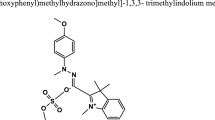
Abstract
Textile effluents containing synthetic dyes are one of the most important sources of water pollution. Several dyes are toxic to the aquatic life and indirectly to humans, and therefore should be treated before rejection to natural water streams. As conventional wastewater treatment systems remain inefficient for treatment of these recalcitrant pollutants, the use of advanced oxidation processed is required. The degradation of the Orange G dye was studied using the photo-Fenton process. Results showed that the use of experimental kinetics rate constants to optimize the ratio R = [H2O2]/[Fe3+] constitutes a suitable way to minimize the occurrence of side reactions. An optimal molar ratio R of 13.8 was found allowing complete degradation of Orange G in 4 min and 93.41% total organic carbon (TOC) removal in 180 min. Based on detected intermediates and end-products, a plausible degradation mechanism of Orange G dye is proposed. Therefore, we demonstrated that the photo-Fenton process can be applied efficiently to the removal of toxic/persistent organic pollutants such as synthetic dyes from water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthraquinonic, azo and heterocyclic dyes are those mostly used in various industries and found in natural water bodies. Particularly, azo dyes represent 60–70% of the usual textile dyes industries (Alinsafi et al. 2007). This sector consumes more than 70% of annual production, estimated about 7.0 × 105 tons (Shanker et al. 2017) and around 2.8 × 105 tons are discharged into the environment through textile effluents (Eren 2012). Orange G is an acid azo dye, used as color marker, pH indicator, to stain keratin and also widely used in food, textile (Divya et al. 2009) and tannery industries. Orange G is reported to show some chromosomal damage and clastogenic activity as special toxic effects (Verma and Kumar 2018). Textile industry is highly polluting due to the high dyes concentrations used in dyeing process and the toxic nature constituting an environment risk factor (Brillas and Martinez-Huitle 2015). The release of colored wastewaters into the ecosystem is a source of aesthetic pollution and also causing perturbation to aquatic life (Akpan and Hameed 2009; Nidheesh et al. 2018; Shukla and Oturan 2015). Moreover, dyes molecules are mostly toxic, carcinogenic, mutagenic or allergenic (Srivastava et al. 2004; Turhan et al. 2012; Chukowry et al. 2017). Dye effluents are generally refractory to bioremediation and conventional treatment techniques (Brillas and Martinez-Huitle 2015). Their removal from wastewater requires the use of more effective processes such as advanced oxidation processes (AOPs) based on the generation of highly reactive and strongly oxidant species like hydroxyl radicals (·OH), able to oxidize any of organic molecules until almost complete mineralization (Oturan and Aaron 2014; Saeed et al. 2018). Among these processes, the photochemical AOPs have the advantage to be non-toxic, speedy and effective (Litter and Quici 2010) in which the in situ produced ·OH reacts non-selectively with organic pollutants either by hydrogen atom abstraction, electrophilic addition, electrons transfer or ipso-substitution (Mousset et al. 2018; Pignatello et al. 2006). Therefore, photochemical-assisted Fenton reaction was effective for contaminant’s oxidation from aqueous solution at optimal pH of around 3. At this pH value, the predominant ferric iron species is Fe(OH)2+ which absorbs strongly UV light. Thus, the photoreduction of this species leads to the formation of Fe2+ with the formation of ·OH (reaction 1). Fe2+ reacts with H2O2 to generate ·OH according to Fenton reaction (reaction 2). Fenton reaction is then catalyzed by reduction of the photocatalyst Fe3+ (reaction 1). The photolysis of H2O2 (reaction 3) constitutes a supplementary source for generation of ·OH. However, the contribution of photolysis of H2O2 (reaction 3) is lesser compared to reactions (1) and (2) because the low absorption coefficient of H2O2.
