and promoting the formation of Cu+ with strong reducing properties on the catalyst surface.
Metal surface oxidation state modification
Metal oxide and metal oxide-derived metal catalysts due to the oxidation state of the metal center play a role in the ligand field and d orbital splitting (Bredar et al. 2020). In other words, metals with the same d electron configuration and metals with a high oxidation state are more likely to have low spin (Fig. 30a), thus promoting catalytic activity (Wu et al. 2021a, b, c). Besides, a stronger oxidation state of metal oxide can improve the electronegativity of catalyst material, enhance the attraction to electrons, and then have stronger adsorption capacity and catalytic capacity (Fig. 30b). What is more, the ability of an oxidized metal to adjust its d-vacant orbital and d-electron distribution (Fig. 30c) and the ability to change energy bands causes the change in relative positions of the conduction band (CB) and valence band (VB), thus creating greater energy differences with the reactants and promoting electron transfer (Fig. 30d) (Wu et al. 2021a, b, c). Because of these advantages, metal oxide catalysts have attracted researchers’ attention. Oxide-derived Cu (OD-Cu) can rapidly remove oxygen from Cu oxide in the electrochemical reduction of CO2, thereby promoting CO absorption and generation of *CO, and further promoting the dimerization mechanism (Ren et al. 2020; Zhang et al. 2020a, b, c, d, e).
Based on this theory, Mistry et al. proposed excellent work in their experiments, in which used O2 and H2 plasmas to treat polycrystalline Cu foil (Mistry et al. 2016). Scanning electron microscope and energy dispersive spectroscope results showed that the surface of Cu foils treated by O2 plasmas formed a highly rough surface, and it was recorded that the number of holes increased by about 64% and the aperture decreased by 50% compared with untreated Cu foils (Fig. 31A). Energy-dispersive spectroscope images show an obvious copper oxide layer and a cuprous oxide layer formed on the surface of Cu foil (Fig. 31B). In electrochemical tests, the selectivity for ethylene was recorded as high as 60% at low potential. Compared with untreated Cu foil, the potential for producing ethylene was increased from − 0.85 to − 0.5 V versus RHE, and the selectivity for ethylene was increased by three times (Mistry et al. 2016). After excluding the effect of surface roughness, Mistry et al. found that Cu+ inhibited methane production, which was similar to Wu et al.’s findings. Because of the suppression of methane production, the production of ethylene becomes more efficient. They suggested that the treatment with the O2 plasmas resulted in high roughness surfaces and low coordination sites, which helped stabilize the oxide during the reaction and thus influence selectivity. Some other works on the modification of Cu based on oxides were also proposed. Yang et al. reported a scheme of mixing CO2 capture material N-doped carbon (NxC) on CuO substance and recorded a selectivity of 36% for ethylene (Yang et al. 2018). Ning et al. proposed a work of loading Cu2O nanotubes in nitrogen-doped reduced graphene oxide (NRGO) (Ning et al. 2019). They achieved 19.7% selectivity toward ethylene and high overpotential (− 1.4 V versus RHE). Therefore, cuprous ions formed on the catalytic surface can significantly improve the selectivity of CO2 reduction to ethylene. Because cuprous ions can effectively inhibit the protonation of *CH2 and methane formation, the selectivity of ethylene is significantly improved.
Morphology of Cu foils catalyst. A Scanning electron microscope images of Cu foils treated with O2 plasma. a, b and c Scanning electron microscope images of Cu foil treated with 2 W for 2 min before and after reaction. d, e, f and g Scanning electron microscope images of Cu foil treated with 100 W for 2 min before and after reaction. h, i and j Scanning electron microscope images of Cu foil treated with 100 W for 10 min before and after reaction. g the sample is first treated with O2 plasma under 100 W for 2 min then followed by H2 plasma treatment at the same power and time. B Energy dispersive spectroscope elemental images of Cu foils treated with O2 plasma. a, b, c, and d treated with O2 under 20 W for 2 min. e, f, g, and h treated with O2 with 100 W for 2 min. g and h have further been treated with H2. Reproduced from reference (Mistry et al. 2016) with permission from Springer
Through reviewing the previous research works, the electrochemical CO2 conversion to formic acid solution is technically feasible. To produce formic acid, the construction of acicular structures through nanotechnology and surface engineering will facilitate the formation of *OCHO intermediates and thus formic acid formation (Han et al. 2019). For ethylene production, Cu is a metal with potential as it can achieve high selectivity toward the dimer intermediate in promoting the catalyst to adsorb *CO intermediates, and producing ethylene through dimerization mechanism, while inhibiting the hydrogen evolution reaction (Ogura 2013). As a whole, these technologies and schemes have made possible commercial applications of electrochemical reduction of CO2 to produce C1 and C2 chemicals.
Table 4 summarizes the recent outstanding modification schemes of catalysts for electrochemical CO2 reduction to ethylene.
Life cycle assessment for electrochemical CO2 reduction
Life cycle assessment is a methodology to quantify the impact of chemicals or chemical processes on the environment and has gradually attracted people's attention to environmental sustainability. However, there are currently several life cycle assessment works on the production of formic acid and ethylene by electrochemical reduction of CO2, especially since the work on ethylene is scarce. To successfully apply technology from the laboratory scale to the relevant industrial scale, assessing the possible environmental and economic impacts of this technology requires a careful assessment based on a systematic perspective (Dominguez-Ramos et al. 2015). Life cycle assessment is a way to quantify the environmental impact of a product over its entire life cycle (Artz et al. 2018). For process design, early decisions can have a profound impact on the future and application of the technology. Life cycle assessment can provide theoretical guidance and prospect description for technologies that are still in the early stage of development (small-scale production) but applied on an industrial scale (large scale) (Arvidsson et al. 2017; Lin et al. 2021), especially for carbon capture and utilization (CCU) based on environmental and economic considerations.
To harmonize the life product lifecycle assessment methodology, the International Standards Organization (ISO) has specified the ISO 14040 and 14044 (Standardization, 2006) standard specifications. According to the ISO standard, the steps of life cycle assessment can be divided into the following stages: goal and scope definition, inventory analysis, impact assessment, and interpretation. The goal and scope definition usually includes clarifying life cycle assessment methodology, functional unit, life cycle assessment system boundary, and classifying different aspects of environmental impact. It should be noted that mainstream research on system boundaries includes cradle-to-gate (Ahn et al. 2019; Khoo et al. 2020; Rumayor et al. 2019a, b; Sternberg et al. 2017; Thonemann 2020; Thonemann and Schulte 2019) and cradle-to-grave (Fernández-Dacosta et al. 2019; Koj et al. 2018). The difference between them lies in that cradle-to-gate life cycle assessment usually only refers to the production process from raw materials to downstream processing of the product, while cradle-to-grave covers the complete life cycle of the product from raw materials to the final disposal. For life cycle assessment analysis of CO2 electrochemical reduction to produce C1 and C2 chemicals, due to the diversity and complexity of downstream utilization of reduction products, cradle-to-grave is usually less studied. To better quantify the impact of electrochemical reduction of CO2 compared with the conventional process, most researchers choose to use the cradle-to-gate system boundary. For inventory analysis, it usually encompasses the quantitative data of input and output for life cycle assessment data analysis, including material and energy resources. Impact assessment relates quantified data to specific environmental impact events. Analysis results are discussed and reviewed in interpretation.
By and large, formic acid is a valuable C1 product. In recent years, some life cycle assessment works for the production of formic acid based on the reduction of CO2 have been proposed (Ahn et al. 2019; Dominguez-Ramos et al. 2015; Rumayor et al. 2019a, b; Sternberg et al. 2017; Thonemann and Schulte 2019). Ahn and his colleagues compared the life cycle assessment of CO2 carbon capture and utilization process with the conventional fossil-based process on formic acid production to determine the production scheme of formic acid with minimal environmental impact (Ahn et al. 2019). In their work, CO2 emissions from fossil power plants and hydrogen generated by electrolysis were used as raw materials. Then CO2 and H2 produced by the electrochemical CO2 reduction process are transformed into formic acid under the action of the catalyst. For the conventional process, they used carbon monoxide (CO) from fossil fuel combustion as input to the system and converted CO to formic acid with the aid of a basic sodium methoxide catalyst (Fig. 32a). In the following hypothesis, the power source and heat source of the system are specified as variables of their study. The environmental impacts of conventional and carbon capture and utilization processes were compared under fossil-based electricity supply cases (burning of hard coal, natural gas) and renewable electricity supply cases (wind power, hydropower). Results show that in the cases where all power is powered by burning fossil fuels, the carbon capture and utilization process effectively reduced climate change by 53.6%, and fossil resource depletion by 28.3%. For carbon capture and utilization processes, the production of H2 through electrochemical CO2 reduction contributed 76% to climate change, while the impact of conventional processes on climate change was mainly due to the combustion of fossil fuels to produce CO (71%) (Fig. 32b). When conventional electricity sources were replaced with renewable hydropower, the production of formic acid through carbon capture and utilization processes yielded significant climate change benefits (130% reduction in impact) (Fig. 32c) (Ahn et al. 2019). In all, it can be found that electrochemical-based carbon capture and utilization processes can achieve greater environmental benefits when using renewable energy than conventional processes. At the same time, their work reflects that the environmental benefits of the electrochemical CO2 reduction process depend heavily on the sustainability of the power source.
Life cycle assessment system boundaries and assessment result of carbon capture and utilization process compared with the conventional process. a Life cycle assessment system boundaries of electrochemical-based carbon capture and utilization process and conventional formic acid production process. FA refer to formic acid, CW refer to cold water. b Contribution analysis results of climate change impact and fossil depletion impact of carbon capture and utilization process compared with the conventional process. c The proportion of impact reduction of carbon capture and utilization process compared with the conventional process when the electricity supply is substituted from burning woodchip (case 1) to hydropower (case 4). Reproduced from reference (Ahn et al. 2019) with permission from Royal Society of Chemistry
In contrast to previous work, Thonemann et al. specifically defined the carbon capture and utilization process as CO2 reduction and conversion to formic acid by electrochemical methods (Thonemann and Schulte 2019). Their life cycle assessment work was first carried out on a lab-scale (6.2 × 10−3 wt% formic acid in output) and then scaled up on an industrial scale (20 wt% formic acid in output) based on 1 kg formic acid product. This work is prospective for industrial applications of electrochemical reduction of CO2. More recently, Kang et al. also conducted modeling and life cycle analysis of the process of producing formic acid through the electrochemical CO2 reduction process (Kang et al. 2021). In their model, material cycling and pretreatment of the electrochemical CO2 reduction process as well as downstream purification processes are considered (Fig. 33a). In their assessment of 18 environmental indicators, the electrochemical CO2 reduction process produced formic acid reduced the climate change by 99.7%, ozone depletion by 51.0% and terrestrial acidification by 32.6% compared with the conventional formic acid production process (Fig. 33b). In terms of contribution analysis, CO2 raw material consumption has a positive impact on climate change, while power consumption has the largest contribution to environmental impact (accounting for 83% of the total) (Fig. 33c) (Kang et al. 2021). At the same time, their work shows that the electrochemical CO2 reduction process can achieve a net environmental benefit when renewable electricity (hydropower) completely replaces the current electricity supply structure. The advantage of electrochemical CO2 reduction processes, therefore, is that they can use otherwise waste CO2 in the atmosphere and reduce the greenhouse effect of CO2, thus contributing to climate change. At the same time, the power supply process plays a decisive role in the final net environmental impact of the electrochemical CO2 reduction process. In conclusion, due to the insufficient catalytic performance of the electrode, insufficient energy conversion efficiency and low CO2 utilization rate, the electrochemical CO2 reduction process is unable to achieve environmental benefits beyond conventional processes under the current energy structure, but in the future, the production of formic acid through electrochemical CO2 reduction process can meet the goal of sustainable development.
