Abstract
Nanomaterials may help to solve issues such as water availability, clean energy generation, control of drug-resistant microorganisms and food safety. Here we review innovative approaches to solve these issues using nanotechnology. The major topics discussed are wastewater treatment using carbon-based, metal-based and polymeric nanoadsorbents for removing organic and metal contaminants; nanophotocatalysis for microbial control; desalination of seawater using nanomembranes; energy conversion and storage using solar cells and hydrogen-sorbents nanostructures; antimicrobial properties of nanomaterials; smart delivery systems; biocompatible nanomaterials such as nanolignocellulosis and starches-based materials, and methods to decrease the toxicity of nanomaterials. Significantly, here it is reviewed two ways to palliate nanomaterials toxicity: (a) controlling physicochemical factors affecting this toxicity in order to dispose of more safe nanomaterials, and (b) harnessing greener synthesis of them to bring down the environmental impact of toxic reagents, wastes and byproducts. All these current challenges are reviewed at the present article in an effort to evaluate environmental implications of nanomaterials technology by means of a complete, reliable and critical vision.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At the nanoscale range, matter shows extraordinary properties that are not shown by bulk materials, due to its increased relative surface area and the dominance of quantum effects in this size range. Altered properties typically result in enhanced chemical reactivity with regard to adsorption and redox processes (Hochella et al. 2008). Boosted biological activity and catalytic behavior compared with larger particles of the same chemical composition are also reported (Garnett and Kallinteri 2006; Nel et al. 2006; Limbach et al. 2007). Importantly, nanoparticles also have greater access to our body’s cells tissues and organs that larger particles of the same material, that means high bioavailability, introducing so new risks of human health and the environment (Sharifi et al. 2012).
Nanoscale science, engineering and technology embrace an exciting and broad scientific frontier with significant impact on nearly all aspects of the global economy, industry and people’s life in the twenty-first century (Gruère et al. 2011; Scott and Chen 2003). Novel properties of nanomaterials have offered many new general applications in strategical areas such as life sciences, pharmacy, food industry, environmental technology and protection, renewable energy, biotechnology science, medicine technologies like neural and brain ones and water (drinking and waste) (Helmut Kaiser Consultancy Group 2015).
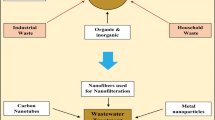
With regard to environmental issues, nanoscale science can specifically help to resolve relevant challenges currently raised such as: (1) wastewater remediation and desalination treatments, (2) alternative clean energy generation to fossil combustibles, (3) control of drug-resistant microorganisms, (4) agricultural applications (Gruère et al. 2011) and (5) sustainable chemical synthesis (Fig. 1). This review is focused to show how nanomaterials science can play nowadays a fundamental role to offer innovative solutions in all these environmental areas, addressing also their associated toxicological risks as well as their control.
Nanomaterials science can play a significant role to solve relevant challenges in some environmental issues such as water quality and availability, clean energy generation, control of drugs resistant microorganisms and sustainable agriculture. Their uncertain toxicity can be palliated by means of controlling physicochemical factors affecting toxicity and by harnessing greener synthesis of them
The provision of clean and affordable water for human needs is a critical challenge in the twenty-first century. Worldwide, water supply struggles to keep up with the fast growing demand, which is exacerbated by population growth, global climate change and water quality deterioration (Xiaolei et al. 2013). Thus, technological innovation with regard to conventional strategies is needed. Nanotechnology holds great potential in advancing wastewater treatments efficiency as well as to augment water supply. This review affords several nanotechnological solutions to resolve these problems like the wastewater treatments where carbon-based, metal-based and polymeric nanoadsorbents have shown great efficiency for removing organic and heavy metal contaminants (Yang et al. 2008; Yang and Xing 2010; Auffan et al. 2008). Nanophotocatalysis has been also used for the treatment of non-biodegradable organic compounds and microbial pathogens in wastewaters, since disinfection of water is another issue where nanomaterials can perform a relevant role (Ditta 2012; Baruah et al. 2016). Lastly, desalination of seawater will likely a major option in the next future and different strategies have considered the incorporation of nanomaterials into membranes to improve the already existing reverse osmosis technology (Pendergast and Hoek 2011; Agboola et al. 2014).
Efficient and sustainable production of energy is nowadays one of the technological goals. The use of nanomaterials for energy conversion and storage is focused in two important topics that are solar cells and hydrogen storage (Zhang et al. 2013). The performance of solar cells can be improved using the unique optical and/or electrical properties of nanomaterials, which basically act increasing optical absorption and improving the electron transport in the solar cell film through new mechanisms or materials (Tetreault et al. 2011; Beard et al. 2010). Hydrogen storage is seen as a new source of clean energy, and nanostructures can be promising sorbents for it. The strategies are based on providing high surface areas and/or trapping hydrogen in microporous media standing out carbon-based, metal–organic frameworks (Sculley et al. 2011) and dual-phase nanocomposite materials (Choucair and Mauron 2015; Yadav et al. 2015).
Nanomaterials are currently considered as a promising approach for controlling drugs resistant microorganisms. This review reports a snapshot about the research conducting in this field focusing on nanomaterials (Santos et al. 2013; Yallappa et al. 2015; Ranjan and Ramalingan 2016), microorganisms and mechanisms of action responsible of antimicrobial behavior (Hajipour et al. 2012; Dasgupta and Ramalingam 2016).
Presently, the agricultural sector is facing various global challenges: climate change, urbanization, sustainable use of resources and other environmental issues such as runoff and accumulation of pesticides and fertilizers. In this sense, nanotechnology can provide tools for a sustainable agricultural development: new products such as nanoherbicides, nanopesticides and nanofertilizers designed by means of new strategies and materials like nanoencapsulation, nanoclays, nanocomposites and nanoparticles. They are smart delivery systems designed with the aim to decrease the amount of needed product and its environmental associated hazards (Chen and Yada 2011). They offer advantages such time releasing or release upon the occurrence of an environmental trigger (temperature, humidity, light) (Ditta 2012; Nanotechnology in Agriculture 2013). There have been also stood out biocompatible nanomaterials like nanolignocellulosis-based ones and starches nanocontainers, traditionally harvested materials, non-toxic and biodegradable, with enhanced physicochemical properties for industrial purposes that even could replace fiberglass and plastics (Balestra 2014; Laborie 2009).
We can conclude from the above statements that implementation of engineers nanomaterials will suppose great advantages, but also a great risk that should be considered: their toxicity is still uncertain. The aim of this section is to contribute to understand physicochemical factors responsible of nanomaterials toxicity such as size, shape and morphology, surface charge, composition, coatings, surface roughness, aggregation and whole reactivity (Sharifi et al. 2012). These physicochemical parameters determine nanoparticles’s mobility, bioavailability, biological uptake and lastly toxicity, that are responsible, in turn, of physiological responses like endocytosis, cell membrane damages and reactive oxygen species (Regulatory Considerations 2014). This knowledge can help to engineer more safe nanomaterials aiding to control their environmental negative impacts too. On the other hand, the synthesis of such nanomaterials using biocompatible reagents like plant extracts and microorganisms as reducing and capping agents could decrease the toxicity of the resulting materials and the environmental impact of the byproducts (Sanchez-Mendieta and Vilchis-Nestor 2012; Varma 2012). So, the field of nanomaterials green design is a research area of growing interest from a sustainable point of view as a contribution for environmental safety (Kharissova et al. 2013; Sukla and Iravani 2017).
Water quality and availability
Nowadays, provision of clean and abundant freshwater is one of the most important challenges faced by the world for both human use and industrial applications (Vörösmarty et al. 2010). Furthermore, large amounts of freshwater are required in agriculture, but in turn, it contributes to groundwater pollution through the use of pesticides, fertilizers and other agricultural chemicals. The research and development in nanotechnology have enabled us to find novel and economically feasible solutions for remediation and purification of this wastewater. On the other hand, accessible water resources are often contaminated with waterborne pathogenic microorganisms like cryptosporidium, coliform bacteria, virus, various salts and metals (Cu, Pb, As, etc.), compounds such as pharmaceuticals and personal care products, endocrine disrupting compounds and radioactive contaminants, either naturally occurring or as the result of anthropogenic activities (Ditta 2012). For improving water quality, nanotechnology has provided novel solutions which are discussed below.