Consequently, in addition to the solution pH (optimal at around 3, the concentration of H2O2 and ferric iron (as catalyst) as well as the irradiation light (λ = 240–300 nm) constitute main parameters in photo-Fenton process (Bouafia-Chergui et al. 2010; Khandelwal and Ameta 2013).
Several studies based on the Fenton-related processes have been successfully achieved for the treatment of chemical dyes in wastewater (Guivarch et al. 2003; Diagne et al. 2009; Soon and Hameed 2011; Cai et al. 2016; Tiya-Djowe et al. 2016). Despite all these studies, just few ones highlighted the fate of degradation by-products or is briefly discussed whenever the case. Therefore, this work is proposed as a breakthrough in the well-established degradation mechanism of Orange G oxidation by ·OH generated in photo-Fenton (homogeneous photocatalysis) process. Optimal conditions for oxidative degradation of Orange G and mineralization of its aqueous solution were established following an empirical method based on the determination of apparent rate constants. In addition, the by-products resulting from oxidation of Orange G were identified and a plausible degradation mechanism was proposed.
Experimental
Chemicals
Orange G dye, H2O2 (30%) and Fe2(SO4)3 were purchased from Sigma-Aldrich and were used without any purification. All solutions were prepared with ultrapure water with resistivity ≥ 18 mΩ cm. Reagent quality Na2CO3 (CAS 497-19-8) and NaHCO3 (CAS 144-55-8) were used for preparation of mobile phase. Sulfuric acid, ammonium acetate and methanol used for liquid chromatography analyses were HPLC grade from Sigma-Aldrich.
Photoreactor
All experiments were performed in 1.3 L working volume photoreactor, equipped with a low-mercury vapor pressure lamp (Heraeus Noblelight-NNI 40/20) emitting at 253.7 nm. A recirculation pump (KNF Neuberger) allowed homogenization of reaction medium at a maximum flow rate of 3 L min−1. The pH of Orange G solution was adjusted to 2.9 ± 0.1 using a pH-meter Cyberscan 1500 from Eutech instruments. Required catalyst and H2O2 amounts are added to the reaction mixture before connecting UV lamp.
Analytical procedures
Orange G concentration was monitored using a Merck Hitachi HPLC device equipped with a pump (LaChrom L-7100), a diode array detector (LaChrom L 7455) and a Purospher STAR RP-18 column under following conditions: mobile phase 40:60 (v/v) methanol/ammonium acetate, flow rate of 0.5 mL min−1, detection at 480 nm and column temperature of 40 °C.
For the identification of carboxylic acids, the same HPLC device was equipped with a column Supelcogel H (id = 4.6 mm × 25 mm), using H2SO4 (1%) at 0.2 mL min−1 with detection at 220 nm. Identification of aromatic intermediates was performed by GC–MS using an ISQ single-quadrupole mass spectrometer coupled with Trace 1300 Series gas chromatograph equipped with a Trace Gold TG-5MS (30 m × 0.25 mm; 0.25 μm) column. For this analysis, experiments were performed using 4 mM of Orange G solution. Prior to the analysis, 20 mL samples of treated solution after t = 2, 4, 6 and 10 min treatment were extracted with 30 mL dichloromethane for two times. Then, the extracts were concentrated with 3 mL of dichloromethane by rotary evaporator at 40 °C. GC–MS temperature program was set as follows: the initial value of 40 °C is held for 10 min, afterward it is increased up to 100 °C with a 12 °C min−1 rate, then to 200 °C with 5 °C min−1 and finally to 270 °C with a 20 °C min−1 rate followed by an isothermal time of 5 min.