Life cycle assessment system boundaries and assessment results of electrochemical CO2 reduction to formic acid. a Production process of electrochemical CO2 reduction to formic acid. b Comparison of environmental impact between the conventional formic acid production process and electrochemical CO2 reduction to formic acid process. c Environmental impact assessment result of 18 mid-point indicators for electrochemical CO2 reduction to formic acid process. Reproduced from reference (Kang et al. 2021) with permission from Elsevier
The environmental impact of ethylene production through electrochemical reduction of CO2 can also be measured through life cycle assessment. Khoo et al. have proposed an electrochemical-based approach to ethylene production life cycle assessment work (Khoo et al. 2020). They regarded 1 g ethylene as a functional unit of life cycle assessment for small-scale process and 1 ton ethylene as a functional unit for large-scale processes. At the same time, natural gas combined cycle (NGCC), renewable hydrogen and bioenergy are considered as energy sources. (Fig. 34a, b) They suggested that in laboratory-scale life cycle assessment, the source of CO2 was usually gas canisters. However, in terms of industrial considerations, industrial processes such as CO2 capture and purification are needed. The environmental impacts of these processes must also be considered in the life cycle of ethylene products. From their results, the use of renewable hydrogen and bioenergy as energy sources in small-scale devices could significantly reduce the impact of global warming (3.70 g of CO2 -eq and 0.98 g of CO2 -eq, respectively) (Fig. 34c) and achieve a net reduction in CO2 (Khoo et al. 2020). Natural gas does not have the potential to reduce CO2 emissions because it produces greenhouse gases when it is burned. A similar reduction in environmental impact was recorded in large-scale devices where a net reduction in CO2 was achieved using renewable hydrogen (3.0 t CO2 -eq) and bioenergy (0.651 t CO2 -eq) (Fig. 34d) (Khoo et al. 2020). Overall, in the life cycle of ethylene production through the electrochemical CO2 reduction process, the power source also has a significant effect on reducing the global warming impact. Only the use of renewable energy can effectively improve the environmental benefits of the electrochemical CO2 reduction process. At the same time, larger scale electrochemical CO2 reduction process plants can lead to more CO2 emission reduction, further reducing the impact of global warming. This demonstrates the potential of building large-scale electrochemical CO2 reduction plants in the future to reduce the impact of global warming.
Life cycle assessment boundary and result of producing ethylene through the electrochemical CO2 reduction process. a Small-scale (lab-scale) electrochemical CO2 reduction process. b Large-scale electrochemical CO2 reduction process. c Life cycle assessment result of global warming potential indicator for small-scale electrochemical CO2 reduction process. d Life cycle assessment result of global warming potential indicator for large-scale electrochemical CO2 reduction process. Reproduced from (Khoo et al. 2020) with permission from Elsevier
According to the IEA's report on the global electricity supply market in 2020, 35% of the world's electricity is currently generated by coal and 24% by gas. Together, the two non-renewable energy sources account for nearly 60% of the world's electricity supply (IEA 2020). Based on the previous analysis, neither producing formic acid nor ethylene through the electrochemical CO2 reduction process can eliminate climate change and global warming using the current electricity supply market mix. However, compared to conventional formic acid and ethylene production processes, the environmental impact is less destructive. In the future, the use of renewable energy can effectively increase the environmental benefits of the electrochemical CO2 reduction process, meaning that the use of electrochemical CO2 reduction processes to produce formic acid and ethylene can improve the current environment. The previous work also shows some deficiencies. First of all, degradation of electrode performance during long-term use has not been fully considered in the modeling process, which will lead to an overly optimistic assessment of environmental impacts caused by the electrode preparation process, such as lower ecosystem toxicity and less water pollution. Second, most of the previous work used single-pass processes without considering CO2 and energy recycling, which will lead to more CO2 consumption and energy consumption. Finally, although the current life cycle assessment work on electrochemical CO2 reduction processes has attracted researchers' attention, more relevant studies are still needed to fully understand the impact of electrochemical CO2 reduction processes on the environment. Future research on the electrochemical CO2 reduction process should pay more attention to the influence of electrode life and also consider the recovery of CO2, raw materials for electrode preparation, electric energy, and heat energy. What is more, the environmental benefits of different products produced through the electrochemical CO2 reduction process should be compared, such as carbon monoxide products, formic acid products, ethylene products, and multi-carbon alcohols species, among others.
Economic assessment for electrochemical CO2 reduction
In the field of producing C1 and C2 chemicals by electrochemical reduction of CO2, the typical C1 and C2 products of formic acid and ethylene was promising (Chatterjee et al. 2021; Chen et al. 2018; Han et al. 2019; Liu et al. 2020a, b, c, d; Yan et al. 2021; Zhao and Quan 2021). From the perspective of economic value, the global formic acid market is growing at a compound annual growth rate of about 5% by 2019 (Formic acid market forecast, global market insights 2019 to 2027, 2019; Zhang et al. 2020a, b, c, d, e). By 2020, the formic acid global market reached 560 million dollars (Global formic acid market report 2021). The growing pharmaceutical industry and food processing industry are important factors driving the development of the global formic acid market, making it a valuable C1 chemical. Electrochemical reduction of CO2 to produce formic acid will be a low-carbon and environmentally friendly way with economic potential in the future. On the other hand, the ethylene market is even more competitive, with the global ethylene market reaching 146.3 billion dollars in 2019 and growing at a compound annual growth rate of 9.8% (Ethylene market share, size 2020–2026, 2020). The technology for producing ethylene is mature with ethylene playing an increasingly important role in terminal applications such as the construction industry and automobile manufacturing. Therefore, the electrochemical production of C2 chemicals such as ethylene would be an environmentally friendly alternative to the current production of ethylene by petrochemical. Therefore, the production of formic acid and ethylene by electrochemical reduction of CO2 is a research subject with economic potential.
In terms of technology, many excellent catalyst modification schemes of predecessors have realized the technical feasibility of producing C1 and C2 products through electrochemical reduction of CO2 in the lab scale (Bai et al. 2017; Fan et al. 2017; Reller et al. 2017; Ren et al. 2020; Yang et al. 2018; Zhang et al. 2020a, b, c, d, e; Zhang et al. 2014a, b). Meanwhile, some related life cycle assessment studies have quantified the environmental impact of electrochemical CO2 reduction technology and analyzed its environmental feasibility (Ahn et al. 2019; Dominguez-Ramos et al. 2015; Rumayor et al. 2019a, b; Sternberg et al. 2017; Thonemann and Schulte 2019). However, an economic feasibility assessment will be important in determining whether the technology will be accepted and selected by manufacturers in future markets. The economic feasibility assessment will help to identify weaknesses at an early stage of current electrochemical technology development, thereby increasing the potential for electrochemical reduction CO2 to produce valuable C1 and C2 technologies for eventual commercial use.
For evaluating the profitability and economic feasibility of technology, common models include net present value, internal rate of return, rate of return, and cost–benefit ratio. The net present value model can fully discount the future cash flow of investment and the value at the beginning of the investment day, which is an important indicator to evaluate whether the technology can be profitable in the future (Fernando 2020a, b). If the net present value is greater than 0, it means that the process can make a profit; otherwise, it will lose money. Similar to net present value, the internal rate of return model is also a model to evaluate the potential profitability of investment by calculating the discount rate when net present value is 0 (Fernando 2020a, b). The rate of return represents the percentage relationship between net income and investments (Kenton 2020). For the current electrochemical CO2 reduction technology, if the rate of the return value is greater than 100% and the net present value is greater than 0, it means that the technology is economically feasible.
For economic feasibility analysis, some excellent work has provided a framework for the assessment of electrochemical reduction of CO2 (Agarwal et al. 2011; Jouny et al. 2018; Rumayor et al. 2019a, b). In Rumayor and his colleagues’ work, they hypothesized an electrochemical reduction CO2 plant in western Europe and performed a top-down approach to assess the costs involved in the process, including capital expenditure (CAPEX) and operating expenditure (OPEX) (Rumayor et al. 2019a, b). In their work, 21% wt formic acid concentration scenario (100% Faradaic efficiency), 85% wt commercial formic acid concentration scenario (100% Faradaic efficiency), traditional formic acid factory scene, and scenario of changing electrochemical cell cathode every 4.45 years were, respectively, conducted. The results showed that the payback period of the scenario in which the cathode was replaced every 4.45 years in the closest realistic scenario was 12.7 years, which was longer than the 5.9 years of the conventional scenario (Fig. 35a) (Rumayor et al. 2019a, b).
Economic assessment result of electrochemical CO2 reduction process. a Net present value and corresponding time (year) of electrochemical reduction of CO2 under different scenarios. Emin-85 represents the production scenario of 85% wt concentration of formic acid at 100% Faradaic efficiency; Emin-20 represents the production scenario of 20% wt concentration of formic acid at 100% Faradaic efficiency; conventional represents a conventional formic acid production scenario; B4.45 represents the scenario of replacing the cathode material every 4.45 years. b Net present value of six studied electrochemical CO2 reduction product. c Cost of ethylene production breakdown for neutral MEA and alkaline flow cell based on lab-level data and optimal case. d, e Cost of ethylene production with crossover ratio and carbonate formation ratio and electrical energy efficiency for neutral MEA and alkaline flow cell. MEA refers to membrane electrolyte assembly. The black line is the reference price of ethylene (1000 $/ton). Reproduced from reference (Jouny et al. 2018; Rumayor et al. 2019a, b; Sisler et al. 2021) with permission from Elsevier and American Chemical Society
Similarly, Agarwal and his colleagues have proposed an economic analysis based on a hypothetical factory (Agarwal et al. 2011). In their analysis, three different CO2 value chains were considered, including direct emissions to the environment, conversion into valuable products, and capture, transfer, and storage. In contrast to Rumayor et al.'s economic analysis assumptions, Agarwal et al.'s assumptions include the geographic distance between capture and storage and transportation costs of CO2. Significantly, Agarwal et al.'s assumptions also cover the recovery and utilization of alkaline wastewater from the electrolytic cell anode recovery and chemicals from downstream treatment wastewater. The assumption of replacing extra NaOH purchases with alkaline wastewater brings their economic analysis closer to an optimized process. Their results show that chemical consumption is a major consideration in the electrochemical reduction of CO2. Similarly, the process payback period for electrochemical reduction of CO2 is still greater than 10 years (Agarwal et al. 2011). A simpler hypothetical framework is proposed in the scheme of Jouny et al. (Jouny et al. 2018). In the work of Jouny et al., they performed an economic assessment based on current electrolytic performance and compared it with optimal electrochemical CO2 reduction performance and optimistic feedstock prices case. The final results showed that the net present value of formic acid and carbon monoxide was greater than 0 and the profit was realized in a variety of electrochemical reduction CO2 products (Jouny et al. 2018).
The key to making electrochemical CO2 reduction profitable is the price of power and the performance of the electrodes. At the current level of technology, the lower current density and higher potential make electrochemical CO2 reduction technology more power-intensive and unable to compete with current mainstream processes in terms of energy consumption per unit of formic acid or ethylene produced. Some technical analyses have given specific requirements for the performance of formic acid and ethylene catalysts respectively. Jouny et al.'s work has analyzed a variety of CO2 reduction products, including propanol, formic acid, CO, ethanol, and ethylene as well methanol (Jouny et al. 2018). According to the results, only formic acid ($39.4 million) and CO ($13.5 million) achieved end-of-life net present value greater than 0 based on 100 tons of products per day (Fig. 35b). The rest of the product is not profitable. At the same time, propanol with four carbon atoms showed the most negative net present value, and methanol was the only product with a negative net present value under the optimal hypothesis. Based on the above analysis, the author believed that the current density of the electrode must be raised to more than 300 mA cm−2, the power conversion efficiency must be higher than 50%, and the Faradaic efficiency must be greater than 50% to achieve profitable electrochemical CO2 reduction production of formic acid (Jouny et al. 2018). More recently, Sisler et al. also conducted a technical–economic analysis of ethylene production by the electrochemical CO2 reduction process. In their work, two electrolysis devices were evaluated, namely neutral membrane electrode assemblies (MEA) and alkaline flow cells (Sisler et al. 2021). Despite the lower cost of MEA compared to the alkaline flow cell, the economic analysis results show that the power cost of the two electrolytic components under the experimental data was still higher than the market reference price of ethylene (1000 $/ton) (Fig. 35c). This indicated that it is necessary to improve the catalytic performance by optimizing the electrode materials. Further analysis shows that for the single-pass electrochemical CO2 reduction process, the CO2 loss per pass also has a significant impact on the final production cost. From the perspective of loss, to achieve profitability at 50% Faradaic efficiency, the mass ratio of CO2 lost to CO2 produced by electrochemical CO2 reduction must not exceed 0.1. Increasing the utilization rate of CO2 through the electrolytic assembly can effectively reduce the cost of ethylene production in an alkaline flow cell, while the effect on MEA electrode assembly is relatively poor (Fig. 35d, e). This demonstrated the importance to recycle CO2 in the overall electrochemical CO2 reduction process, as well as the need to build a circular economy in future development.