Wastewater treatment
Carbon-based, metal-based and polymeric nanoadsorbents
Adsorption is a very useful strategy to remove organic and inorganic contaminants in wastewater treatment. In the adsorption process, the adsorbents having both a large adsorption capacity and adsorption rate are preferable (Duman and Ayranci 2006).The most commonly used adsorbent for the treatment of organic compounds such as phenolic ones (Ayranci and Duman 2005), anilinic ones (Duman and Ayranci 2005), aromatic organic acids (Ayranci and Duman 2006), surfactants (Ayranci and Duman 2007; Duman and Ayranci 2010a), dyes (Ayranci and Duman 2009), benzene and naphthalene sulfonates (Ayranci and Duman 2010) and also for the treatment of inorganic compounds such as metal ions (Duman and Ayranci 2010b), in industrial wastewater treatment systems, has been activated carbon due to its large specific surface area (Duman et al. 2015). Nevertheless, the efficiency of conventional adsorbents is usually limited by the surface area or active sites, the lack of selectivity and the adsorption kinetics. In this sense, nanoadsorbents offer significant improvement with their extremely high specific surface area and associated sorption sites, short intraparticle diffusion distance, tunable pore size and surface chemistry. There are mainly three approaches: carbon-based, metal-based and polymeric nanoadsorbents (Xiaolei et al. 2013; Xue et al. 2017).
Carbon-based nanoadsorbents have shown a great efficiency for organic removal. For instance, carbon nanotubes present high adsorption capacity because of their larger specific surface area and of the diverse organic contaminants–carbon nanotube interactions (hydrophobic, electrostatic, π–π, hydrogen and covalent bonding, etc.) (Yang and Xing 2010). So, the rich π electron surface of carbon nanotubes allows π–π interactions with organic molecules with C=C bonds or benzene rings, such as polycyclic aromatic hydrocarbons and polar aromatic compounds. Organic compounds with carboxyl, hydroxyl or amine functional groups could also form hydrogen bonds with the graphitic carbon nanotube surface which donates electrons (Yang et al. 2008). As mentioned before, electrostatic attraction facilitates the adsorption of positively charged organic chemicals such as some antibiotics at suitable pH (Ji et al. 2009).
Oxidized carbon nanotubes can also present a high efficiency for heavy metal removal due to their adsorption capacity for metal ions with fast kinetics. The surface functional groups (e.g., carboxyl, hydroxyl and phenol) of carbon nanotubes are the major adsorption sites for metal ions, mainly through electrostatic attraction and chemical bonding (Rao et al. 2007). As a result, surface oxidation can significantly enhance the adsorption capacity of carbon nanotubes. Several studies show that carbon nanotubes are better adsorbents than activated carbon for heavy metals (e.g., Cu2+, Pb2+, Cd2+ and Zn2+) (Lu et al. 2006) since the adsorption kinetics is faster on carbon nanotubes due to the highly accessible adsorption sites and the short intraparticle diffusion distance. In addition, the surface modification of oxidized carbon nanotubes with anionic polysaccharides is an alternative way to improve the adsorption performance of carbon nanotubes for the removal of cationic pollutants from waters (Duman et al. 2016a, b).
Metal-based nanoadsorbents such as iron oxide, titanium dioxide and alumina are effective and low-cost adsorbents for heavy metals like arsenic, lead, mercury, copper, cadmium, chromium, and nickel (Sharma et al. 2009) and radionuclides. The sorption is mainly based in a complexation reaction between dissolved metals and the oxygen in metal oxides (Koeppenkastrop and Decarlo 1993). This process involves a fast adsorption of metal ions on the external surface, followed by the rate-limiting intraparticle diffusion along the micropore walls (Trivedi and Axe 2000). At nanoscale, this process presents higher adsorption capacity and faster kinetics because of the higher specific surface area, shorter intraparticle diffusion distance and larger number of surface reaction sites (i.e., corners, edges, vacancies). For instance, as the particle size of nanomagnetite decreased from 300 to 11 nm, its arsenic adsorption capacity increased more than 100 times (Auffan et al. 2008, 2009), suggesting a “nanoscale effect.” This “nanoscale effect” was attributed to the change of magnetite surface structure which creates new adsorption sites (vacancies) (Auffan et al. 2009). In addition to high adsorption capacity, some iron oxide nanoparticles, e.g., nanomaghemite and nanomagnetite, can be superparamagnetic. As the size decreases, magnetic particles become superparamagnetic, which allows easy separation and recovery by a low-gradient magnetic field. These magnetic nanoparticles can be either used directly as adsorbents or as the core material in a core–shell nanoparticle structure (Fig. 2), where the shell provides the desired function while the magnetic core performs the magnetic separation (Yavuz et al. 2006).

From Xiaolei et al. (2013), with permission from Elsevier
Multi-functional magnetic nanoparticles. These magnetic nanoparticles can be either used directly as adsorbents or as the core material in a core–shell nanoparticle structure where the shell provides the desired function while the magnetic core realizes magnetic separation. Silica coating helps functionalization due to the rich silica chemistry.
With regard to polymeric nanoadsorbents, recent works have been focussed on dendrimers, tailored adsorbents capable to remove both organics and heavy metals. Their interior shells can be hydrophobic for sorption of organic compounds, while the exterior branches can be tailored (e.g., hydroxyl- or amine-terminated) for adsorption of heavy metals. The sorption can be based on complexation, electrostatic interactions, hydrophobic effect and hydrogen bonding. A dendrimer ultrafiltration system was designed to recover metal ions from aqueous solutions (Diallo 2009). This system achieved almost complete removal of Cu2+ ions with an initial concentration of 10 ppm by polyamidoamine dendrimer–NH2 ratio of 0.2. After adsorption, the metal ions laden into dendrimer were recovered by ultrafiltration and regenerated by decreasing pH to 4.
Nanophotocatalysis
One of the main applications of nanoparticles is their ability to act as catalysts in presence of light. The mechanism of this reaction is based on the exposure to ultraviolet light of an absorbing nanosized compound; thus, the electrons in the outermost shell (valence electrons) are excited resulting in the formation of electron hole pairs. Nanoparticles that work as excellent oxidizable agents are metal oxides like TiO2, ZnO, SnO2 as well as sulfides like ZnS (Ditta 2012), since a good photocatalyst should absorb light efficiently in the visible or near ultraviolet part of the electromagnetic spectrum, as they do. Sufficient electron vacant states need to be present also to inhibit recombination of electron hole pairs upon light exposure. Nanostructured photocatalysts offer large surface-to-volume ratios allowing higher adsorption of the target molecules too, reason why these ones have very efficient rate of degradation and disinfection, presenting also a tremendous increase in their chemical reactivity and other physicochemical properties related to photocatalysis, photoluminescence, etc. Intensive research over the past decade for theirs implementation in the purification of drinking water (Mahmood 2011) or even industrial and agricultural effluents (Gaya and Abdullah 2008) can be found in the literature.
Photocatalytic oxidation is therefore an advanced oxidation process for removal of trace contaminants and microbial pathogens in wastewater. It is also a useful pretreatment for hazardous and non-biodegradable organic contaminants to enhance their biodegradability. The major barrier for its wide application is the slow kinetics due to limited light fluency and photocatalytic activity. Current research focuses on increasing photocatalytic reaction kinetics and photoactivity range (Xiaolei et al. 2013). TiO2 is the most widely used photocatalyst in wastewaters treatment owing to its low toxicity and chemical stability. It generates an electron/hole (e−/h+) pair upon absorbs an ultraviolet photon, which later can migrate to the surface and forms reactive oxygen species or either undergoes undesired recombination. The photoactivity of nano-TiO2 can be improved by optimizing particle size and shape, reducing e−/h+ recombination by noble metal doping, maximizing reactive facets and with surface treatment to enhance contaminant adsorption. The size of TiO2 plays an important role in its solid-phase transformation, sorption and e−/h+ dynamics. With regard to crystal structure, rutile is the most stable for particles larger than 35 nm, while anatase (more efficient for producing reactive oxygen species) is the most stable for those smaller than 11 nm (Xiaolei et al. 2013; Fujishima et al. 2008).