Dionex ionic chromatography system ICS-1000 equipped with an autonomous suppressor (ASRS-Ultra II (anions) and CSRS-Ultra II (cations) was used for the detection of ions. An anion-exchange column (IonPac AS4ASC, 25 cm × 4 mm) was connected to an IonPac AG4A-SC column for anions (SO42− and NO3−) analysis, while a cation-exchange column (IonPac CS12A, 25 cm × 4 mm) fitted with an IonPac CG12A column guard was used for NH4+ analysis. A mixture of 1.8 mM Na2CO3 and 1.7 mM NaHCO3 at 2 mL min−1 was used as mobile phase for anions, while a 9 mM H2SO4 solution at 1 mL min−1 was used for cations, respectively, as mobile phase. Mineralization degree of photo-Fenton treated solutions was monitored from total organic carbon removal and was performed with a Shimadzu TOC analyzer VCSH with ± 2% accuracy using the non-purgeable organic carbon method.
Results and discussion
Optimization of the operating parameters
The removal efficiency of organic contaminants from aqueous solution by photo-Fenton process depends on several parameters such as the initial pH, light irradiation and wavelength, the initial concentration of H2O2 and Fe3+ as well as their ratio [H2O2]/[Fe3+] and the initial pollutant concentration. The effect and limiting values of these parameters were studied in detail in Bouafia-Chergui et al. (2010) and Khandelwal and Ameta (2013). In this study, H2O2 and Fe3+ concentration and their ratio are optimized keeping the others parameters constant. As a main parameter in photo-Fenton process, the concentration of H2O2 plays an important role on photo-Fenton process efficiency for degradation of organics pollutants in water. Therefore, the effect of different initial concentration of H2O2 on oxidative degradation of 0.1 mM Orange G solution was studied in the presence of 0.4 mM Fe3+ (photocatalyst) at pH 3 and results are depicted in Fig. 1. As can be seen from Fig. 1a, the increase in H2O2 initial concentration from 1 to 6 mM improved dye degradation efficiency due to the higher amount of ·OH production from reactions (1)–(3). From the kinetic point of view, the decay of Orange G follows pseudo-first-order reaction kinetics with R2 values above 0.988 in all the cases. Therefore, the apparent rate constants (kapp) of 0.39, 0.53, 0.75, 1.05 and 1.01 min−1 were determined from the slope of the straight lines for 1, 2, 4, 6 and 8 mM of H2O2, respectively, pointing out the value of 6 mM as optimal initial H2O2 concentration. Further increase in H2O2 concentration decreased degradation efficiency due to the competition of the following wasting reaction (4) because the increase in its reaction rate in excess of H2O2.
Influence of operating parameters on the degradation and mineralization of 0.1 mM Orange G (OG) dye solution: Effect of H2O2 initial concentration (filled square: 2 mM, filled red circle: 6 mM, filled pointed up blue triangle: 8 mM) on degradation kinetics (a) and total organic carbon (TOC) removal efficiency (b) in presence of 0.4 mM Fe3+ and effect of initial Fe3+ concentration (filled square: 0.2 mM, filled red circle: 0.4 mM, filled pointed up blue triangle: 0.6 mM) on OG decay (c) and total organic carbon (TOC) removal efficiency (d) in the presence of 6 mM H2O2 at pH 3. The results obtained from these figures highlight 6 mM H2O2 and 0.4 mM Fe3+ initial concentrations as optimal values for effective treatment of 0.1 mM Orange G solution
Similar effects of H2O2 concentration were also observed on the mineralization of Orange G solution in terms of TOC removal rate (Fig. 1b). The maximum mineralization degree of 92.2% was attained for 6 mM H2O2 concentration after 180 min of treatment. Thus, 6 mM was chosen as the optimal H2O2 concentration value for the following experiments.
The effect of initial Fe3+ concentration on the photo-Fenton efficiency was then investigated at pH 3 and fixed (6 mM) H2O2 initial concentration, and the results are shown in Fig. 1c, d. Fe3+ behaves as photocatalyst in photo-Fenton process; its photocatalytic regeneration [reactions (1) and (2)] allows the use of low concentrations avoiding ferric hydroxide sludge formation. The results shown in Fig. 1c, d highlight 0.4 mM initial Fe3+ concentration as optimal value for treatment of 0.1 mM Orange G solution. Therefore, the apparent rate constants (kapp) of 0.36, 0.68, 0.85, 1.05 and 0.93 min−1 for 0.1, 0.2, 0.3, 0.4 and 0.6 mM Fe3+ concentration, respectively, were determined from kinetic analysis. Hence, the value of 0.4 mM was selected as the optimal concentration of Fe3+. For concentrations higher than 0.4 mM, the wasting reaction (5) becomes competitive and quenches part of generated ·OH.