Production of formic acid by electrochemical CO2 reduction is approaching commercial application, but production of ethylene is still some way off. In 2019, formic acid in the US market was sold at 0.5 $/kg, and the cost of production through the electrochemical CO2 reduction process was 0.96 ± 0.78 $/kg. Ethylene's selling price is 0.58 $/kg, but the electrochemical CO2 reduction process costs 2.48 ± 1.83 $/kg (Jordaan and Wang 2021; Orella et al. 2019). Relevant metrics indicate that increasing the electrochemical CO2 reduction energy efficiency above 60% and current density above 100 mA cm−2 are key parameters to improve the competitiveness of the production of formic acid by the electrochemical CO2 reduction process (Rumayor et al. 2021). For ethylene production via electrochemical CO2 reduction, the current density is required to be as high as 200 mA cm−2 (Jordaan and Wang 2021).
In the current technological context, the electrochemical reduction of CO2 to produce valuable products is less commercially efficient than traditional production processes, embodied in longer return cycles and lower returns. The durability of electrode materials and the consumption of electricity and chemicals during the electrochemical reduction of CO2 are the main influencing factors, which limit technological readiness. At present, electrochemical CO2 reduction technology shows excellent potential in environmental sustainability, but it still faces problems such as high cost and slow investment return in the economic aspect. The high cost of power use and power consumption can only be reduced by improving the performance of the catalyst. Future electrochemical CO2 reduction technologies should be developed from optimizing material properties, improving the energy efficiency of the overall process, reducing environmental toxicity and building a sustainable economic model, which necessitates cooperation from all parties and disciplines such as academics and industry players.
Conclusion
Today, the world is paying more and more attention to the environmental impact of our industrial activities. As such, the efficient utilization and conversion of CO2 into valuable chemicals is a research topic of epochal significance. This work reviewed and assessed the current electrochemical reduction of CO2 to produce formic acid and ethylene from the aspects of technology, economy, and environment. The fundamental principles and reaction pathways of electrochemical reduction of CO2 were discussed at the technical level. Sn, Pb, In, Bi, Cu, and other single metal catalysts for the electrochemical production of formic acid and ethylene and their modification schemes were systematically reviewed. These include structural engineering, construction of metal alloys, design of metal and nonmetal composites, defect engineering, the development of single-atom catalyst, and metal-functional polymers. For the production of formic acid, state-of-the-art Bi metal-based is one of the auspicious materials to potentially achieve high performance and efficiency due to its highly inhibitory properties on hydrogen evolution reaction. Other metals such as Sn and In also manifest potential for large-scale applications due to their better adsorption affinity for oxygen atoms or stronger adsorption capacity for the key intermediate *OCHO. On the other hand, for the production of ethylene, Cu metal catalyst and its modification schemes have been widely employed for realizing C2 or C2+ products due to its special promoting effect on carbon–carbon coupling and affinity for *CO intermediate. However, the performance of the current electrochemical CO2 reduction technology for C2 product catalyst scheme is far inferior to that for C1 product catalyst scheme in terms of Faradaic efficiency, current density and stability. In addition, the single-atom catalyst scheme, which has become increasingly popular in recent years, has thrown light in its intriguing properties due to its high atomic utilization and the ability to improve the electronic structure of the catalytic active sites. Additionally, metal and functional polymer composites, which employ polymer to improve the electrode surface solution, are gaining momentum recently. With substantial advancement in the realm of catalyst design over the past few years, the electrochemical CO2 reduction process has become more practical, which will serve as a new paradigm in renewable technology. Some medium-scale electrochemical CO2 reduction trials have been reported to date (Masel et al. 2021; Yan et al. 2021; Zhang et al. 2021a, b, c, d). A visual summary of the various discussed modification schemes is shown in Table 5.
Apart from experimental design, more focus should be placed on first-principles calculation as it gives us insight into the reaction sites, reaction kinetics and compatibility of the catalyst. This can then be used to verify experimental results, as well as inform future research works. The selection of catalyst materials by machine learning combined with density functional theory will accelerate the innovation of catalyst materials effectively. Current applications of machine learning include the screening of high-throughput catalyst materials and rapid prediction of surface reactivity of alloy catalysts through free energy calculations (Chen et al. 2020a, b, c; Guo et al. 2021; Ma et al. 2015; Ren et al. 2022). Notably, recent work has been reported that the ethylene selective Cu catalysts is able to achieve over 400 mA cm−2 current density, which is designed by combining machine learning prediction and experimental validation (Wu et al. 2021a, b, c; Zhong et al. 2020). Scientific breakthroughs can only be achieved through joint cooperation between the two ventures. Combined with computational analysis, these methods can aid in reducing the guesswork needed for traditional experimental analysis. It is also important to note that the accuracy of the calculated results depends on the standardization of current experimental works, with more dependable benchmark data being the key toward artificial intelligence-aided catalyst design (Lim and Ong 2021). For example, the current density, type of electrode, and reaction conditions should be reported along with the experimental results.
The current life cycle assessment study focuses on the comparison between conventional and new methods to selectively produce C1 and C2 chemicals. A number of life cycle assessment works have shown that a current lab-level electrochemical CO2 reduction process is inferior to conventional formic acid and ethylene production processes in terms of global warming potential and ecosystem toxicity. The reasons for this phenomenon are the loss caused by the non-recycling of unreacted CO2 in the electrochemical CO2 reduction process, the high material consumption caused by the regeneration of electrode materials, and the consumption of natural resources and greenhouse gas emissions caused by the high electricity power consumption. Therefore, in future research on electrochemical CO2 reduction technology, priority should be given to the optimization of electrode performance, while more consideration should be given to the regeneration process of electrode materials and the cycle of CO2 products. The environmental benefits of electrochemical production of formic acid and ethylene, as well as the merits of using electrochemistry combined with clean energy, require systematic life cycle assessment.
As for the economic assessment, although the electrochemical CO2 reduction process is still not profitable with current technology, the results of the study indicate its promising potential in the future. Recent techno-economic assessments have shown that the cost of producing formic acid by electroreduction of CO2 is close to 0.59 $/kg, which is close to the conventional process. Ethylene production is still 2.5 $/kg higher than the conventional process. Relevant metrics have indicated that more than 50% energy efficiency, 5 years electrode lifetime, 30% single-pass conversion, and less than 0.01 $/kWh electricity price are the key to realizing commercial-scale application (Shin et al. 2021). Novel design strategies for catalytic electrode materials will be necessary to achieve commercialization goals. Biomimetic molecular design strategy is a potential solution. For example, reducing the nucleophilicity of metal active sites by improving the molecular structure of catalyst materials and thus inhibiting hydrogen evolution reactions, using the electrical difference between metal alloy to promote the CO2 adsorption to electro-rich center, and changing the environment hydrophobicity to inhibit the proton adsorption by introduced local cations. In addition, for C2+ products, the C–C bond coupling process can be promoted by secondary sphere interaction (Shafaat and Yang 2021).
In the future, the electrochemical CO2 reduction process needs to combine with lower cost and sustainable energy sources such as hydropower, wind power and solar power. This will effectively reduce the overall environmental impact and production costs. Based on the current energy supply structure, it is still hard to achieve a mass renewable supply to the electrochemical CO2 reduction process. Therefore, to fully realize the potential of electrocatalytic CO2 conversion, relevant researchers, practitioners, and governments still need to support the development of electrochemical CO2 reduction in terms of technological innovation, industrial evolution and policy support. Relying on renewable sources of electricity also means building a circular economy of electrochemical CO2 reduction processes, linked to upstream carbon-intensive industries, with a cycle of carbon emissions to carbon capture to electrochemical CO2 reduction and finally reusing of chemicals. Therefore, infrastructures including abundant renewable energy, renewable electricity networks, and efficient carbon capture systems are essential to building such a sustainable cycle.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Agarwal AS, Zhai Y, Hill D, Sridhar N (2011) The electrochemical reduction of carbon dioxide to formate/formic acid: Engineering and economic feasibility. Chemsuschem 4:1301–1310. https://doi.org/10.1002/cssc.201100220