WO3 doped with Pt has also shown potential to be used in photocatalytic water treatment (Kim et al. 2010). Fullerene derivatives, such as aminofullerenes can also generate 1O2 under visible light irradiation (<550 nm), that has been employed to degrade pharmaceutical compounds and inactivate viruses (Lee et al. 2010). Fullerol and fullerenes encapsulated with poly(N-vinylpyrrolidone) can produce 1O2 and superoxides under ultraviolet light (Brunet et al. 2009). Nevertheless, fullerenes are currently much more expensive and not as readily available as TiO2. A noticeable report on the topic can be found in the recent review written by Baruah et al. (2016).
Disinfection and microbial control
Nanoparticles are able to exhibit a powerful long known antimicrobial action; this will be extensively later afforded. Antimicrobial nanomaterials are envisaged to find their applications in three critical challenges in wastewater systems: disinfection, membrane biofouling control and biofilm control on other relevant surfaces (Xiaolei et al. 2013). Ag nanoparticles (nano-Ag) have a great potential for application in water disinfection treatments. Ag nanoparticles have been incorporated into ceramic microfilters as a barrier for pathogens (Peter-Varbanets et al. 2009). Commercial devices using it are already available, e.g., MARATHON® and Aquapure® systems. The antimicrobial properties, fibrous shape and high conductivity of carbon nanotubes enable novel filters for both bacteria and virus removal. The thin layer of carbon nanotubes can effectively remove bacteria and viruses by size exclusion and depth filtration mechanisms, respectively (Brady-Estevez et al. 2010). With a small intermittent voltage, multi-walled carbon nanotubes can directly oxidize attached bacteria and viruses leading to their inactivation in seconds (Rahaman et al. 2012; Vecitis et al. 2011). The application of nanomaterials in membrane biofouling control is next addressed in “Desalination” section.
Desalination
Due to limited resources of freshwater, likely in the near future, desalination of seawater will become a major source of freshwater. Conventional desalination technologies like reverse osmosis are being used now, but these are costly due to the large amount of energy required. Nanotechnology can play a very important role for developing a number of low-energy alternatives, among which the most promising are protein-polymer biomimetic membranes, aligned-carbon nanotube membranes and thin-film nanocomposite membranes (Hoek and Ghosh 2009). A significant number of studies on membrane nanotechnologies are focussed on creating synergism by adding nanomaterials into polymeric or inorganic membranes; such materials are usually metal oxides (Al2O3, TiO2 and zeolites), some of them with photocatalytic, antimicrobial activity, etc. (Ag, carbon nanotubes, etc.). The goal of this strategy is to reduce fouling by increasing the hydrophilicity of the membrane and water permeability. These inorganic nanoparticles also help enhance the mechanical and thermal stability of polymeric membranes, reducing the negative impact of compaction and heat on membrane permeability (Pendergast et al. 2010). These technologies have shown up to 1000 times better desalination efficiencies than reverse osmosis since they have high water permeability.
Some of these membranes are involved in the integration of other processes like disinfection, deodorizing, de-fouling and self-cleaning. These technologies may be introduced in the market place in the near future, but scale-up fabrication, practical desalination effectiveness and long-term stability are the most critical challenges to be considered before their successful commercialization. A recent review ranked current membrane nanotechnologies based on their potential performance enhancement and state of commercial readiness (Pendergast and Hoek 2011). This review also includes comparative tables between conventional reverse osmosis and nanotechnology-based membranes such as inorganic (zeolite thin-film nanocomposite), organic (aquaporin biomimetic) and mixed matrix (carbon nanotubes biomimetic), reporting about their performance in terms of salt rejection and water permeability. Characterization of nanofiltration membranes by methods such as scanning electron microscopy, thermal gravimetric analysis, attenuated total reflection Fourier transform infrared spectroscopy and atomic-force microscopy can help to advance in the design and development of membrane chemical structures and properties (Agboola et al. 2014). Recent advances have also included complex mathematical prediction studies of nanofiltration membranes performances under different operating conditions (Agboola et al. 2015).
Energy conversion and storage
Energy generation from conventional fossil fuels has been identified as the main culprit of environmental quality degradation and environmental pollution. In order to address these issues, nanotechnology plays an essential role for revolutionizing the device applications in energy conversion and storage. Some of the most promising topics in this area are next exposed.
Solar cells
The basic design principle for solar cells is to increase the optical absorption of the active layer and/or reduce the electron leak during transport. Nanostructures can be employed to improve the performance of solar cells by four different approaches (Zhang et al. 2013):
-
(a)
Developing new mechanisms, such as the multiple exciton generation effect in quantum dots. The multiple exciton generation effect describes the generation of two or more excitons with one photon excitation, in contrast with the conventional case where excitation by one photon can only produce a single electron–hole pair (or exciton). Taking this effect into account, the maximum efficiency for quantum dot solar cells has been predicted to be about 42% (Beard et al. 2010; Nozik 2008) which is a value much higher than the Shockley–Queisser efficiency limit for any type of bulk semiconductor-based single junction solar cell (31%).
-
(b)
Providing larger surface area, like the nanocrystalline films used for dye-sensitized solar cells. Many nanostructures such as nanoparticles, nanowires/nanorods, nanotubes and core–shell structures of oxides like TiO2, ZnO, SnO2 and Nb2O5 have been extensively studied for dye-sensitized solar cells, with the aim to increase the internal surface area in the photoelectrode film, but also for giving other functions to enhance the optical absorption or electron transport (Zhang et al. 2012).
-
(c)
Generating unique optical effects to either reduce the light losses or enhance the optical absorption (Zhang et al. 2013). With this aim nanostructures have been employed to improve the performance of solar cells through, for example, in the following ways: (1) serving as an antireflection layer to reduce the loss of incident light at the front interface of solar cells, (2) generating surface plasmon resonance or (3) causing light scattering to enhance the optical absorption of the active layer in dye-sensitized solar cells.
-
(d)
Improving the electrons transport and/or their collection through the use of (1) one-dimensional nanostructures, such as ZnO nanowires/nanorods and TiO2 nanotubes, especially useful in polymer solar cells and dye-sensitized solar cells, for reducing the recombination of photogenerated electrons with holes before to get the collecting electrode or (2) purposely designed three-dimensional structures, such as host–passivation–guest hollow core–shell spheres, to exploit the high-electron-mobility material for achieving highly efficient electrons transport and collection in dye-sensitized solar cells, as recently developed by Tetreault et al. (2011).
Hydrogen storage
Hydrogen is seen as an important new source of energy due to its inherent pollution-free nature to clean combustion, resource availability and provision of the highest energy efficiency (Lim et al. 2010). Hydrogen storage materials are nowadays subject of intensive investigation and research, standing out exhaustive reviews on the topic (Sculley et al. 2011; Liu et al. 2010). These materials should simultaneously satisfy the following requirements: relatively high storage density, moderate decomposition temperatures (60–120 °C), good reversibility, fast kinetics of the hydrogen absorption and desorption process and low manufacturing cost. Research in solid-state hydrogen storage appears to provide the highest probability for producing a material that can satisfy these generic criteria (Zhang et al. 2013).
Nanostructures are promising sorbents for hydrogen storage (McNicholas et al. 2010) by reducing the gravimetric and the volumetric storage densities. Storage mainly occurs through a process called physisorption, where the forces involved are weak intermolecular forces, which generally resulting in fast kinetics and reversibility, that means that significant hydrogen adsorption often takes place only at a cryogenic temperature. Nanostructured materials may offer advantages over bulk materials for molecular hydrogen storage by providing high surface areas or by encapsulating or trapping hydrogen in microporous media since the increased surface area and porosity in nanostructures offer additional binding sites that could increase storage capacity. With this aim, different nanostructured materials such as porous carbons, carbon nanotubes, graphene, zeolites, metal–organic frameworks, polymers with intrinsic microporosity and nanocomposites have been studied. All of them are exposed in the exhaustive review of Zhang et al. (2013).
With regard carbon-based nanostructures, carbon nanotubes have shown potential advantages to store hydrogen, mainly carbon nanotubes composite storage materials, such as single-walled carbon nanotubes doped with alkali or transition metals and single-walled carbon nanotubes coated with metal hydrides (Chin et al. 2012). These carbon nanotubes composite storage materials have shown high hydrogen storage capacities, in addition to the advantages of storing and releasing hydrogen at ambient conditions, as well as the ability of metal hydrides to adsorb certain amount of additional hydrogen molecules (Durgun et al. 2008; Lochan and Head-Gordon 2006). Different metal hydrides have been coated on single-walled carbon nanotubes to facilitate the adsorption of hydrogen molecules and to increase the hydrogen storage capacity like aluminum hydride, boron hydride, lithium hydride and nickel hydride (Surya et al. 2009, 2010; Iyakutti et al. 2009). Recent studies were developed on carbon aerogels, another class of amorphous porous carbon structures with high surface area (Robertson and Mokaya 2013; Biener et al. 2011), and on graphene nanosheets (Pumera 2011) trying to optimize and to improve storage capacity and the sorption–desorption kinetics.