The effect of Fe3+ concentration on TOC removal was also evaluated as shown in Fig. 1d. The mineralization rates are enough high (> 83%) even for lower Fe3+ concentrations, while the highest value of 92.2% is reached for 0.4 mM Fe3+ initial concentration after 180 min irradiation.
Effect of Fenton’s reagent ratio [H2O2]/[Fe3+]
In the photo-Fenton process, the ratio R = [H2O2]/[Fe3+] is a key parameter for optimization because of the ·OH wasting reactions (4) and (5) mentioned above due to their high rate constants (k4 = 2.7 × 107 M−1 s−1 and k5 = 3.0 × 108 M−1 s−1 (De Laat and Gallard 1999). To minimize their occurrence, a suitable ratio R was established using the apparent rate constants. Figure 2 points out that the relationship between kapp and R is a straight line (Eq. 6) as a function of [H2O2] for a fixed [Fe3+] = 0.4 mM.
Optimization of Fenton’s reagent molar ratio (R = [H2O2]/[Fe3+]) for oxidation of 0.1 mM Orange G solution using apparent rate constants obtained at 0.4 mM Fe3+ (filled square) and 6 mM H2O2 (filled pointed up red triangle). The analysis based on the variation of apparent rates constants highlights an R value of 13.8 (corresponding to 6 mM H2O2 and 0.435 mM Fe3+ concentrations) as optimal for effective treatment of 0.1 mM Orange G solution
The variation of R for different [Fe3+] at [H2O2] = 6 mM is also a straight line (Eq. 7).
At the optimal R value, the straight lines from Eqs. (6) and (7) are equal and correspond to the intercept of the two straight lines of Fig. 2. At this point, the scavenging rates of ·OH by H2O2 and/or Fe2+ overdosed are at the lower limit and equal, corresponding to analytical derived R ratio of 13.8. This result is in agreement with the mathematical derived molar ratio of 11 for Fenton oxidation of double bond unsaturated organics like azo dyes (Tang 2003). On the other hand, the work evaluating the discoloration of Acid Blue 161 azo dye by Fenton oxidation and using similar data analysis reported an optimal molar ratio of 14.30 (Trovó et al. 2016).
The complete mineralization of Orange G by ·OH the following Eq. (8) can be represented by reaction (8):
From Eq. (8), 8.4 mM of ·OH is required for complete mineralization of 0.1 mM of Orange G aqueous solution. Assuming the formation ·OH from reaction (1) added to the concentration of 6 mM found above for H2O2 as optimal value seems to be in agreement with the theoretical value of 8.4 mM for mineralization of 0.1 mM Orange G solution.
Comparative study of various processes involves in photo-Fenton process
Oxidative degradation of Orange G was performed by 3 different photochemical processes: direct photolysis, H2O2 photolysis and photo-Fenton (Fig. 3). To show the effect of light irradiation in photo-Fenton process, a supplementary experiment (Fenton-like reaction) (reaction 9) was carried out without light irradiation. After 4 min of treatment, 4.0, 27.8, 46.1 and 100% of degradation rate were achieved using UValone, Fe3+/H2O2, UV/H2O2 and photo-Fenton processes, respectively (Fig. 3a) evidencing the catalytic role of Fe3+ under UV light irradiation. On the other hand, TOC removal degrees of 5.0, 30.0, 65.8 and 93.4% were attained at 180 min of treatment for UValone, Fenton-like (Fe3+/H2O2), UV/H2O2 and photo-Fenton processes, respectively, at the optimal R value established above (Fig. 3b). Once more again the effect of light irradiation on catalytic regeneration of Fe2+ and H2O2 photolysis was demonstrated since photo-Fenton process provided significantly best results compared to Fenton-like reaction.