Ahn Y, Byun J, Kim D, Kim B-S, Lee C-S, Han J (2019) System-level analysis and life cycle assessment of CO2 and fossil-based formic acid strategies. Green Chem 21:3442–3455. https://doi.org/10.1039/c9gc01280j
Alfath M, Lee CW (2020) Recent advances in the catalyst design and mass transport control for the electrochemical reduction of carbon dioxide to formate. Catalysts 10:859. https://doi.org/10.3390/catal10080859
An X, Li S, Hao X, Xie Z, Du X, Wang Z, Hao X, Abudula A, Guan G (2021) Common strategies for improving the performances of tin and bismuth-based catalysts in the electrocatalytic reduction of CO2 to formic acid/formate. Renew Sust Energ Rev 143:110952. https://doi.org/10.1016/j.rser.2021.110952
Artz J, Muller TE, Thenert K, Kleinekorte J, Meys R, Sternberg A, Bardow A, Leitner W (2018) Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem Rev 118:434–504. https://doi.org/10.1021/acs.chemrev.7b00435
Arvidsson R, Tillman AM, Sandén BA, Janssen M, Nordelöf A, Kushnir D, Molander S (2017) Environmental assessment of emerging technologies: recommendations for prospective LCA. J Ind Ecol 22:1286–1294. https://doi.org/10.1111/jiec.12690
Back S, Kim JH, Kim YT, Jung Y (2016) On the mechanism of high product selectivity for HCOOH using Pb in CO2 electroreduction. Phys Chem Chem Phys 18:9652–9657. https://doi.org/10.1039/c6cp00542j
Bagger A, Ju W, Varela AS, Strasser P, Rossmeisl J (2017) Electrochemical CO2 reduction: a classification problem. ChemPhysChem 18:3266–3273. https://doi.org/10.1002/cphc.201700736
Bagger A, Ju W, Varela AS, Strasser P, Rossmeisl J (2019) Electrochemical CO2 reduction: classifying Cu facets. ACS Catal 9:7894–7899. https://doi.org/10.1021/acscatal.9b01899
Bai X, Chen W, Zhao C, Li S, Song Y, Ge R, Wei W, Sun Y (2017) Exclusive formation of formic acid from CO2 electroreduction by a tunable Pd-Sn alloy. Angew Chem Int Ed Engl 56:12219–12223. https://doi.org/10.1002/anie.201707098
Baruch MF, Pander JE, White JL, Bocarsly AB (2015) Mechanistic insights into the reduction of CO2 on tin electrodes using in situ ATR-IR spectroscopy. ACS Catal 5:3148–3156. https://doi.org/10.1021/acscatal.5b00402
Bashir S, Hossain SS, Rahman S, Ahmed S, Amir A-A, Hossain MM (2016) Electrocatalytic reduction of carbon dioxide on SnO2/MWCNT in aqueous electrolyte solution. J CO2 Util 16:346–353. https://doi.org/10.1016/j.jcou.2016.09.002
Benson EE, Kubiak CP, Sathrum AJ, Smieja JM (2009) Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem Soc Rev 38:89–99. https://doi.org/10.1039/b804323j
Bohlen B, Wastl D, Radomski J, Sieber V, Vieira L (2020) Electrochemical CO2 reduction to formate on indium catalysts prepared by electrodeposition in deep eutectic solvents. Electrochem Commun 110:106597. https://doi.org/10.1016/j.elecom.2019.106597
Bok J, Lee SY, Lee BH, Kim C, Nguyen DLT, Kim JW, Jung E, Lee CW, Jung Y, Lee HS, Kim J, Lee K, Ko W, Kim YS, Cho SP, Yoo JS, Hyeon T, Hwang YJ (2021) Designing atomically dispersed Au on tensile-strained Pd for efficient CO2 electroreduction to formate. J Am Chem Soc 143:5386–5395. https://doi.org/10.1021/jacs.0c12696
Bredar ARC, Chown AL, Burton AR, Farnum BH (2020) Electrochemical impedance spectroscopy of metal oxide electrodes for energy applications. ACS Appl Energy Mater 3:66–98. https://doi.org/10.1021/acsaem.9b01965
Bulushev DA, Ross JRH (2018) Towards sustainable production of formic acid. Chemsuschem 11:821–836. https://doi.org/10.1002/cssc.201702075
Bushuyev OS, De Luna P, Dinh CT, Tao L, Saur G, van de Lagemaat J, Kelley SO, Sargent EH (2018) What should we make with CO2 and how can we make it? Joule 2:825–832. https://doi.org/10.1016/j.joule.2017.09.003
Cai Y, Fu J, Zhou Y, Chang YC, Min Q, Zhu JJ, Lin Y, Zhu W (2021) Insights on forming N, O-coordinated Cu single-atom catalysts for electrochemical reduction CO2 to methane. Nat Commun 12:586. https://doi.org/10.1038/s41467-020-20769-x
Calle-Vallejo F, Koper MT (2013) Theoretical considerations on the electroreduction of CO to C2 species on Cu(100) electrodes. Angew Chem Int Ed Engl 52:7282–7285. https://doi.org/10.1002/anie.201301470
Chaplin RPS, Wragg AA (2003) Effects of process conditions and electrode material on reaction pathways for carbon dioxide electroreduction with particular reference to formate formation. J Appl Electrochem 33:1107–1123. https://doi.org/10.1023/B:JACH.0000004018.57792.b8
Chatterjee S, Dutta I, Lum Y, Lai Z, Huang K-W (2021) Enabling storage and utilization of low-carbon electricity: power to formic acid. Energy Environ Sci 14:1194–1246. https://doi.org/10.1039/d0ee03011b
Chen C, Khosrowabadi Kotyk JF, Sheehan SW (2018) Progress toward commercial application of electrochemical carbon dioxide reduction. Chem 4:2571–2586. https://doi.org/10.1016/j.chempr.2018.08.019
Chen A, Zhang X, Chen L, Yao S, Zhou Z (2020a) A machine learning model on simple features for CO2 reduction electrocatalysts. J Phys Chem C 124:22471–22478. https://doi.org/10.1021/acs.jpcc.0c05964
Chen X, Chen J, Alghoraibi NM, Henckel DA, Zhang R, Nwabara UO, Madsen KE, Kenis PJA, Zimmerman SC, Gewirth AA (2020b) Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat Catal 4:20–27. https://doi.org/10.1038/s41929-020-00547-0
Chen X, Ma D-D, Chen B, Zhang K, Zou R, Wu X-T, Zhu Q-L (2020c) Metal–organic framework-derived mesoporous carbon nanoframes embedded with atomically dispersed Fe–N active sites for efficient bifunctional oxygen and carbon dioxide electroreduction. Appl Catal B 267:118720. https://doi.org/10.1016/j.apcatb.2020.118720
Chen G, Ye D, Chen R, Li J, Zhu X, Liao Q (2021) Enhanced efficiency for carbon dioxide electroreduction to formate by electrodeposition Sn on Cu nanowires. J CO2 Util 44:101409. https://doi.org/10.1016/j.jcou.2020.101409
Choi SY, Jeong SK, Kim HJ, Baek I-H, Park KT (2016) Electrochemical reduction of carbon dioxide to formate on tin–lead alloys. ACS Sustain Chem Eng 4:1311–1318. https://doi.org/10.1021/acssuschemeng.5b01336
Clark EL, Hahn C, Jaramillo TF, Bell AT (2017) Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J Am Chem Soc 139:15848–15857. https://doi.org/10.1021/jacs.7b08607
Climent V, Feliu JM (2011) Thirty years of platinum single crystal electrochemistry. J Solid State Electrochem 15:1297–1315. https://doi.org/10.1007/s10008-011-1372-1
Daiyan R, Saputera WH, Masood H, Leverett J, Lu X, Amal R (2020) A disquisition on the active sites of heterogeneous catalysts for electrochemical reduction of CO2 to value-added chemicals and fuel. Adv Energy Mater 10:1902106. https://doi.org/10.1002/aenm.201902106
Damas GB, Miranda CR, Sgarbi R, Portela JM, Camilo MR, Lima FHB, Araujo CM (2019) On the mechanism of carbon dioxide reduction on Sn-based electrodes: insights into the role of oxide surfaces. Catalysts 9:636. https://doi.org/10.3390/catal9080636
Dominguez-Ramos A, Singh B, Zhang X, Hertwich EG, Irabien A (2015) Global warming footprint of the electrochemical reduction of carbon dioxide to formate. J Clean Prod 104:148–155. https://doi.org/10.1016/j.jclepro.2013.11.046
Dong WJ, Hong DM, Park JY, Kim S, Yoo CJ, Lee J-L (2021) Kinetic-controlled growth of Bi nanostructures for electrocatalytic CO2 reduction. J Electrochem Soc Jpn 168:016514. https://doi.org/10.1149/1945-7111/abdc6f
Duan YX, Liu KH, Zhang Q, Yan JM, Jiang Q (2020) Efficient CO2 reduction to HCOOH with high selectivity and energy efficiency over Bi/rGO catalyst. Small Methods 4:1900846. https://doi.org/10.1002/smtd.201900846
Ethylene market share, size 2020–2026 (2020) Polaris Market Research. Retrieved 20/12 from https://www.polarismarketresearch.com/industry-analysis/ethylene-market
Fan M, Garbarino S, Botton GA, Tavares AC, Guay D (2017) Selective electroreduction of CO2 to formate on 3D [100] Pb dendrites with nanometer-sized needle-like tips. J Mater Chem A 5:20747–20756. https://doi.org/10.1039/c7ta06528k
Fan T, Ma W, Xie M, Liu H, Zhang J, Yang S, Huang P, Dong Y, Chen Z, Yi X (2021) Achieving high current density for electrocatalytic reduction of CO2 to formate on bismuth-based catalysts. Cell Rep 2:100353. https://doi.org/10.1016/j.xcrp.2021.100353
Feaster JT, Shi C, Cave ER, Hatsukade T, Abram DN, Kuhl KP, Hahn C, Nørskov JK, Jaramillo TF (2017) Understanding selectivity for the electrochemical reduction of carbon dioxide to formic acid and carbon monoxide on metal electrodes. ACS Catal 7:4822–4827. https://doi.org/10.1021/acscatal.7b00687
Feng Y, Li Z, Liu H, Dong C, Wang J, Kulinich SA, Du X (2018) Laser-prepared CuZn alloy catalyst for selective electrochemical reduction of CO2 to ethylene. Langmuir 34:13544–13549. https://doi.org/10.1021/acs.langmuir.8b02837
Fernández-Dacosta C, Shen L, Schakel W, Ramirez A, Kramer GJ (2019) Potential and challenges of low-carbon energy options: Comparative assessment of alternative fuels for the transport sector. Appl Energy 236:590–606. https://doi.org/10.1016/j.apenergy.2018.11.055
Fernando J (2020a) Internal rate of return (IRR). Investopedia. Retrieved 13/11 from https://www.investopedia.com/terms/i/irr.asp
Fernando J (2020b) Net present value (NPV). Investopedia. Retrieved 13/11 from https://www.investopedia.com/terms/n/npv.asp
Foo JJ, Ng S-F, Ong W-J (2022) Dimensional heterojunction design: the rising star of 2D bismuth-based nanostructured photocatalysts for solar-to-chemical conversion. J Nano Res https://doi.org/10.1007/s12274-021-4045-0
Formic acid market forecast, global market insights 2019 to 2027 (2019) FACT.MR. Retrieved 12/20 from https://www.factmr.com/report/4279/formic-acid-market
Francke R, Schille B, Roemelt M (2018) Homogeneously catalyzed electroreduction of carbon dioxide-methods, mechanisms, and catalysts. Chem Rev 118:4631–4701. https://doi.org/10.1021/acs.chemrev.7b00459
Fu S, Liu X, Ran J, Jiao Y, Qiao S-Z (2021) CO2 reduction by single copper atom supported on g-C3N4 with asymmetrical active sites. Appl Surf Sci 540:148293. https://doi.org/10.1016/j.apsusc.2020.148293
Gao D, Zhou H, Cai F, Wang J, Wang G, Bao X (2018) Pd-containing nanostructures for electrochemical CO2 reduction reaction. ACS Catal 8:1510–1519. https://doi.org/10.1021/acscatal.7b03612