There has been a recent increase in research concerning metal organic frameworks, which are microporous materials consisting of metal cluster building units connected by organic ligands. These materials have high crystallinity, high purity and very high specific surface areas with controllable pore sizes. The structure and chemistry can be easily tuned by varying the metal clusters, length and functionalization of the organic ligands. For hydrogen storage, the strategy is the enhancement of the number of adsorption sites in a material for increasing the adsorption of hydrogen. This can be accomplished by using thin organic ligands with constituents available for gas adsorption. A reference table with all the recently reported metal organic frameworks for hydrogen storage and the effects of their sample preparation and activation on their hydrogen storage performance is discussed in an exhaustive review written by Sculley et al. (2011).
There are noticeable efforts for developing a dual-phase nanocomposite hydrogen storage material, where the two phases consist of a highly porous support material and a hydride hydrogen storage material. As hydride materials sodium aluminum hydride (NaAlH4), ammonia borane (NH3BH3) and lithium borohydride (LiBH4) have been reported. As porous materials have been explored graphene, carbon aerogels and nanoporous silica scaffolds among others, because of their high surface area and pore volume. They can contribute significantly to a solid-state hydride-based hydrogen storage composite material by providing a structural support matrix, as well as size confinement for hydrides and a percolated heat conduction network (Choucair and Mauron 2015; Yadav et al. 2015; Kim et al. 2009). A scheme illustrating a coherent nanocomposite of ammonia borane (AB) within a carbon cryogel network (CC) is depicted in Fig. 3. AB has high gravimetric and volumetric hydrogen content, as well as a reasonable decomposition temperature. The addition of AB to the carbon cryogel resulted in hydrogen release at a much lower temperature than pure AB, showing evidence of faster kinetics.

Reproduced from Zhang et al. (2013), with permission of The Royal Society of Chemistry
a Illustration of a dual-phase nanocomposite hydrogen storage material, consisting of a carbon cryogel network (CC) as a highly porous support material and a hydride (ammonia borane, AB) as storage hydrogen, b comparison of dehydration peaks between AB and CC–AB nanocomposites at different pore size taken at 5 °C min−1. The addition of AB and CC resulted in hydrogen release at a much lower temperature than pure AB, showing evidence of faster kinetics.
Antimicrobial activity
Drug-resistant microorganisms are a serious and increasing public health problem. New strategies for controlling bacteria activity are urgently needed, and nanomaterials can be a very promising approach (Santos et al. 2013). This section focuses on nanotechnology strategies for inhibiting cellular adhesion and attachment, for interfering on bacterial physiology and communication (quorum sensing) and for avoiding biofilm development. The antimicrobial activity of nanomaterials is certainly a function of their size and other features such as high surface area, unusual crystal morphologies (edges and corners) and reactive sites (Allaker 2010) (see “Toxicity section” for a complete explanation). Although the whole mechanism is not fully understood yet, there are four major hypothesis considered as responsible of antimicrobial behavior of nanomaterials (Chwalibog et al. 2010; Sweet et al. 2012). They are:
Cell membrane damage
The mechanism of action of nanoparticles on cell membranes is recognized as non-specific, but there is no doubt about cell permeability is altered in contact to nanoparticles. Experiments indicated the formation of a “hole” or “pore” in the living cell membranes as a possible mechanistic hypothesis (Leroueil et al. 2007). Although the meaning of the term “hole” or “pore” still requires clarification, images of cell damage have given clear evidence of this effect. In more serious or extreme cases, a literal hole in the bilayer membrane is located which promotes the complete loss of the plasma membrane (Leroueil et al. 2007). These membrane ruptures have been confirmed by membrane integrity tests and by scanning electron microscopy images in a recent work developed by Dasgupta and Ramalingam (2016), where nanosilver antimicrobial mechanism was reported.
Release of toxic ions
It has been demonstrated that Cd2+, Zn2+ and Ag+ ions can react in bacteria with different groups of proteins. The ability of Ag+ to form low solubility salts is also considered as one of its major mechanisms for attacking bacteria cells. For instance, cell respiration is inhibited when the chloride ions precipitate as silver chloride in the cytoplasm of the cells. The antimicrobial efficiency of silver nanoparticles is also well known, especially against Gram-negative bacteria such as Escherichia coli since silver ions penetrate into cells interfering in their metabolic systems. Thus, there are evidences that Ag+ ions can also damage deoxyribonucleic acid by inhibiting its replication (Niskanen et al. 2010). Cd2+ and Zn2+ ions can also bind to sulfur-containing proteins of the cell membrane and interfere in cell permeability.
Interruption of electron transport, protein oxidation and membrane collapse
There is a strong evidence that the positive charge of nanoparticles is critical for antimicrobial activity since bacteria cell membrane is negatively charged. Although the clear mechanism is still under debate, it has been suggested that ions (e.g., silver) can affect respiratory enzymes membrane bound as well as affect efflux bombs of ions that can result in cell death (Allaker 2010). The contact of bacteria with CeO2 or fullerenes has also given insights about key enzymes that can be inactivated. Upon this mechanism, the cascade of events after nanoparticle contact with bacteria can start with possible oxidation of respiratory enzymes, which facilitate the production of reactive oxygen species and radical species that will eventually affect cell physiology and promote deoxyribonucleic acid degradation (Xia et al. 2008).
Generation of reactive oxygen species
The oxygen is a powerful oxidant agent, and although it results to be the best acceptor of electrons during respiration, it can be lethal for some bacteria. During many processes, H2O2 is formed by the respiratory burst which consumes O2 with the production of free radicals. Hence, the production of free hydroxyl radicals is certainly the basis of hydrogen peroxide action which leading to oxidation of deoxyribonucleic acid, proteins and membrane lipids (Hajipour et al. 2012). Bacteria affected by reactive oxygen species lose the integrity of their membranes progressively, presenting oxidative stress related to them, which affects their functions and communication capacities (Dasgupta and Ramalingam 2016). Nano-Ag is the most applied antimicrobial nanomaterial. Its strong antimicrobial activity, broad antimicrobial spectrum, low human toxicity and ease of use make it a promising choice for water disinfection and microbial control. It is now well accepted that the antimicrobial activity of nanosilver largely stems from the release of silver ions (Xiu et al. 2011, 2012), which can damage enzymes and even prevent deoxyribonucleic acid replication. Thus, the release rate and bioavailability of silver ions are crucial for the toxicity of nano-Ag. Studies have suggested physicochemical properties of nano-Ag play an important role in its antimicrobial activity (as it will be explained in “Toxicity” section). So, the size, shape, coating and crystallographic facets of nanomaterial strongly affect the release kinetics of silver ions. The presence of common ligands reduces the bioavailability of silver ions and mitigates their toxicity (Xiu et al. 2011). A recent study carried out by Dasgupta and Ramalingam (2016) explains in detail the antimicrobial activity of nano-Ag as a result of a sequence of events that begins with reactive oxygen species generation, followed by damage in DNA, mitochondria, protein and membrane rupture (Fig. 4).

Reproduced from Hajipour et al. (2012), with permission from Elsevier
Mechanisms of toxicity of nanomaterials against bacteria’s cell. Ions of nanoparticles (e.g., silver and zinc) produce free radicals, resulting in induction of oxidative stress related to reactive oxygen species. These reactive oxygen species lead to irreversibly damage in bacteria, for instance, DNA in nucleus, mitochondria, protein and cell membrane disruption, bringing bacterial death.