Comparison of processes for oxidative degradation (A) and total organic carbon (TOC) removal of 0.1 mM Orange G (b). (filled pointed up triangle): UV alone (filled pointed down triangle): UV/H2O2 (6 mM), (filled circle): Fe3+/H2O2 ([H2O2] = 6 mM, R = 13.8), filled square: photo-Fenton ([H2O2] = 6 mM, R = 13.8) for oxidative degradation (a) and total organic carbon (TOC) removal. The results highlight the superiority of photo-Fenton process
By-products analysis and mineralization pathway
From the practical point of view, oxidative degradation products may be more harmful than parent pollutant (Oturan et al. 2008a, b), and therefore, their analysis is a key factor so that the treated effluent contains only harmless compounds such as short-chain carboxylic acids. The analysis by ion-exclusion chromatography showed that concentration of oxalic and glyoxylic acids reaches their maximum value around 20 min of treatment before gradually mineralized to CO2 by ·OH. Formic and fumaric acids were detected at trace level while the concentration of oxamic acid increased continuously to reach 0.01 mM at the end of 180 min of treatment (results not shown). The residual TOC of 1.26 mg L−1 at the end of treatment is composed of remaining carboxylic acids, mainly oxalic and oxamic acids. On the other hand, the analysis of released inorganic ions pointed out that the almost complete S atom is released as sulfate ions at 180 min of treatment, whereas the total nitrogen concentration released as majorly NO3− (and in a less extend NH4+) is about 0.1 mM corresponding to about 50% of initial N of Orange G. This non-balance in N can be explained by the N atom present at the remaining oxamic acid and formation of volatile N-species such as N2 and/or other NxOy species (Ganzenko et al. 2018).
Based on IC and GC–MS results of the present work as well as the previous studies (Meetani et al. 2011; Wang et al. 2015), a plausible oxidation mechanism of Orange G by ·OH during photo-Fenton process is proposed on Fig. 4. The various detected intermediates arise from an indiscriminate attack of ·OH radical at several sites. However, the starting identified intermediates such as aromatic hydroxyl amines (B and C), nitroso (A and J) and phenolic substituted compounds (D, K, L, M) suggested oxidative cleavage of high electron density azo bonds as the primarily sites of ·OH attack according to the literature data (Oturan et al. 2008a, b; Brillas and Martinez-Huitle 2015). Then, the activated sulfonate groups were attacked conducting their release as sulfates ions. The formed primary intermediates are then oxidized by O2 and successive hydroxylation giving rise to hydroxylated aromatic intermediates. Further hydroxylation of theses aromatics ultimately causes the ring opening, leading to the formation of short-chain carboxylic acids that are complexed by Fe(III) in excess at the end of treatment. The concomitant mineralization of free carboxylic acids by ·OH and Fe(III)–carboxylate complexes photo-decarboxylation completes the mineralization process of orange G.
Proposed mineralization reaction pathway of Orange G induced by ·OH generated in photo-Fenton process. Aromatic (polyhydroxylated and quinone) intermediates undertake oxidative ring opening reactions leading to the formation of carboxylic acids that are the end organic products before complete mineralization
Conclusions
The present work has demonstrated that the persistent organic pollutant like Orange G azo dye can be removed effectively from water by photo-Fenton process at optimized operating conditions, i.e., 6 mM H2O2, 0.435 mM Fe3+ corresponding to the reagent ratio [H2O2]/[Fe3+] = 13.8 for 0.1 mM (45.2 g L−1) initial dye concentration. Under these conditions, Orange G dye solution was completely degraded at only 4 min irradiation, whereas 93.4% mineralization rate was achieved at 180 min of treatment. Identification of aromatic intermediates and short-chain carboxylic acids as well as released inorganic ions allowed proposing a plausible mineralization pathway of Orange G by photo-Fenton generated hydroxyl radicals.