Gao D, Sinev I, Scholten F, Aran-Ais RM, Divins NJ, Kvashnina K, Timoshenko J, Roldan Cuenya B (2019) Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte-driven nanostructuring. Angew Chem Int Ed Engl 58:17047–17053. https://doi.org/10.1002/anie.201910155
Gao Y, Wu Q, Liang X, Wang Z, Zheng Z, Wang P, Liu Y, Dai Y, Whangbo MH, Huang B (2020) Cu2O nanoparticles with both 100 and 111 facets for enhancing the selectivity and activity of CO2 electroreduction to ethylene. Adv Sci 7:1902820. https://doi.org/10.1002/advs.201902820
Garcia de Arquer FP, Bushuyev OS, De Luna P, Dinh CT, Seifitokaldani A, Saidaminov MI, Tan CS, Quan LN, Proppe A, Kibria MG, Kelley SO, Sinton D, Sargent EH (2018) 2D metal oxyhalide-derived catalysts for efficient CO2 electroreduction. Adv Mater 30:e1802858. https://doi.org/10.1002/adma.201802858
Garg S, Li M, Weber AZ, Ge L, Li L, Rudolph V, Wang G, Rufford TE (2020) Advances and challenges in electrochemical CO2 reduction processes: an engineering and design perspective looking beyond new catalyst materials. J Mater Chem A 8:1511–1544. https://doi.org/10.1039/c9ta13298h
Global formic acid market report 2021. (2021, 01/02/2021). ARC Report Store (OPC) Pvt. Ltd. https://arcreportsstore.com/report/2020-26-formic-acid-market-58567?utm_source=Neighbor0203&utm_medium=KD7604
Global monitoring laboratory-carbon cycle greenhouse gases. (2021). Global Monitoring Laboratory. https://gml.noaa.gov/ccgg/trends/global.html
Gómez-Marín AM, Feliu JM (2012) Pt(111) surface disorder kinetics in perchloric acid solutions and the influence of specific anion adsorption. Electrochim Acta 82:558–569. https://doi.org/10.1016/j.electacta.2012.04.066
Greeley J, Jaramillo TF, Bonde J, Chorkendorff IB, Norskov JK (2006) Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat Mater 5:909–913. https://doi.org/10.1038/nmat1752
Gu J, Heroguel F, Luterbacher J, Hu X (2018) Densely packed, ultra small SnO nanoparticles for enhanced activity and selectivity in electrochemical CO2 reduction. Angew Chem Int Ed Engl 57:2943–2947. https://doi.org/10.1002/anie.201713003
Guo Y, He X, Su Y, Dai Y, Xie M, Yang S, Chen J, Wang K, Zhou D, Wang C (2021) Machine-learning-guided discovery and optimization of additives in preparing cu catalysts for CO2 reduction. J Am Chem Soc 143:5755–5762. https://doi.org/10.1021/jacs.1c00339
Han N, Ding P, He L, Li Y, Li Y (2019) Promises of main group metal–based nanostructured materials for electrochemical CO2 reduction to formate. Adv Energy Mater 10:1902338. https://doi.org/10.1002/aenm.201902338
Han H, Noh Y, Kim Y, Park S, Yoon W, Jang D, Choi SM, Kim WB (2020) Selective electrochemical CO2 conversion to multicarbon alcohols on highly efficient N-doped porous carbon-supported Cu catalysts. Green Chem 22:71–84. https://doi.org/10.1039/c9gc03088c
Hoang TTH, Verma S, Ma S, Fister TT, Timoshenko J, Frenkel AI, Kenis PJA, Gewirth AA (2018) Nanoporous copper-silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J Am Chem Soc 140:5791–5797. https://doi.org/10.1021/jacs.8b01868
Hori Y, Wakebe H, Tsukamoto T, Koga O (1994) Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim Acta 39:1833–1839. https://doi.org/10.1016/0013-4686(94)85172-7
Hori Y, Takahashi R, Yoshinami Y, Murata A (1997) Electrochemical reduction of CO at a copper electrode. J Phys Chem B 101:7075–7081. https://doi.org/10.1021/jp970284i
Hori Y (2008) Electrochemical CO2 reduction on metal electrodes. In: Modern aspects of electrochemistry, vol n/a, pp 89–189. Springer
Hou Y, Liang Y-L, Shi P-C, Huang Y-B, Cao R (2020) Atomically dispersed Ni species on N-doped carbon nanotubes for electroreduction of CO2 with nearly 100% CO selectivity. Appl Catal B 271:118929. https://doi.org/10.1016/j.apcatb.2020.118929
IEA (2020) Electricity market report-December 2020. IEA. https://www.iea.org/reports/electricity-market-report-december-2020
Ikeda S, Takagi T, Ito K (1987) Selective formation of formic acid, oxalic acid, and carbon monoxide by electrochemical reduction of carbon dioxide. Bull Chem Soc Jpn 60:2517–2522. https://doi.org/10.1246/bcsj.60.2517
Innocent B, Pasquier D, Ropital F, Hahn F, Léger JM, Kokoh KB (2010) FTIR spectroscopy study of the reduction of carbon dioxide on lead electrode in aqueous medium. Appl Catal B 94:219–224. https://doi.org/10.1016/j.apcatb.2009.10.027
Jia M, Fan Q, Liu S, Qiu J, Sun Z (2019) Single-atom catalysis for electrochemical CO2 reduction. Curr Opin Green Sustain Chem 16:1–6. https://doi.org/10.1016/j.cogsc.2018.11.002
Jia C, Ren W, Chen X, Yang W, Zhao C (2020) (N, B) Dual heteroatom-doped hierarchical porous carbon framework for efficient electroreduction of carbon dioxide. ACS Sustain Chem Eng 8:6003–6010. https://doi.org/10.1021/acssuschemeng.0c00739
Jia S, Zhu Q, Chu M, Han S, Feng R, Zhai J, Xia W, He M, Wu H, Han B (2021a) Hierarchical metal-polymer hybrids for enhanced CO2 electroreduction. Angew Chem Int Ed Engl 60:10977–10982. https://doi.org/10.1002/anie.202102193
Jia S, Zhu Q, Chu M, Han S, Feng R, Zhai J, Xia W, He M, Wu H, Han B (2021b) Hierarchical metal-polymer hybrids for enhanced CO2 electroreduction. Angew Energy Environ Sci 60:10977–10982. https://doi.org/10.1002/anie.2021b02193
Jiang J, Feng X, Yang M, Wang Y (2020a) Comparative technoeconomic analysis and life cycle assessment of aromatics production from methanol and naphtha. J Clean Prod 277:123525. https://doi.org/10.1016/j.jclepro.2020.123525
Jiang X, Wang Q, Xiao X, Chen J, Shen Y, Wang M (2020b) Interfacial engineering of bismuth with reduced graphene oxide hybrid for improving CO2 electroreduction performance. Electrochim Acta 357:136840. https://doi.org/10.1016/j.electacta.2020.136840
Jiang H, Wang L, Li Y, Gao B, Guo Y, Yan C, Zhuo M, Wang H, Zhao S (2021) High-selectivity electrochemical CO2 reduction to formate at low overpotential over Bi catalyst with hexagonal sheet structure. Appl Surf Sci 541:148577. https://doi.org/10.1016/j.apsusc.2020.148577
Jiménez C, García J, Martínez F, Camarillo R, Rincón J (2020) Cu nanoparticles deposited on CNT by supercritical fluid deposition for electrochemical reduction of CO2 in a gas phase GDE cell. Electrochim Acta 337:135663. https://doi.org/10.1016/j.electacta.2020.135663
Jordaan SM, Wang C (2021) Electrocatalytic conversion of carbon dioxide for the Paris goals. Nat Catal 4:915–920. https://doi.org/10.1038/s41929-021-00704-z
Jouny M, Luc W, Jiao F (2018) General techno-economic analysis of CO2 electrolysis systems. Ind Eng Chem Res 57:2165–2177. https://doi.org/10.1021/acs.iecr.7b03514
Kaneco S, Iwao R, Iiba K, Ohta K, Mizuno T (1998) Electrochemical conversion of carbon dioxide to formic acid on Pb in KOH/methanol electrolyte at ambient temperature and pressure. Energy 23:1107–1112. https://doi.org/10.1016/S0360-5442(98)00054-1
Kang D, Byun J, Han J (2021) Electrochemical production of formic acid from carbon dioxide: a life cycle assessment study. Chem Eng J 9:106130. https://doi.org/10.1016/j.jece.2021.106130
Kapusta S, Hackerman N (1983) The electroreduction of carbon dioxide and formic acid on tin and indium electrodes. J Electrochem Soc Jpn 130:607
Kapusta S, Hackerman N (2019) The electroreduction of carbon dioxide and formic acid on tin and indium electrodes. J Electrochem Soc 130:607–613. https://doi.org/10.1149/1.2119761
Karaba A, Dvořáková V, Patera J, Zámostný P (2020) Improving the steam-cracking efficiency of naphtha feedstocks by mixed/separate processing. J Anal Appl Pyrolysis 146:104768. https://doi.org/10.1016/j.jaap.2019.104768
Kas R, Yang K, Bohra D, Kortlever R, Burdyny T, Smith WA (2020) Electrochemical CO2 reduction on nanostructured metal electrodes: Fact or defect? Chem Sci 11:1738–1749. https://doi.org/10.1039/c9sc05375a
Kenton W (2020) Rate of return (RoR). Investopedia. Retrieved 31/3 from https://www.investopedia.com/terms/r/rateofreturn.asp
Khan K, Tareen AK, Aslam M, Zhang Y, Wang R, Ouyang Z, Gou Z, Zhang H (2019) Recent advances in two-dimensional materials and their nanocomposites in sustainable energy conversion applications. Nanoscale 11:21622–21678. https://doi.org/10.1039/c9nr05919a
Khoo HH, Halim I, Handoko AD (2020) LCA of electrochemical reduction of CO2 to ethylene. J CO2 Util 41:101229. https://doi.org/10.1016/j.jcou.2020.101229
Kim J, Choi W, Park JW, Kim C, Kim M, Song H (2019) Branched copper oxide nanoparticles induce highly selective ethylene production by electrochemical carbon dioxide reduction. J Am Chem Soc 141:6986–6994. https://doi.org/10.1021/jacs.9b00911
Koj JC, Wulf C, Linssen J, Schreiber A, Zapp P (2018) Utilisation of excess electricity in different power-to-transport chains and their environmental assessment. Transp Res Part D 64:23–35. https://doi.org/10.1016/j.trd.2018.01.016
Komatsu S, Yanagihara T, Hiraga Y, Tanaka M, Kunugi A (1995) Electrochemical reduction of CO2 at Sb and Bi electrodes in KHCO3 solution. Bunseki Kagaku 63:217–224. https://doi.org/10.5796/kogyobutsurikagaku.63.217
Kortlever R, Shen J, Schouten KJ, Calle-Vallejo F, Koper MT (2015) Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J Phys Chem Lett 6:4073–4082. https://doi.org/10.1021/acs.jpclett.5b01559
Koshy DM, Nathan SS, Asundi AS, Abdellah AM, Dull SM, Cullen DA, Higgins D, Bao Z, Bent SF, Jaramillo TF (2021) Bridging thermal catalysis and electrocatalysis: catalyzing CO2 conversion with carbon-based materials. Angew Chem Int Ed Engl 60:17472–17480. https://doi.org/10.1002/anie.202101326
Kuhl KP, Cave ER, Abram DN, Jaramillo TF (2012) New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ Sci 5:7050. https://doi.org/10.1039/c2ee21234j
Lai Q, Yang N, Yuan G (2017) Highly efficient In–Sn alloy catalysts for electrochemical reduction of CO2 to formate. Electrochem Commun 83:24–27. https://doi.org/10.1016/j.elecom.2017.08.015
Lei F, Liu W, Sun Y, Xu J, Liu K, Liang L, Yao T, Pan B, Wei S, Xie Y (2016) Metallic tin quantum sheets confined in graphene toward high-efficiency carbon dioxide electroreduction. Nat Commun 7:12697. https://doi.org/10.1038/ncomms12697
Lei Z, Xue Y, Chen W, Qiu W, Zhang Y, Horike S, Tang L (2018) MOFs-based heterogeneous catalysts: new opportunities for energy-related CO2 conversion. Adv Energy Mater 8:1801587. https://doi.org/10.1002/aenm.201801587