Nanomaterials subject of antimicrobial studies have been mainly metallic nanoparticles like silver, copper, gold, bismuth and even some alloys of them (e.g., Au–Ag) (Table 1). Several nanoparticles oxides such as TiO2, CuO, ZnO, Fe3O4 and even composites of iron oxides have also shown antimicrobial activity, mainly antibacterial one (Ranjan and Ramalingan 2016). It must be pointed out some of these metals and metal oxides have been coated onto several materials. Other strategies lie to incorporate these metals into a substrate such as polymethylmethacrylate or dental restorative materials (Boldyryeva et al. 2005; Aruguete et al. 2013). According to some recent reports, carbon-based nanomaterials such as fullerenes, carbon nanotubes, especially single-walled carbon nanotubes and graphene oxide nanomaterials exhibit also potent antimicrobial properties (Table 1). Carbon nanotubes kill bacteria by causing physical perturbation of the cell membrane, oxidative stress or disruption of a specific microbial process (Vecitis et al. 2010) upon direct contact with bacterial cells. Graphene and graphite materials exhibit also antimicrobial properties through similar mechanisms (Liu et al. 2011). A later review written by Dizaj et al. (2015) summarized the antimicrobial activity of these carbon-based nanoparticles together with their mechanism of action.
With respect microorganisms, the most usual candidates for microbial experiments are bacteria and fungi such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia, Bacillus subtilis. Among fungi species, different Candida strains such albicans (I and II), tropicalis and parapsilosis, Trychophyton mentagrophytes were the most common. An exhaustive overview of antimicrobial activity exhibited by different nanomaterials species on bacteria and fungi is summarized in Table 1.
Sustainable agriculture and food production
Plant-based agricultural production is the basis of broad agriculture systems providing food, fibber, fire (thermal energy) and even fuel, through advancements in materials science and biomass conversion technologies. While the demand for crop yield will rapidly increase in the future, the natural resources such as land, water and soil fertility are limited. Other production inputs including synthetic fertilizers and pesticides are predicted to be much more expensive due to constrains of known petroleum reserves. So, precision farming is hence an important area of study to reduce production inputs and maximize agriculture production outputs. Given that nanotechnology may allow the precise control of manufacturing at the nanometer scale, novel techniques nanotechnology-based are available for the improvement of precision farming sustainable practices (Chen and Yada 2011; Ditta 2012; Nanotechnology in Agriculture 2013). In this section, some potential applications of nanoscale science, engineering and technology for agriculture are discussed; despite a wide industrial interest in this area, there are not too many available commercial products yet. Most applications are still in research and development stage.
Nanoscale carriers
Nanoscale carriers can be utilized for the efficient delivery of fertilizers, pesticides, herbicides, plan growth regulators, etc. Mechanisms involved in the efficient delivery, better storage and control release include: encapsulation and entrapment, polymers and dendrimers, ionic surface and weak bond attachments among others (Ditta 2012). These mechanisms help improve stability against degradation in the environment and reduce the amount to be applied which also reduces chemical runoff and alleviates environmental problems. The nanoscale delivery “vehicles” may be designed to anchor to plant roots or to the surrounding soil structures and organic matter by means of molecular or conformational affinity between the delivery nanoscale carrier and the targeted structures and matters in soil (Johnston 2010). Controlled release mechanisms allow the active ingredients to be slowly taken up, hence avoiding temporal overdose, reducing the amount of used chemical and minimizing the input and waste. These strategies have been involved in the controlled delivery of nanopesticides, nanoherbicides, nanofertilizers, seed germinators, etc.
Nanopesticides
In order to protect the active ingredient from the adverse environmental conditions and to promote persistence, a nanotechnology approach “nanoencapsulation” is used. Nanoencapsulation comprised nanosized particles of the active ingredients within a thin-walled sac or shell (protective coating). The shell can be constituted by different elements, such as polymers, lipids, viral capsids or nanoclays. Its main function is to protect the active compound until it is released, but it can also improve the solubility and the penetration of the compound into the plant tissues. Nanoencapsulation of insecticides, fungicides or nematicides help to produces a formulation which offers effective control of pests while preventing accumulation of residues in soil. Several pesticides’s manufacturers are developing nanoencapsulated pesticides (OECD and Allianz 2008). These pesticides may be time released or released upon the occurrence of an environmental trigger (temperature, humidity, light) that means “a controlled release of the active ingredient”, which would improve the effectiveness of the formulation and thus decrease the amount of needed pesticide and its environmental associated hazards. So, for instance, carbofuran (Table 2) is one of the most toxic carbamate pesticides, it can be manufactured as polymeric nanocapsules, where the rate release of active ingredient can be adjusted from 7.5 to 55 days depending on the amount and molecular weight of used polymer (Regulatory Considerations 2014). Natural Nano, a start-up company from Rochester, has found a way to apply halloysite, a natural clay nanotube (aluminosilicate nanotube), which is used as nanocarrier for pesticides, with low costs, extended release and better contact with plants. It is estimated this technology could reduce the amount of applied pesticide in a 70–80% range, decreasing the costs of chemical product as well as the impact in water streams (Murphy 2008).
Porous hollow silica nanoparticles (Table 2) are also promising carriers for applications requiring sustained pesticide release, especially for photosensitive active ingredients. They have a shell thickness of approximately 15 nm, a pore diameter of 4–5 nm and facilitate a high pesticide loading. The UV shielding properties of porous hollow nanoparticle have been demonstrated to improve significantly the photostability of avermectin entrapped in the hollow core of the nanoparticle carrier besides a time-release behavior (Li et al. 2007). Controlled delivery from porous hollow silica has been also reported for the water-soluble pesticide validamycin (Liu et al. 2006). Moreover nanoparticles of defined concentrations could be successfully used for the control of several plant diseases caused by different phytopathogens. So, recent studies report about the development of new pesticides based on nanosilver, nanoaluminosilicates, nano-TiO2, carbon-based nanomaterials, magnetic nanoparticles, etc. (Singh et al. 2015; Yao et al. 2009). The presumed active mechanism of titanium dioxide nanoparticles includes protection against diseases and increasing of photosynthesis in cowpeas too (Owolade and Ogunleti 2008; Moaveni et al. 2011) (Table 2). Otherwise, nanoparticles of silver and copper were trialed in laboratory, glasshouse and field studies, and they showed to curtail fungal and bacterial plant pathogens (Table 2) (Rai et al. 2012; Lamsal et al. 2011).
Nanoherbicides
Nanoencapsulated herbicides are becoming relevant, keeping in view the needing to design and produce a nanoherbicide protected under natural environment and that only acts when there is a spell of rainfall. Thus, the aim is to develop a specific herbicide molecule (encapsulated as nanoparticle) for specific receptors in the roots of target weeds, which enter into roots system and is translocated to the rest (Chinnamuthu and Kokiladevi 2007).
Nanofertilizers
In the past decades, efficiencies of N, P and K fertilizers have remained constant between 30–35, 18–20 and 35–40%, respectively (Nanotechnology in Agriculture 2013), leaving a major portion of added fertilizers to accumulate in the soil or enter into aquatic system causing eutrophication. Therefore, it is important to evolve a nano-based fertilizer formulation that addresses those issues. Currently research is underway to develop nanocomposites to supply all these required essential macronutrients in suitable proportion through smart delivery system, providing so a balanced fertilization (Tarafdar et al. 2012a). These new nutrient delivery systems that exploit the porous parts of plants would reduce N, P and K losses by increasing plan uptake at nanoscale level. Fertilizers manufactured as nanoparticles will increase the uptake of nutrients since a significant increase in yields has been observed due to foliar application of nanoparticles, e.g., nanophosphorous as fertilizer (Tarafdar et al. 2012b). Nanoclays are also employed as hosts for the controlled release of plant grow regulator α-naphthaleneacetate (Table 2) (Bin Hussein et al. 2005).
Khodakovskaya et al. (2009) have reported the use of carbon nanotubes for improving the germination of tomato seeds through better permeation of moisture (Table 2). Their data show that carbon nanotubes serve as new pores for water permeation. This process facilitates germination which can be exploded in rainfed agricultural system as stimulator growth (Tripathi et al. 2011). With regard to macronutrients nanofertilizers (N, P, K, Mg, S, etc.), micronutrients nanoformulations (Fe, Mn, Zn) and nanoparticulate fertilizers (TiO2, SiO2, carbon nanotubes) is noticeable the summary reported by Chhipa (2017) where an extensive compilation is performed about this new kind of nanofertilizers, created for an efficient targeted delivery. Other nanotechnological approaches such as nanoemulsions (MAXX, Syngenta), solid lipid nanoparticles, nanodispersions and nanogels have been currently designed to supply hydrophobic organic agrochemicals (Table 2) (Regulatory considerations 2014). These chemicals have to be properly coated in order to be dissolved into usual aqueous media.