References
Akpan UG, Hameed BH (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater 170:520–529. https://doi.org/10.1016/j.jhazmat.2009.05.039
Alinsafi A, Evenou F, Abdulkarim EM, Pons MN, Zahraa O, Benhammou A, Yaacoubi A, Nejmeddine A (2007) Treatment of textile industry wastewater by supported photocatalysis. Dyes Pigments 74:439–445. https://doi.org/10.1016/j.dyepig.2006.02.024
Bouafia-Chergui S, Oturan N, Khalaf H, Oturan MA (2010) Parametric study on the effect of the ratios [H2O2]/[Fe3+] and [H2O2]/[substrate] on the photo-Fenton degradation of cationic azo dye basic blue 41. J Environ Sci Health A 45:622–629. https://doi.org/10.1080/10934521003595746
Brillas E, Martinez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B: Environ 166:603–643. https://doi.org/10.1016/j.apcatb.2014.11.016
Cai M, Su J, Lian G, Wei X, Dong C, Zhang H, Jin M, Wei Z (2016) Sono-advanced Fenton decolorization of azo dye Orange G: analysis of synergistic effect and mechanisms. Ultrason Sonochem 31:93–200. https://doi.org/10.1016/j.ultsonch.2015.12.017
Chukowry PK, Mudhoo A, Santchurn SJ (2017) Bacillus algicola decolourises more than 95% of some textile azo dyes. Environ Chem Lett 15:531–536. https://doi.org/10.1007/s10311-017-0627-1
De Laat J, Gallard H (1999) Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: mechanism and kinetic modeling. Environ Sci Technol 33:2726–2732. https://doi.org/10.1021/es981171v
Diagne M, Oturan N, Oturan MA, Sirés I (2009) UV-C light-enhanced photo-Fenton oxidation of methyl parathion. Environ Chem Lett 7:261–265. https://doi.org/10.1007/s10311-008-0162-1
Divya N, Bansal A, Jana AK (2009) Degradation of acidic orange g dye using UV-H2O2 in batch photoreactor. Int J Biol Chem Sci 3:54–62. https://doi.org/10.4314/ijbcs.v3il.42735
Eren Z (2012) Ultrasound as a basic and auxiliary process for dye remediation: a review. J Environ Manag 104:127–141. https://doi.org/10.1016/j.jenvman.2012.03.028
Ganzenko O, Oturan N, Sirés I, Huguenot D, Van Hullebusch ED, Esposito G, Oturan MA (2018) Fast and complete removal of the 5-fluorouracil drug from water by electro-Fenton oxidation. Environ Chem Lett 16:281–286. https://doi.org/10.1007/s10311-017-0659-6
Guivarch E, Trevin S, Lahitte C, Oturan MA (2003) Degradation of azo dyes in water by Electro-Fenton process. Environ Chem Lett 1(1):38–44. https://doi.org/10.1007/s10311-002-0017-0
Khandelwal DH, Ameta R (2013) Use of photo-fenton reagent for the degradation of Basic yellow 2 in aqueous medium. Res J Recent Sci 2:39–43
Litter IM, Quici N (2010) Photochemical advanced oxidation processes for water and wastewater treatment. Recent Pat Eng 4:217–241. https://doi.org/10.2174/187221210794578574
Meetani MA, Rauf MA, Hisaindee S, Khaleel A, Alzamly A, Ahmed A (2011) Mechanistic studies of photoinduced degradation of Orange G using LC/MS. RSC Adv 1:490–497. https://doi.org/10.1039/c1ra00177a
Mousset E, Oturan N, Oturan MA (2018) An unprecedented route of ·OH radical reactivity: ipso-substitution with perhalogenocarbon compounds. Appl Catal B: Environ 226:135–156. https://doi.org/10.1016/j.apcatb.2017.12.028
Nidheesh PV, Zhou M, Oturan MA (2018) An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 197:210–227. https://doi.org/10.1016/j.chemosphere.2017.12.195