Li F, Chen L, Xue M, Williams T, Zhang Y, MacFarlane DR, Zhang J (2017a) Towards a better Sn: Efficient electrocatalytic reduction of CO2 to formate by Sn/SnS2 derived from SnS2 nanosheets. Nano Energy 31:270–277. https://doi.org/10.1016/j.nanoen.2016.11.004
Li Q, Fu J, Zhu W, Chen Z, Shen B, Wu L, Xi Z, Wang T, Lu G, Zhu JJ, Sun S (2017b) Tuning Sn-catalysis for electrochemical reduction of CO2 to CO via the core/shell Cu/SnO2 structure. J Am Chem Soc 139:4290–4293. https://doi.org/10.1021/jacs.7b00261
Li D, Wu J, Liu T, Liu J, Yan Z, Zhen L, Feng Y (2019) Tuning the pore structure of porous tin foam electrodes for enhanced electrochemical reduction of carbon dioxide to formate. Chem Eng J 375:122024. https://doi.org/10.1016/j.cej.2019.122024
Li F, Gu GH, Choi C, Kolla P, Hong S, Wu T-S, Soo Y-L, Masa J, Mukerjee S, Jung Y, Qiu J, Sun Z (2020a) Highly stable two-dimensional bismuth metal-organic frameworks for efficient electrochemical reduction of CO2. Appl Catal B 277:119241. https://doi.org/10.1016/j.apcatb.2020.119241
Li H, Xiao N, Wang Y, Liu C, Zhang S, Zhang H, Bai J, Xiao J, Li C, Guo Z, Zhao S, Qiu J (2020b) Promoting the electroreduction of CO2 with oxygen vacancies on a plasma-activated SnOx/carbon foam monolithic electrode. J Mater Chem A 8:1779–1786. https://doi.org/10.1039/c9ta12401b
Li J, Zhu M, Han YF (2020c) Recent advances in electrochemical CO2 reduction on indium-based catalysts. ChemCatChem 13:514–531. https://doi.org/10.1002/cctc.202001350
Li M, Wang H, Luo W, Sherrell PC, Chen J, Yang J (2020d) Heterogeneous single-atom catalysts for electrochemical CO2 reduction reaction. Adv Mater 32:e2001848. https://doi.org/10.1002/adma.202001848
Li Q, Zhang X, Zhou X, Li Q, Wang H, Yi J, Liu Y, Zhang J (2020e) Simply and effectively electrodepositing Bi-MWCNT-COOH composite on Cu electrode for efficient electrocatalytic CO2 reduction to produce HCOOH. J CO2 Util 37:106–112. https://doi.org/10.1016/j.jcou.2019.12.003
Liang H-Q, Zhao S, Hu X-M, Ceccato M, Skrydstrup T, Daasbjerg K (2021) Hydrophobic copper interfaces boost electroreduction of carbon dioxide to ethylene in water. ACS Catal 11:958–966. https://doi.org/10.1021/acscatal.0c03766
Lim XB, Ong W-J (2021) A current overview of the oxidative desulfurization of fuels utilizing heat and solar light: from materials design to catalysis for clean energy [10.1039/D1NH00127B]. Nanoscale Horiz 6:588–633. https://doi.org/10.1039/D1NH00127B
Lin S, Ng S-F, Ong W-J (2021) Life cycle assessment of environmental impacts associated with oxidative desulfurization of diesel fuels catalyzed by metal-free reduced graphene oxide. Environ Pollut 288:117677. https://doi.org/10.1016/j.envpol.2021.117677
Ling GZS, Ng S-F, Ong W-J (2022) Tailor-engineered 2D cocatalysts: Harnessing electronhole redox center of 2D g-C3N4 photocatalysts toward solar-to-chemical conversion and environmental purification. Adv Funct Mater. https://doi.org/10.1002/adfm.202111875
Liu M, Pang Y, Zhang B, De Luna P, Voznyy O, Xu J, Zheng X, Dinh CT, Fan F, Cao C, de Arquer FP, Safaei TS, Mepham A, Klinkova A, Kumacheva E, Filleter T, Sinton D, Kelley SO, Sargent EH (2016) Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537:382–386. https://doi.org/10.1038/nature19060
Liu G, Tran-Phu T, Chen H, Tricoli A (2018) A review of metal- and metal-oxide-based heterogeneous catalysts for electroreduction of carbon dioxide. Adv Sustain Syst 2:1800028. https://doi.org/10.1002/adsu.201800028
Liu S, Lu XF, Xiao J, Wang X, Lou XWD (2019) Bi2O3 nanosheets grown on multi-channel carbon matrix to catalyze efficient CO2 electroreduction to HCOOH. Angew Chem Int Ed Engl 58:13828–13833. https://doi.org/10.1002/anie.201907674
Liu A, Gao M, Ren X, Meng F, Yang Y, Gao L, Yang Q, Ma T (2020a) Current progress in electrocatalytic carbon dioxide reduction to fuels on heterogeneous catalysts. J Mater Chem A 8:3541–3562. https://doi.org/10.1039/c9ta11966c
Liu G, Li Z, Shi J, Sun K, Ji Y, Wang Z, Qiu Y, Liu Y, Wang Z, Hu P (2020b) Black reduced porous SnO2 nanosheets for CO2 electroreduction with high formate selectivity and low overpotential. Appl Catal B 260:118134. https://doi.org/10.1016/j.apcatb.2019.118134
Liu H, Zhu Y, Ma J, Zhang Z, Hu W (2020c) Recent advances in atomic-level engineering of nanostructured catalysts for electrochemical CO2 reduction. Adv Energy Mater 30:1910534. https://doi.org/10.1002/adfm.201910534
Liu Q, Zhu Y, He Z, Jin S, Chen Y (2020d) A facile top-down approach for constructing perovskite oxide nanostructure with abundant oxygen defects as highly efficient water oxidation electrocatalyst. Int J Hydrog Energy 45:22808–22816. https://doi.org/10.1016/j.ijhydene.2020.06.137
Lu P, Gao D, He H, Wang Q, Liu Z, Dipazir S, Yuan M, Zu W, Zhang G (2019) Facile synthesis of a bismuth nanostructure with enhanced selectivity for electrochemical conversion of CO2 to formate. Nanoscale 11:7805–7812. https://doi.org/10.1039/c9nr01094g
Lu X-L, Rong X, Zhang C, Lu T-B (2020) Carbon-based single-atom catalysts for CO2 electroreduction: progress and optimization strategies. J Mater Chem A 8:10695–10708. https://doi.org/10.1039/d0ta01955k
Lu P, Tan X, Zhao H, Xiang Q, Liu K, Zhao X, Yin X, Li X, Hai X, Xi S, Wee ATS, Pennycook SJ, Yu X, Yuan M, Wu J, Zhang G, Smith SC, Yin Z (2021) Atomically dispersed indium sites for selective CO2 electroreduction to formic acid. ACS Nano 15:5671–5678. https://doi.org/10.1021/acsnano.1c00858
Lyu Z, Zhu S, Xie M, Zhang Y, Chen Z, Chen R, Tian M, Chi M, Shao M, Xia Y (2021) Controlling the surface oxidation of Cu nanowires improves their catalytic selectivity and stability toward C2+ products in CO2 reduction. Angew Chem Int Ed Engl 60:1909–1915. https://doi.org/10.1002/anie.202011956
Ma X, Li Z, Achenie LE, Xin H (2015) Machine-learning-augmented chemisorption model for CO2 electroreduction catalyst screening. J Phys Chem Lett 6:3528–3533. https://doi.org/10.1021/acs.jpclett.5b01660
Ma S, Sadakiyo M, Heima M, Luo R, Haasch RT, Gold JI, Yamauchi M, Kenis PJ (2017) Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu-Pd catalysts with different mixing patterns. J Am Chem Soc 139:47–50. https://doi.org/10.1021/jacs.6b10740
Masel RI, Liu Z, Yang H, Kaczur JJ, Carrillo D, Ren S, Salvatore D, Berlinguette CP (2021) An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat Nanotechnol 16:118–128. https://doi.org/10.1038/s41565-020-00823-x
Masood ul Hasan I, Peng L, Mao J, He R, Wang Y, Fu J, Xu N, Qiao J (2020) Carbon-based metal-free catalysts for electrochemical CO2 reduction: Activity, selectivity, and stability. Carbon 3:24–49. https://doi.org/10.1002/cey2.87
Mc Murry JE, Fleming MP (1974) A new method for the reductive coupling of carbonyls to olefins. Synthesis of beta-carotene. J Am Chem Soc 96:4708–4709. https://doi.org/10.1021/ja00821a076
Mistry H, Varela AS, Bonifacio CS, Zegkinoglou I, Sinev I, Choi YW, Kisslinger K, Stach EA, Yang JC, Strasser P, Cuenya BR (2016) Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat Commun 7:12123. https://doi.org/10.1038/ncomms12123
Mizuno T, Ohta K, Sasaki A, Akai T, Hirano M, Kawabe A (1995) Effect of temperature on electrochemical reduction of high-pressure CO2 with In, Sn, and Pb electrodes. Energy Sources 17:503–508. https://doi.org/10.1080/00908319508946098
Ng S-F, Foo JJ, Ong W-J (2022) Solar-powered chemistry: Engineering low-dimensional carbon nitride-based nanostructures for selective CO2 conversion to C1–C2 products. InfoMat. https://doi.org/10.1002/inf2.12279
Ning H, Mao Q, Wang W, Yang Z, Wang X, Zhao Q, Song Y, Wu M (2019) N-doped reduced graphene oxide supported Cu2O nanocubes as high active catalyst for CO2 electroreduction to C2H4. J Alloys Compd 785:7–12. https://doi.org/10.1016/j.jallcom.2019.01.142
Nitopi S, Bertheussen E, Scott SB, Liu X, Engstfeld AK, Horch S, Seger B, Stephens IEL, Chan K, Hahn C, Norskov JK, Jaramillo TF, Chorkendorff I (2019) Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem Rev 119:7610–7672. https://doi.org/10.1021/acs.chemrev.8b00705
Ochedi FO, Liu D, Yu J, Hussain A, Liu Y (2020) Photocatalytic, electrocatalytic and photoelectrocatalytic conversion of carbon dioxide: a review. Environ Chem Lett 19:941–967. https://doi.org/10.1007/s10311-020-01131-5
Ogura K (2013) Electrochemical reduction of carbon dioxide to ethylene: Mechanistic approach. J CO2 Util 1:43–49. https://doi.org/10.1016/j.jcou.2013.03.003
Ong W-J, Shak KPY (2020) 2D/2D Heterostructured photocatalysts: an emerging platform for artificial photosynthesis. Sol RRL 4:2000132. https://doi.org/10.1002/solr.202000132
Ong W-J, Putri LK, Mohamed AR (2020) Rational design of carbon-based 2D nanostructures for enhanced photocatalytic CO2 reduction: a dimensionality perspective. Chem Eng J 26:9710–9748. https://doi.org/10.1002/chem.202000708
Orella MJ, Brown SM, Leonard ME, Román-Leshkov Y, Brushett FR (2019) A general technoeconomic model for evaluating emerging electrolytic processes. Energy Technol 8:1900994. https://doi.org/10.1002/ente.201900994
Paik W, Andersen TN, Eyring H (1969) Kinetic studies of the electrolytic reduction of carbon dioxide on the mercury electrode. Electrochim Acta 14:1217–1232. https://doi.org/10.1016/0013-4686(69)87019-2
Pan F, Li B, Sarnello E, Fei Y, Gang Y, Xiang X, Du Z, Zhang P, Wang G, Nguyen HT, Li T, Hu YH, Zhou HC, Li Y (2020) Atomically dispersed iron-nitrogen sites on hierarchically mesoporous carbon nanotube and graphene nanoribbon networks for CO2 reduction. ACS Nano 14:5506–5516. https://doi.org/10.1021/acsnano.9b09658
Peng J, Chen X, Ong W-J, Zhao X, Li N (2019) Surface and heterointerface engineering of 2D MXenes and their nanocomposites: Insights into electro- and photocatalysis. Chem 5:18–50. https://doi.org/10.1016/j.chempr.2018.08.037
Peterson AA, Abild-Pedersen F, Studt F, Rossmeisl J, Nørskov JK (2010) How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ Sci 3:1311–1315. https://doi.org/10.1039/c0ee00071j
Popovic S, Smiljanic M, Jovanovic P, Vavra J, Buonsanti R, Hodnik N (2020) Stability and degradation mechanisms of copper-based catalysts for electrochemical CO2 reduction. Angew Chem Int Ed Engl 59:14736–14746. https://doi.org/10.1002/anie.202000617