Naturally occurring nanoparticles for agriculture
Recent researches have been focussed to the development of starch-based nanocontainers for the delivery of nutrients, biostimulants and crop protection molecules into the plants tissues. The clear advantage of this approach is that starch is biocompatible, biodegradable and non-toxic for plants, animals and environment (Balestra 2014). It can be employed to deliver nutrients into plants tissues at slower release rates for the long-term feeding of plants and to protect phosphorus and micronutrients (e.g., iron, manganese, zinc) in alkaline soils. Biostimulant compounds can also be slowly released through nanocontainers according to the plant needs, while being protected from microbial degradation before plant uptake. Moreover, starch nanocontainers can be developed for the delivery of plant protection products, e.g., antibacterial active principles, which can also be suitable for organic agriculture (e.g., vegetal extracts, copper) and thereby used in smaller amounts.
Recently, nanosized lignocellulosic materials have been produced from crops and trees. It opens up a whole new market for innovative and value-added nanobiomaterials, e.g., nanosized cellulosic crystals have been used as lightweight reinforcement in polymeric matrix as nanocomposite (Laborie 2009; Mathew et al. 2009). Their applications can include food packaging, construction and transportation vehicle body structures. Cellullosic nanowhisker production technology from wheat straw has been developed by Michigan Biotechnology Incorporate (MBI) International and is expected to make biodegradable nanocomposite that could substitute to fiberglass and plastics in multiple applications (Leistritz et al. 2007).
Field sensing systems to monitor the environmental stresses and crop conditions
Nanotechnology may be developed and deployed for real-time monitoring of the crop growth and field conditions including soil fertility, temperature, nutrient status, insects, plant diseases and weeds. Networks of wireless nanosensors positioned across cultivated fields provide essential data leading to the best agronomic processes (Chen and Yada 2011). For instance, there have been developed electronic nose (e-nose) devices (Sugunan et al. 2005), where ZnO nanowires are used as gas biosensors to identify compounds associated with specified physiological, pathological and environmental problems of crops (Table 2) or either enzymatic biosensors where enzymes on a immobilization surface can act as a sensing element by means of their attachment to certain biomolecules (Sassolas et al. 2012).
Toxicity
The incorporation of manufactured nanomaterials into strategic areas involves human and environmental toxicological effects from direct and indirect exposure to these materials. Because of their very small size, nanoparticles are capable to enter to the human body by inhalation, ingestion, skin penetration or injections and they have the potential to interact with intracellular structures and macromolecules for long periods of time (Sharifi et al. 2012). There is a growing evidence that some manufactured nanomaterials will be more toxic peer unit of mass than their respective bulk particles. For example, titanium dioxide is considered to be biologically inert in bulk form and is widely used as food additive being considered as Generally Recognized As Safe (GRAS) by Food and Drugs Administration. However, in vitro experiments show that as a nanoparticle, it damages deoxyribonucleic acid, disrupts the function of cells and can provoke inflammation (Ashwood et al. 2007; Wang et al. 2007).
Another important example to illustrate this difference is silver, widely used in food packing, storage containers, liners refrigerators, cutting boards, etc., since in its ionic form can be considered either as a powerful antibacterial agent as well as a powerful toxic in cell cultures. Since in the nanoscopic form its surface area is much greater than in the macroscopic one, Ag nanoparticles are much more reactive and therefore more easily ionizable, thus exhibiting higher antimicrobial and toxic nature. In fact it has been shown that for similar concentrations, the nanoparticles of silver have higher antibacterial activity than the corresponding macroscopic ions (Lok et al. 2006) as result of the unique physicochemical properties of nanomaterials in terms of size, shape and characteristics surface (Brunner et al. 2006). It has been also documented that these nanoparticles are associated with the production of reactive oxygen species, which in turn can interfere with cell metabolism, causing damage to proteins, membranes and deoxyribonucleic acid because of its toxicity (Hussain et al. 2005; Nel et al. 2006).
Recent researches suggest the current increase in the immune system dysfunction diseases, as well as the gastrointestinal inflammation tract, such as Crohn’s disease, can be directly related to exposure to increasing levels of nanoparticles, either in the workplace or well as food components in an intentionally or accidentally way (Ashwood et al. 2007; Schneider 2007). Toxicity from nanomaterials at workplace deserves special consideration. Recent research has revealed links between occupational exposure to nanoparticles and health damage. Specifically, a study published in the European Respiratory Journal in 2009 says that seven Chinese workers developed severe lung damage (inflammation, pulmonary fibrosis and pleural granuloma) after inhaling polyacrylate nanoparticles produced in an ink factory for a period between 5 and 13 months (Song et al. 2009). Despite above evidences, the unique toxicological properties of nanomaterials and their long-term impact on human health are still poorly studied (Papp et al. 2008; Wang et al. 2007). As a new nanomaterials-based technology begins, it will be essential to have a framework where its potential toxicity can be evaluated. There is a considerable gap between the available data about the nanomaterials production and toxicity evaluations, reason that prevents the safe design of nanoparticles. The aim of this section is to contribute to the better control and understanding of nanomaterials’ toxicity by addressing two relevant aspects such as (a) knowledge of biophysicochemical properties affecting toxicity, since on this factor lie the tools to build and manage nanomaterials with lower risks and (b) greener synthesis of nanomaterials to bring down the environmental impact of toxic reagents, wastes and byproducts.
Characteristic parameters of nanoparticles, such as agglomeration state, chemical composition, size, shape, crystal structure, surface charge, surface morphology and surface coating among others, influence the biological interaction of nanomaterials, reason why becomes important to evaluate these properties to characterize the toxic potential of them (Sharifi et al. 2012). The following text describes the effect of the most significant physiochemical parameters over biological responses, as well as the influence of these parameters among them.
Size influence
Particle size and surface area are crucial characteristics from a toxicological point of view since interactions between nanomaterials and biological organisms typically take place at the surface of the nanoparticle. As the particles size decreases, the surface area exponentially increases and a greater proportion of the particles (atoms or molecules) will be displayed on the surface rather than within the bulk of the material. Thus, the nanomaterial surface becomes more reactive toward itself or surrounding biological components with decreasing size, increasing also the available catalytic surface for chemical reactions.
Although some aspects of size-dependent nanoparticle toxicity can be reasonably predicted by in vitro techniques, the wide range of concentrations, exposure routes and nanomaterial nature make difficult to determine when the observed cytotoxicity is clinically relevant. While in vitro applications require less strict toxicological characterization, in vivo use of nanoparticle requires a comprehensive understanding of the toxicokinetic of nanomaterials (absorption, distribution, metabolism and elimination) which depend of their nature and exposure routes (Lewinski et al. 2008). To our knowledge, few data are still available in the literature related to the in vivo size-dependent evaluation of nanomaterials. In general terms, from the existing research, it can be concluded that nanomaterials with smaller size/dimensions could potentially have enhanced mobility, reactivity (i.e., increased surface area), biological uptake via passive transport and toxicity (Sharifi et al. 2012; Regulatory Considerations 2014).
Particle shape and morphology influence
Nanomaterials can have very different shapes including fibers, spheres, tubes, rings and planes. Most of the knowledge about shape-dependent toxicity is again based on in vitro experiments. In vivo shape-dependent toxicity of nanomaterials is usually evaluated through its adverse effect on endocytosis or clearance by macrophages. For example, it has been suggested that endocytosis of spherical nanoparticles is easier and faster compared to rod-shaped or fiber-like ones (Champion and Mitragotri 2006). For instance, a shape-dependent toxicity has been observed with silica and TiO2 allotropes among other nanomaterials. Thus, amorphous silica is a Food and Drug Administration-approved food additive, whereas crystalline silica is a suspected human carcinogen and is involved in the pathogenesis of silicosis (Petersen and Nelson 2010). Different toxicity behavior has also been observed for TiO2 nanoparticles with their different crystal structures rutile and anatase (Gurr et al. 2005).