Oturan MA, Aaron J (2014) Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit Rev Environ Sci Technol 44:2577–2641. https://doi.org/10.1080/10643389.2013.829765
Oturan MA, Guivarch E, Oturan N, Sirés I (2008a) Oxidation pathways of malachite green by Fe3+-catalysed electro-Fenton process. Appl Catal B: Environ 82:244–254. https://doi.org/10.1016/j.apcatb.2008.01.016
Oturan N, Trajkovska S, Oturan MA, Couderchet M, Aaron JJ (2008b) Study of the toxicity of diuron and its metabolites formed in aqueous medium during application of the electrochemical advanced oxidation process “electro-Fenton”. Chemosphere 73:1550–1556. https://doi.org/10.1016/j.chemosphere.2008.07.082
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–284. https://doi.org/10.1080/10643380500326564
Saeed M, Ahmad A, Boddula R, Inamuddin Haq Au, Azhar A (2018) Ag@MnxOy: an effective catalyst for photo-degradation of rhodamine B dye. Environ Chem Lett 16:287–294. https://doi.org/10.1007/s10311-017-0661-z
Shanker U, Rani M, Jassal V (2017) Degradation of hazardous organic dyes in water by nanomaterials. Environ Chem Lett 15:623–642. https://doi.org/10.1007/s10311-017-0650-2
Shukla S, Oturan MA (2015) Dye removal via electrochemistry and oxidation using semiconductor oxides nanotubes. Environ Chem Lett 13:157–172. https://doi.org/10.1007/s10311-015-0501-y
Soon AN, Hameed BH (2011) Heterogeneous catalytic treatment of synthetic dyes in aqueous media using Fenton and photo-assisted Fenton process. Desalination 269:1–16. https://doi.org/10.1016/j.desal.2010.11.002
Srivastava S, Sinha R, Roy D (2004) Toxicological effects of malachite green. Aquat Toxicol 66:319–329. https://doi.org/10.1016/j.aquatox.2003.09.008
Tang WZ (2003) Advanced oxidation processes. In: Physicochemical treatment of hazardous wastes. CRC Press. ISBN: 9781566769273
Tiya-Djowe A, Nzali S, Njoyim ET, Laminsi S, Gaigneaux EM (2016) Thermal treatment of plasma-synthesized goethite improves Fenton-like degradation of orange II dye. Environ Chem Lett 14:515–519. https://doi.org/10.1007/s10311-016-0578-y
Trovó AG, Hassan AK, Sillanpää M, Tang WZ (2016) Degradation of acid blue 161 by Fenton and photo-Fenton processes. Int J Environ Sci Technol 13:147–158. https://doi.org/10.1007/s13762-015-0854-6
Turhan K, Ilknur D, Ozturkcan SA, Turgut Z (2012) Decolorization of textile basic dye in aqueous solution by ozone. Dyes Pigments 92:897–901. https://doi.org/10.1016/j.dyepig.2011.07.012
Verma P, Kumar S (2018) Microwave-enhanced advanced oxidation processes for the degradation of dyes in water. Environ Chem Lett. https://doi.org/10.1007/s10311-018-0739-2
Wang Y, Priambodo R, Zhang H, Huang YH (2015) Degradation of the azo dye Orange G in a fluidized bed reactor using iron oxide as a heterogeneous photo-Fenton catalyst. RSC Adv 5:45276–45283. https://doi.org/10.1039/C5RA04238K
Acknowledgements
The authors are grateful to the “Service de la Cooperation et d’Action Culturelle” (SCAC) at the French Embassy in Cameroon for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarkwa, JB., Oturan, N., Acayanka, E. et al. Photo-Fenton oxidation of Orange G azo dye: process optimization and mineralization mechanism. Environ Chem Lett 17, 473–479 (2019). https://doi.org/10.1007/s10311-018-0773-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-0773-0