Qin Z, Jiang X, Cao Y, Dong S, Wang F, Feng L, Chen Y, Guo Y (2021) Nitrogen-doped porous carbon derived from digested sludge for electrochemical reduction of carbon dioxide to formate. Sci Total Environ 759:143575. https://doi.org/10.1016/j.scitotenv.2020.143575
Reller C, Krause R, Volkova E, Schmid B, Neubauer S, Rucki A, Schuster M, Schmid G (2017) Selective electroreduction of CO2 toward ethylene on nano dendritic copper catalysts at high current density. Adv Energy Mater 7:1602114. https://doi.org/10.1002/aenm.201602114
Ren T, Patel M, Blok K (2006) Olefins from conventional and heavy feedstocks: energy use in steam cracking and alternative processes. Energy 31:425–451. https://doi.org/10.1016/j.energy.2005.04.001
Ren M, Zheng H, Lei J, Zhang J, Wang X, Yakobson BI, Yao Y, Tour JM (2020) CO2 to formic acid using Cu-Sn on laser-induced graphene. ACS Appl Mater Interfaces 12:41223–41229. https://doi.org/10.1021/acsami.0c08964
Ren Y, Foo JJ, Zeng D, Ong W-J (2022) ZnIn2S4-based nanostructures in artificial photosynthesis: Insights into photocatalytic reduction toward sustainable energy production. Small. https://doi.org/10.1002/sstr.202200017
Rong X, Wang HJ, Lu XL, Si R, Lu TB (2020) Controlled synthesis of a vacancy-defect single-atom catalyst for boosting CO2 electroreduction. Angew Chem Int Ed Engl 59:1961–1965. https://doi.org/10.1002/anie.201912458
Rumayor M, Dominguez-Ramos A, Irabien A (2019a) Environmental and economic assessment of the formic acid electrochemical manufacture using carbon dioxide: Influence of the electrode lifetime. Sustain Prod Consum 18:72–82. https://doi.org/10.1016/j.spc.2018.12.002
Rumayor M, Dominguez-Ramos A, Perez P, Irabien A (2019b) A techno-economic evaluation approach to the electrochemical reduction of CO2 for formic acid manufacture. J CO2 Util 34:490–499. https://doi.org/10.1016/j.jcou.2019b.07.024
Rumayor M, Fernández-González J, Domínguez-Ramos A, Irabien A (2021) Deep decarbonization of the cement sector: A prospective environmental assessment of CO2 recycling to methanol. ACS Sustain Chem Eng 10:267–278. https://doi.org/10.1021/acssuschemeng.1c06118
Schouten KJP, Kwon Y, van der Ham CJM, Qin Z, Koper MTM (2011) A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem Sci 2:1902. https://doi.org/10.1039/c1sc00277e
Senanayake SD, Waterhouse GI, Idriss H, Madey TE (2005) Coupling of carbon monoxide molecules over oxygen-defected UO2(111) single crystal and thin film surfaces. Langmuir 21:11141–11145. https://doi.org/10.1021/la0519103
Shafaat HS, Yang JY (2021) Uniting biological and chemical strategies for selective CO2 reduction. Nat Catal 4:928–933. https://doi.org/10.1038/s41929-021-00683-1
Shin HC, Dong J, Liu M (2003) Nanoporous structures prepared by an electrochemical deposition process. Adv Mater 15:1610–1614. https://doi.org/10.1002/adma.200305160
Shin H, Hansen KU, Jiao F (2021) Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat Sustain 4:911–919. https://doi.org/10.1038/s41893-021-00739-x
Si R, Raitano J, Yi N, Zhang L, Chan S-W, Flytzani-Stephanopoulos M (2012) Structure sensitivity of the low-temperature water-gas shift reaction on Cu–CeO2 catalysts. Catal Today 180:68–80. https://doi.org/10.1016/j.cattod.2011.09.008
Sisler J, Khan S, Ip AH, Schreiber MW, Jaffer SA, Bobicki ER, Dinh C-T, Sargent EH (2021) Ethylene electrosynthesis: a comparative techno-economic analysis of alkaline vs membrane electrode assembly vs CO2–CO–C2H4 tandems. ACS Energy Lett 6:997–1002. https://doi.org/10.1021/acsenergylett.0c02633
Standardization I O f (2006) Environmental management: Life cycle assessment; principles and framework. ISO. Retrieved 2006 from https://www.iso.org/standard/37456.htmlACS Appl Mater Interfaces
Sternberg A, Jens CM, Bardow A (2017) Life cycle assessment of CO2-based C1-chemicals. Green Chem 19:2244–2259. https://doi.org/10.1039/c6gc02852g
Sun X, Lu L, Zhu Q, Wu C, Yang D, Chen C, Han B (2018) MoP nanoparticles supported on indium-doped porous carbon: Outstanding catalysts for highly efficient CO2 electroreduction. Angew Chem Int Ed Engl 57:2427–2431. https://doi.org/10.1002/anie.201712221
Thonemann N (2020) Environmental impacts of CO2-based chemical production: a systematic literature review and meta-analysis. Appl Energy 263:114599. https://doi.org/10.1016/j.apenergy.2020.114599
Thonemann N, Schulte A (2019) From laboratory to industrial scale: A prospective LCA for electrochemical reduction of CO2 to formic acid. Environ Sci Technol 53:12320–12329. https://doi.org/10.1021/acs.est.9b02944
Todoroki M, Hara K, Kudo A, Sakata T (1995) Electrochemical reduction of high pressure CO2 at Pb, Hg and In electrodes in an aqueous KHCO3 solution. J Electroanal Chem 394:199–203. https://doi.org/10.1016/0022-0728(95)04010-l
Tonner SP, Trimm DL, Wainwright MS, Cant NW (2002) Dehydrogenation of methanol to methyl formate over copper catalysts. Ind Eng Chem Res 23:384–388. https://doi.org/10.1021/i300015a012
Top 10 emerging technologies of 2021 (2021) World economic forum. Retrieved Decemeber 14 from https://www.weforum.org/agenda/2021/11/these-are-the-top-10-emerging-technologies-of-2021/
Tsujiguchi T, Kawabe Y, Jeong S, Ohto T, Kukunuri S, Kuramochi H, Takahashi Y, Nishiuchi T, Masuda H, Wakisaka M, Hu K, Elumalai G, Fujita J-i, Ito Y (2021) Acceleration of electrochemical CO2 reduction to formate at the Sn/reduced graphene oxide interface. ACS Catal 11:3310–3318. https://doi.org/10.1021/acscatal.0c04887
Varela AS, Ju W, Strasser P (2018) Molecular nitrogen-carbon catalysts, solid metal organic framework catalysts, and solid metal/nitrogen-doped carbon (MNC) catalysts for the electrochemical CO2 reduction. Adv Energy Mater 8:1703614. https://doi.org/10.1002/aenm.201703614
Vasileff A, Zheng Y, Qiao SZ (2017) Carbon solving carbon’s problems: Recent progress of nanostructured carbon-based catalysts for the electrochemical reduction of CO2. Adv Energy Mater 7:1700759. https://doi.org/10.1002/aenm.201700759
Vasileff A, Zhu Y, Zhi X, Zhao Y, Ge L, Chen HM, Zheng Y, Qiao SZ (2020) Electrochemical reduction of CO2 to ethane through stabilization of an ethoxy intermediate. Angew Chem Int Ed 132:19817–19821. https://doi.org/10.1002/ange.202004846
Wang H, Chen Y, Hou X, Ma C, Tan T (2016a) Nitrogen-doped graphenes as efficient electrocatalysts for the selective reduction of carbon dioxide to formate in aqueous solution. Green Chem 18:3250–3256. https://doi.org/10.1039/c6gc00410e
Wang J, Wang H, Han Z, Han J (2016b) Electrodeposited porous Pb electrode with improved electrocatalytic performance for the electroreduction of CO2 to formic acid. Front Chem Sci Eng 9:57–63. https://doi.org/10.1007/s11705-014-1444-8
Wang H, Jia J, Song P, Wang Q, Li D, Min S, Qian C, Wang L, Li YF, Ma C, Wu T, Yuan J, Antonietti M, Ozin GA (2017) Efficient electrocatalytic reduction of CO2 by nitrogen-doped nanoporous carbon/carbon nanotube membranes: a step towards the electrochemical CO2 refinery. Angew Chem Int Ed Engl 56:7847–7852. https://doi.org/10.1002/anie.201703720
Wang A, Li J, Zhang T (2018) Heterogeneous Single-Atom Catalysis Nat Rev Chem 2:65–81. https://doi.org/10.1038/s41570-018-0010-1
Wang T, Wang Z, Salvatierra RV, McHugh E, Tour JM (2020) Top-down synthesis of graphene nanoribbons using different sources of carbon nanotubes. Carbon 158:615–623. https://doi.org/10.1016/j.carbon.2019.11.033
Wang J, Cheng T, Fenwick AQ, Baroud TN, Rosas-Hernandez A, Ko JH, Gan Q, Goddard WA III, Grubbs RH (2021) Selective CO2 electrochemical reduction enabled by a tricomponent copolymer modifier on a copper surface. J Am Chem Soc 143:2857–2865. https://doi.org/10.1021/jacs.0c12478
Wanninayake N, Ai Q, Zhou R, Hoque MA, Herrell S, Guzman MI, Risko C, Kim DY (2020) Understanding the effect of host structure of nitrogen doped ultrananocrystalline diamond electrode on electrochemical carbon dioxide reduction. Carbon 157:408–419. https://doi.org/10.1016/j.carbon.2019.10.022
Wen G, Lee DU, Ren B, Hassan FM, Jiang G, Cano ZP, Gostick J, Croiset E, Bai Z, Yang L, Chen Z (2018) Orbital interactions in Bi-Sn bimetallic electrocatalysts for highly selective electrochemical CO2 reduction toward formate production. Adv Energy Mater 8:1802427. https://doi.org/10.1002/aenm.201802427
Wu D, Dong C, Wu D, Fu J, Liu H, Hu S, Jiang Z, Qiao SZ, Du X-W (2018) Cuprous ions embedded in ceria lattice for selective and stable electrochemical reduction of carbon dioxide to ethylene. J Mater Chem A 6:9373–9377. https://doi.org/10.1039/c8ta01677a
Wu D, Liu J, Liang Y, Xiang K, Fu XZ, Luo JL (2019) Electrochemical transformation of facet-controlled bioi into mesoporous bismuth nanosheets for selective electrocatalytic reduction of CO2 to formic acid. Chemsuschem 12:4700–4707. https://doi.org/10.1002/cssc.201901724
Wu D, Chen W, Wang X, Fu X-Z, Luo J-L (2020a) Metal-support interaction enhanced electrochemical reduction of CO2 to formate between graphene and Bi nanoparticles. J CO2 Util 37:353–359. https://doi.org/10.1016/j.jcou.2020a.02.007
Wu D, Huo G, Chen W, Fu X-Z, Luo J-L (2020b) Boosting formate production at high current density from CO2 electroreduction on defect-rich hierarchical mesoporous Bi/Bi2O3 junction nanosheets. Appl Catal B 271:118957. https://doi.org/10.1016/j.apcatb.2020.118957
Wu D, Zhang J, Cheng M-J, Lu Q, Zhang H (2021a) Machine learning investigation of supplementary adsorbate influence on copper for enhanced electrochemical CO2 reduction performance. J Phys Chem C 125:15363–15372. https://doi.org/10.1021/acs.jpcc.1c05004
Wu Z-Z, Gao F-Y, Gao M-R (2021b) Regulating the oxidation state of nanomaterials for electrocatalytic CO2 reduction. Energy Environ Sci 14:1121–1139. https://doi.org/10.1039/d0ee02747b
Wu Z, Wu H, Cai W, Wen Z, Jia B, Wang L, Jin W, Ma T (2021c) Engineering bismuth-tin unterface in bimetallic aerogel with a 3D porous structure for highly selective electrocatalytic CO2 reduction to HCOOH. Angew Chem Int Ed Engl 60:12554–12559. https://doi.org/10.1002/anie.202102832
Xu H, Rebollar D, He H, Chong L, Liu Y, Liu C, Sun C-J, Li T, Muntean JV, Winans RE, Liu D-J, Xu T (2020) Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat Eng 5:623–632. https://doi.org/10.1038/s41560-020-0666-x