Surface charge influence
Surface charge also plays a role in toxicity, as it influences the adsorption of ions and biomolecules that may change organism or cellular responses toward particles. In addition, surface charge is a major determinant of colloidal behavior, which influences the organism response by changing the shape and size of nanoparticles through aggregates or agglomerates formation (Hoshino et al. 2004). In general, it is believed that cationic surfaces are more toxic than anionic ones, since cationic surfaces are more likely to induce hemolysis and platelet aggregation, whereas neutral surfaces are the most biocompatible (Goodman et al. 2004). This may be due to the affinity of cationic particles to the negative phospholipid head groups or protein domains on cell membranes. For example, Saxena et al. showed that acid-functionalized single-wall carbon nanotubes caused markedly greater in vivo toxicity compared to pristine nanotubes. This higher toxicity could result either from a possible greater bioavailability or from the high negative charge of acid-functionalized single-wall carbon nanotubes, or even from both simultaneously (Saxena et al. 2007).
The effect of surface charge on the dissolution and uptake of Au nanoparticles by different plant species was investigated by Zhu’s group (Zhu et al. 2012). In this study, the group found that positively charged Au nanoparticles were most readily taken up by plant roots which are predominantly negatively charged. Zeta potential parameter is a measure of surface charge and governs interactions with environmental media and biota. In water media works as a practical measure of the potential stability of a colloidal system. This parameter is affected by surface chemistry as well as by the properties and composition of solution, such as pH, ionic strength and dissolved organic matter. It provides an indication of the degree of repulsion between adjacent, similarly charged particles, i.e., low values indicate poor stability and potential aggregation of particles. Zeta potential and hence surface charge have also implications for other environmental processes such as adsorption, complexation (e.g., with organic matter) and interaction with biological systems such as cell membranes, and it will be a very useful parameter in aquatic and soil environments (Milani et al. 2012; Stebounova et al. 2011), where nanoparticle interaction with other materials will be more likely.
Composition influence
Although it has been suggested that size or surface area may be more important than chemical composition for conferring nanoparticles toxicity, particle chemistry becomes more relevant in relation to cell molecular chemistry and oxidative stress species (Sharifi et al. 2012).
Coatings influence
Surface chemistry of a nanomaterial has implications for its dissolution, aggregation, reactivity and toxicity. In different formulations, the nanomaterial surface could be coated or modified with multiple organic and inorganic coatings. These modifications are typically done for the following reasons: to passivate the surface of the nanoparticles (remove free electrons and dangling bonds), control the size of the nanoparticles, render the nanoparticles dispersible in a particular solvent or formulation and, in some cases, to improve specificity. The adverse effects of nanoparticles may be mitigated or eliminated by incorporation of surface coatings. Proper surface coatings can stabilize particles and avoid agglomeration. Coating is also an effective means for preventing the dissolution and releasing of toxic ions (Kirchner et al. 2005). Furthermore, the steric hindrance of coatings can retard the cellular uptake and accumulation of nanoparticles, or on the opposite, some coatings can facilitate their endocytosis. Surface coatings can modify the surface charge or surface composition, which can impact intracellular distribution (mobility) and the production of reactive oxygen species. Otherwise, many coatings are environmentally labile or degradable and may shed or degrade after exposure to biological media, thus rendering an initially non-toxic material in hazardous one (Kirchner et al. 2005).
The influence of surface chemistry on uptake and toxicity has been demonstrated in different studies. For example, Chompoosor et al. (2010) investigated the effect of surface hydrophobicity on toxicity, mainly cytotoxicity and deoxyribonucleic acid damage, using gold nanoparticles (2 nm core) linked quaternary ammonium functionalized with varied hydrophobic alkyl chain. In this study, it was found that increasing hydrophobicity on the surface of the nanoparticles resulted in higher cytotoxicity with concomitant reactive oxygen species production. In some nanoparticles such as quantum dots, a coating is unavoidable because of the metallic core is hydrophobic, and the core itself is toxic since it is composed of heavy metals such as cadmium. Thus, a secondary coating is needed to increase the quantum dot core’s durability, to prevent ion leaching, and to increase water dispersibility (Zhao et al. 2010). The type of surface in the secondary coating may affect the toxicity of the quantum dot complex. For example, Chen et al. coated quantum dots with silica and the lack of genotoxicity was related to the silica coating, which successfully prevented the interaction of Cd, Se, Zn and S with proteins and deoxyribonucleic acid in the nucleus (Chen and Gerion 2004).
One of the common strategies reported for coating the nanoparticles surface is nanoencapsulation, for instance, “core–shell” models, where the core would be the nanoparticle and the shell was the protective coating. As coatings, different approaches have been described like dendritic macromolecules (Namazi et al. 2007), surfactants (Guang et al. 2012), single-crystal ZnO nanorods (Liu et al. 2014), used upon the nature of immobilized nanomaterial and or the pursued application.
Surface roughness influence
Surface roughness along with hydrophobicity and cationic charge is the main factors involved in non-specific binding forces that promote cellular uptake (Mahmoudi and Serpooshan 2011). Small-radii surface coarseness can significantly minimize electrostatic or hydrophobic–hydrophilic repulsive interactions, promoting so cell adhesion.
Aggregation influence
Proper and stable dispersion of nanoparticles in the delivery medium is very important for their biological distribution and subsequent activity. Due to agglomeration, nanoparticles may not form a stable suspension suitable for in vivo exposure in physiological solutions. So, aggregation (homo- and hetero-) is an important property that determines the fate and toxicity of nanomaterials in the environment (Batley et al. 2012). This process is heavily influenced by the properties of the suspension such as pH, ionic strength and the presence of other colloids. Since nanoparticles can aggregate between themselves (homoaggregation) and with other natural particles (heteroaggregation), the main route for the removal of most nanoparticles in the environment will be through aggregation, followed by sedimentation. Heteroaggregation of engineered nanomaterials with natural colloids is therefore likely the simplest way to control the fate of most nanoparticles. This aggregation process may change the overall size of the nanomaterials, surface area and reactivity that will affect their mobility and ecotoxicity in the environment. Sometimes dispersion or wetting agents are added in media to improve nanomaterials solubility, but it also may adversely affect their toxicity (Regulatory Considerations 2014).
Reactivity influence
Evaluation of nanomaterial reactivities (catalytic, redox, radical formation, etc.) is particularly important when we have to consider potential ecotoxicity (i.e., nanomaterials facilitating the formation of toxic reactive oxygen species). Reactivity is closely associated with other fundamental properties such as size, surface area, composition and crystalline structure. For example, TiO2, in its anatase form, is known for its photocatalytic activity, i.e., TiO2 nanoparticles are used to enhance pesticide degradation (Khot et al. 2012), while it is photostable in rutile form, i.e., TiO2 nanoparticles are used to protect a system from photodegradation (Gogos et al. 2012).
Green chemistry
Although different techniques such as ultraviolet irradiation, aerosol technologies, laser ablation, ultrasonic fields, photochemical reduction have been used successfully to produce nanoparticles, they remain expensive and involve the use of hazardous chemicals. Therefore, there is a significant interest in the development of environmental-friendly and sustainable methods (Kharissova et al. 2013; Narayanan and Sakthivel 2010). At the same time, despite intensive developments of the nanotechnology, the adverse effects of nanomaterials are still relatively unknown. So, the synthesis of such materials using environmental-friendly and biocompatible reagents could decrease the toxicity of the resulting materials and the environmental impact of the byproducts (Sanchez-Mendieta and Vilchis-Nestor 2012; Varma 2012).To reach this goal, non-toxic solvents (preferably water), closed reactors, “green” techniques without contact between reaction media and air (ultrasound, microwave, hydrothermal, magnetic and biological methods) and low temperatures can be used as environmental-friendly tools.
Methods for obtaining nanoparticles using naturally occurring reagents such as vitamins, sugars, plant extracts, biodegradable polymers and microorganisms as reducing and capping agents can be considered attractive for nanotechnology. This kind of synthesis has been limited so far to the fabrication of a small number of inorganic nanoparticles, mainly metal nanoparticles, although several metallic oxides and salts have been also produced. Among the reagents above mentioned, plant-based materials seem to be the best candidates and they are suitable for large-scale “biosynthesis” of nanoparticles (Iravani 2011). Plant parts (leaf, root, latex) and products derived from them such as extracts from tea, coffee, banana, simple amino acids, as well as wine, table sugar and glucose, have been utilized as reagents. A considerable number of efforts are devoted to tea extracts, which owing to a high amount of presented polyphenols, can act as both chelating and reducing and capping agents for the nanoparticles. Moreover, the resulted particles are protected from further reactions and aggregations, which increase their stability and longevity. Greener methods involving plant materials are generally single-pot reactions, without the use of additional surfactants, capping agents and templates. Furthermore, the technique developed for a given metal and metal oxide nanoparticle can also be frequently applied for other metals (Kharissova et al. 2013). As green reagent, it should be highlighted chitosan, biomolecule from marine origin, whose use is very popular due to its function as both stabilizer and reducing agent for colloidal particles providing stabilization of them for months and reaching smaller size and narrower size distribution too (Adlim and Bakar 2008, 2013).