Yan Y, Ke L, Ding Y, Zhang Y, Rui K, Lin H, Zhu J (2021) Recent advances in Cu-based catalysts for electroreduction of carbon dioxide. Mater Chem Front 5:2668–2683. https://doi.org/10.1039/d0qm01127d
Yang HJ, Yang H, Hong YH, Zhang PY, Wang T, Chen LN, Zhang FY, Wu QH, Tian N, Zhou ZY, Sun SG (2018) Promoting ethylene selectivity from CO2 electroreduction on CuO supported onto CO2 capture materials. Chemsuschem 11:881–887. https://doi.org/10.1002/cssc.201702338
Yang Q, Yang CC, Lin CH, Jiang HL (2019) Metal-organic-framework-derived hollow N-doped porous carbon with ultrahigh concentrations of single Zn atoms for efficient carbon dioxide conversion. Angew Chem Int Ed Engl 58:3511–3515. https://doi.org/10.1002/anie.201813494
Yang Z, Qi Y, Wang F, Han Z, Jiang Y, Han H, Liu J, Zhang X, Ong WJ (2020) State-of-the-art advancements in photo-assisted CO2 hydrogenation: recent progress in catalyst development and reaction mechanisms. J Mater Chem A 8:24868–24894. https://doi.org/10.1039/D0TA08781E
Yang X, Cheng J, Yang X, Xu Y, Sun W, Liu N, Liu J (2021) Boosting electrochemical CO2 reduction by controlling coordination environment in atomically dispersed Ni@NxCy catalysts. ACS Sustain Chem Eng 9:6438–6445. https://doi.org/10.1021/acssuschemeng.1c01364
Yoo JS, Christensen R, Vegge T, Norskov JK, Studt F (2016) Theoretical insight into the trends that guide the electrochemical reduction of carbon dioxide to formic acid. Chemsuschem 9:358–363. https://doi.org/10.1002/cssc.201501197
Yu X, Ng S-F, Putri LK, Tan L-L, Mohamed AR, Ong W-J (2021) Point-defect engineering: leveraging imperfections in graphitic carbon nitride (g-C3N4) photocatalysts toward artificial photosynthesis. Small 17:2006851. https://doi.org/10.1002/smll.202006851
Yuan H, Qian X, Luo B, Wang L, Deng L, Chen Y (2020a) Carbon dioxide reduction to multicarbon hydrocarbons and oxygenates on plant moss-derived, metal-free, in situ nitrogen-doped biochar. Sci Total Environ 739:140340. https://doi.org/10.1016/j.scitotenv.2020.140340
Yuan T, Hu Z, Zhao Y, Fang J, Lv J, Zhang Q, Zhuang Z, Gu L, Hu S (2020b) Two-dimensional amorphous SnOx from liquid metal: Mass production, phase transfer, and electrocatalytic CO2 reduction toward formic acid. Nano Lett 20:2916–2922. https://doi.org/10.1021/acs.nanolett.0c00844
Zeng J, Jagdale P, Lourenço MAO, Farkhondehfal MA, Sassone D, Bartoli M, Pirri CF (2021) Biochar-supported BiOx for effective electrosynthesis of formic acid from carbon dioxide reduction. Crystals 11:363. https://doi.org/10.3390/cryst11040363
Zhang S, Kang P, Meyer TJ (2014a) Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J Am Chem Soc 136:1734–1737. https://doi.org/10.1021/ja4113885
Zhang S, Kang P, Ubnoske S, Brennaman MK, Song N, House RL, Glass JT, Meyer TJ (2014b) Polyethylenimine-enhanced electrocatalytic reduction of CO2 to formate at nitrogen-doped carbon nanomaterials. J Am Chem Soc 136:7845–7848. https://doi.org/10.1021/ja5031529
Zhang J, Zheng Y, Wang Z, Wang S, Zhang R, Yang S, Zhao D, Yang L, Zhang D, Chen L (2016a) Structural characterization, optical properties, and phase transitions of In1–xSnx alloy thin films. J Phys Chem Lett 120:7822–7828. https://doi.org/10.1021/acs.jpcc.5b12288
Zhang Z, Chi M, Veith GM, Zhang P, Lutterman DA, Rosenthal J, Overbury SH, Dai S, Zhu H (2016b) Rational design of Bi nanoparticles for efficient electrochemical CO2 reduction: the elucidation of size and surface condition effects. ACS Catal 6:6255–6264. https://doi.org/10.1021/acscatal.6b01297
Zhang Y, Li F, Zhang X, Williams T, Easton CD, Bond AM, Zhang J (2018) Electrochemical reduction of CO2 on defect-rich Bi derived from Bi2S3 with enhanced formate selectivity. J Mater Chem A 6:4714–4720. https://doi.org/10.1039/c8ta00023a
Zhang B, Zhang J, Hua M, Wan Q, Su Z, Tan X, Liu L, Zhang F, Chen G, Tan D, Cheng X, Han B, Zheng L, Mo G (2020a) Highly Electrocatalytic Ethylene Production from CO2 on Nanodefective Cu Nanosheets. J Am Chem Soc 142:13606–13613. https://doi.org/10.1021/jacs.0c06420
Zhang T, Han X, Yang H, Han A, Hu E, Li Y, Yang XQ, Wang L, Liu J, Liu B (2020b) Atomically dispersed nickel(I) on an alloy-encapsulated nitrogen-doped carbon nanotube array for high-performance electrochemical CO2 reduction reaction. Angew Chem Int Ed Engl 59:12055–12061. https://doi.org/10.1002/anie.202002984
Zhang W, Huang C, Xiao Q, Yu L, Shuai L, An P, Zhang J, Qiu M, Ren Z, Yu Y (2020c) Atypical oxygen-bearing copper boosts ethylene selectivity toward electrocatalytic CO2 reduction. J Am Chem Soc 142:11417–11427. https://doi.org/10.1021/jacs.0c01562
Zhang W, Mohamed AR, Ong W-J (2020d) Z-scheme photocatalytic systems for carbon dioxide reduction: Where are we now? Angew Chem Int Ed 59:22894–22915. https://doi.org/10.1002/anie.201914925
Zhang Z, Feng C, Liu C, Zuo M, Qin L, Yan X, Xing Y, Li H, Si R, Zhou S, Zeng J (2020e) Electrochemical deposition as a universal route for fabricating single-atom catalysts. Nat Commun 11:1215. https://doi.org/10.1038/s41467-020-14917-6
Zhang B, Jiang Y, Gao M, Ma T, Sun W, Pan H (2021a) Recent progress on hybrid electrocatalysts for efficient electrochemical CO2 reduction. Nano Energy 80:105504. https://doi.org/10.1016/j.nanoen.2020.105504
Zhang J, Guo Y, Shang B, Fan T, Lian X, Huang P, Dong Y, Chen Z, Yi X (2021b) Unveiling the synergistic effect between graphitic carbon nitride and Cu2O toward CO2 electroreduction to C2 H4. Chemsuschem 14:929–937. https://doi.org/10.1002/cssc.202002427
Zhang W, Yang S, Jiang M, Hu Y, Hu C, Zhang X, Jin Z (2021c) Nanocapillarity and nanoconfinement effects of pipet-like bismuth@carbon nanotubes for highly efficient electrocatalytic CO2 reduction. Nano Lett 21:2650–2657. https://doi.org/10.1021/acs.nanolett.1c00390
Zhang Y, Cao C, Wu X-T, Zhu Q-L (2021d) Three-dimensional porous copper-decorated bismuth-based nanofoam for boosting the electrochemical reduction of CO2 to formate. Inorg Chem Front 8:2461–2467. https://doi.org/10.1039/d1qi00065a
Zhao K, Quan X (2021) Carbon-based materials for electrochemical reduction of CO2 to C2+ oxygenates: Recent progress and remaining challenges. ACS Catal 11:2076–2097. https://doi.org/10.1021/acscatal.0c04714
Zhao K, Zhu W, Liu S, Wei X, Ye G, Su Y, He Z (2020a) Two-dimensional metal–organic frameworks and their derivatives for electrochemical energy storage and electrocatalysis. Nanoscale Adv 2:536–562. https://doi.org/10.1039/c9na00719a
Zhao M, Gu Y, Gao W, Cui P, Tang H, Wei X, Zhu H, Li G, Yan S, Zhang X, Zou Z (2020b) Atom vacancies induced electron-rich surface of ultrathin Bi nanosheet for efficient electrochemical CO2 reduction. Appl Catal B 266:118625. https://doi.org/10.1016/j.apcatb.2020.118625
Zhao S, Qin Y, Guo T, Li S, Liu X, Ou M, Wu Y, Chen Y (2021a) SnS nanoparticles grown on Sn-atom-modified N, S-codoped mesoporous carbon nanosheets as electrocatalysts for CO2 reduction to formate. ACS Appl Nano Mater 4:2257–2264. https://doi.org/10.1021/acsanm.0c03361
Zhao X-H, Chen Q-S, Zhuo D-H, Lu J, Xu Z-N, Wang C-M, Tang J-X, Sun S-G, Guo G-C (2021b) Oxygen vacancies enriched Bi based catalysts for enhancing electrocatalytic CO2 reduction to formate. Electrochim Acta 367:137478. https://doi.org/10.1016/j.electacta.2020.137478
Zheng X, De Luna P, García de Arquer FP, Zhang B, Becknell N, Ross MB, Li Y, Banis MN, Li Y, Liu M, Voznyy O, Dinh CT, Zhuang T, Stadler P, Cui Y, Du X, Yang P, Sargent EH (2017) Sulfur-modulated tin sites enable highly selective electrochemical reduction of CO2 to formate. Joule 1:794–805. https://doi.org/10.1016/j.joule.2017.09.014
Zheng Y, Vasileff A, Zhou X, Jiao Y, Jaroniec M, Qiao SZ (2019) Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J Am Chem Soc 141:7646–7659. https://doi.org/10.1021/jacs.9b02124
Zhi X, Jiao Y, Zheng Y, Davey K, Qiao S-Z (2021) Directing the selectivity of CO2 electroreduction to target C2 products via non-metal doping on Cu surfaces. J Mater Chem A 9:6345–6351. https://doi.org/10.1039/d0ta11604a
Zhong M, Tran K, Min Y, Wang C, Wang Z, Dinh CT, De Luna P, Yu Z, Rasouli AS, Brodersen P, Sun S, Voznyy O, Tan CS, Askerka M, Che F, Liu M, Seifitokaldani A, Pang Y, Lo SC, Ip A, Ulissi Z, Sargent EH (2020) Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581:178–183. https://doi.org/10.1038/s41586-020-2242-8
Zhou Y, Che F, Liu M, Zou C, Liang Z, De Luna P, Yuan H, Li J, Wang Z, Xie H, Li H, Chen P, Bladt E, Quintero-Bermudez R, Sham TK, Bals S, Hofkens J, Sinton D, Chen G, Sargent EH (2018) Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat Chem 10:974–980. https://doi.org/10.1038/s41557-018-0092-x
Zhou Y, Guo X, Li X, Fu J, Liu J, Hong F, Qiao J (2020) In-situ growth of CuO/Cu nanocomposite electrode for efficient CO2 electroreduction to CO with bacterial cellulose as support. J CO2 Util 37:188–194. https://doi.org/10.1016/j.jcou.2019.12.009
Zhu DD, Liu JL, Qiao SZ (2016) Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv Mater 28:3423–3452. https://doi.org/10.1002/adma.201504766
Funding
The authors would like to acknowledge the financial support provided by the Ministry of Higher Education (MOHE) Malaysia under the Fundamental Research Grant Scheme (FRGS) (Ref no: FRGS/1/2020/TK0/XMU/02/1) and also Guangdong Basic and Applied Basic Research Foundation (Ref no: 2021A1515111019). This work is also funded by Xiamen University Malaysia Investigatorship Grant (Grant no: IENG/0038), Xiamen University Malaysia Research Fund (XMUMRF/2021-C8/IENG/0041 and XMUMRF/2019-C3/IENG/0013), and Hengyuan International Sdn. Bhd. (Grant no: EENG/0003).
Author information
Authors and Affiliations
Contributions
Ling Ai performed writing—original draft preparation, and formal analysis. Sue-Faye Ng performed writing—original draft preparation, and writing—review and editing. Wee-Jun Ong contributed to conceptualization, supervision, writing-review and editing, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ai, L., Ng, SF. & Ong, WJ. Carbon dioxide electroreduction into formic acid and ethylene: a review. Environ Chem Lett 20, 3555–3612 (2022). https://doi.org/10.1007/s10311-022-01470-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-022-01470-5