Microorganisms have been also employed, but the reached synthesis methods are slow and limited to get some specific sizes and shapes of nanomaterials (Dhillon et al. 2012, Park et al. 2016). Nanoparticles’ bioproduction by fungi has some practical advantages over other microorganisms, such as bacteria, yeasts, algae and viruses. Specifically fungi are gaining worldwide popularity since they better tolerate higher metal concentrations than bacteria and secrete abundant extracellular redox proteins to reduce soluble metal ions to their insoluble form and eventually to nanocrystals (Kitching et al. 2014). Fungi can support high concentrations of metal ions because of their inherent ability to produce higher concentrations of proteins which aid in the reduction of metal ions to less toxic forms. The higher the production of enzymes, the more will be the reduction of metal ions. On the opposite, in the case of bacteria, most metal ions are toxic, and therefore, reduction of ions or formation of water insoluble complexes is a defense mechanism developed by bacteria to overcome such toxicity (Dhillon et al. 2012). Furthermore, fungi are a versatile group which can adapt and grow under different extreme conditions of pH, temperature, and nutrient availability (Anand et al. 2006). The size and shape of the nanoparticles synthesized using fungi appear to depend on the type of microorganisms and the factors, such as temperature and pH of the medium. Exhaustive descriptions about the influence of these factors on morphology and composition of different nanomaterials were performed in the excellent review of Dhillon et al. (2012).
Overall, fabrication of metal, metal oxides and salt nanoparticles using natural substances is a very promising area in nanotechnology, where invaluable nanomaterials can be produced at more safe scale because the biomaterial-based routes eliminate the need to use harsh or toxic chemicals (Parsons et al. 2007) and since even waste products are relatively innocuous because they are mostly composed of leftover natural plant extracts. Mechanisms of these bioreductive transformations (Zhou et al. 2010; Huang et al. 2011) as well as catalytic properties and spectroscopic characterization have been discussed in different papers (Du et al. 2011; Zhan et al. 2012; Sukla and Iravani 2017).
Most studies reported to date are dedicated to obtain nanoparticles of silver, perhaps due to their easy preparation and importance in the science of disinfection. The green synthesis of gold nanoparticles also exhibits considerable interest, although in a much lesser degree than those of Ag. Gold nanoparticles present also a considerable interest, although in much lesser degree than Ag. Other pure metals (Fe, Pt, Pd, Cu), hybrids of them (alloys or core–shell), oxides and salts have also been obtained by “greener” methods. The state of the art, with some representative examples, their respective precursors and green reagents are summarized in Table 3.
In conclusion, green synthesis of nanomaterials provides great advantages like: (a) use of only naturally occurring nonhazardous materials, (b) no hazardous waste neither, (c) reduced processing efforts, (d) the obtained nanomaterials are more stable (Adlim and Bakar 2008) easily stored and easily transported, and (e) the materials can be more readily produced around the world using common and often biorenewable materials. (Varma 2012).
But, on the contrary, nowadays it still presents serious deficiencies like such investigations have been just carried out at research laboratories in small scale, whereas there are no reports on pilot plant or industrial-scale fabrication of nanomaterials using natural products. So, it’s time to pay attention on scaling up the processes of the preparation (Kharissova et al. 2013; Sukla and Iravani 2017). On the other hand, the lack of control over final characteristics of obtained nanomaterials also supposes a great disadvantage. Then, efforts are necessary to improve the optimization of the reaction conditions, the extraction and purification of the produced nanoparticles and their separation according to size and shape (Sukla and Iravani 2017).
Conclusion
Nanomaterials science can solve challenges in some environmental issues such as wastewater and desalination sea treatments, clean energy conversion and storage, control of drug-resistant microorganisms and new nanomaterials and strategies for a more sustainable agriculture. The review tries to show the state of the art of the most promising applications in these different fields, where relevant profits from the research about nanomaterials are still expected. Current water supplies and wastewater treatment technologies and infrastructures are reaching their limit for providing adequate water quality to meet human and environmental needs. Nanotechnology aims to provide new treatment capabilities such as nanoadsorbents, nanotechnology-enabled membranes and nanophotocatalysts for expanding water supplies in the near future.
The advantages of nanostructured materials for energy production and storage have been documented in this review, but there are still several issues to improve in terms of controllable fabrication with regard to the fully desired morphology, insightful understanding of the relationship between the device performance and the nanomaterial structure and the development of new mechanisms relying on nanostructures. Nanomaterials represent an innovative strategy to develop new pharmaceutical formulations based on metallic nanoparticles with efficacious antimicrobial properties in the fight against multi-drug-resistant microorganisms. The possibility of developing microbial resistance cannot be ruled out. Therefore, preclinical and clinical trials are needed for a better understanding of potentiality and limitations. Nanotechnology has also a great potential for contributing to sustainable agriculture and environment by means of new technological materials and strategies such as smart nanocarrier systems for controlled releasing of chemicals (pesticides, fertilizers, etc.) and development of innovative harvested nanomaterials, non-toxic and biodegradable, with industrial purposes.
The enormous potential benefits offered by nanotechnology must be weighed against the potential risks of use and abuse of nanomaterials, and in large part these risks are not evaluated yet. Thus, they should not be discarded and it must be seriously addressed its biggest disadvantage: the uncertain toxicity. This review has addressed two ways to palliate nanomaterials toxicity: (a) to control physicochemical factors affecting toxicity in order to dispose of more safe nanomaterials and (b) to harness greener synthesis of them to bring down the environmental impact of toxic reagents, wastes and byproducts.
Physicochemical properties play a crucial role for determining the toxicity of nanomaterials. Thus, the toxicity of chemically identical materials can be altered significantly by the manipulation of some physicochemical properties. In order to reduce the considerable knowledge gap between the production and the in vivo toxicity evaluation of nanoparticles, a considerable effort is needed by the scientific community to study the physiological effects of acute and chronic exposure to them.
Safety and health related to nanomaterials are currently facing a situation in which almost all aspects to consider present knowledge gaps due to limited information. There is a significant lack of reliable knowledge on toxicology, effects on human health and absence of the appropriate metric for determining exposure to nanomaterials (INSHT 2015). So, with regard to legislation, although European legislation has not a specific framework for nanomaterials, the rules to be applied are depending on the situation they are used and their hazard characteristics, as dictates the European Commission (European Commission 2014).
References
Adlim A, Bakar MA (2008) Preparation of Chitosan-gold nanoparticles: Part 2. The role of Chitosan. Indo J Chem 8(3):320–326
Adlim A, Bakar M (2013) The properties of Pd/Au bimetallic colloidal catalysts stabilized by chitosan and prepared by simultaneous and stepwise chemical reduction of the precursor ions. Kinet Catal 54(5):586–596. doi:10.1134/S0023158413050017
Agboola O, Maree J, Mbaya R (2014) Characterization and performance of nanofiltration membranes. Environ Chem Lett 12:241–255. doi:10.1007/s10311-014-0457-3
Agboola O, Maree J, Kolesnikov A, Mbaya R, Sadiku R (2015) Theoretical performance of nanofiltration membranes for waste water treatment. Environ Chem Lett 13:37–47. doi:10.1007/s10311-014-0486-y
Ahamed M, Alhadlaq H, Khan M, Karuppiah P, Al-Dhab N (2014) Synthesis, characterization and antimicrobial activity of copper oxide nanoparticles. J Nanomater. doi:10.1155/2014/637858
Ahluwalia V, Kumar J, Sisodia R, Shakil N, Walia S (2014) Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind Crops Prod 55:202–206. doi:10.1016/j.indcrop.2014.01.026
Ahmed M, Murtaza G, Mehmood A, Bhatti T (2015) Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: characterization and antibacterial activity. Mater Lett 153:10–13. doi:10.1016/j.matlet.2015.03.143
Allaker R (2010) The use of nanoparticles to control oral biofilm formation. J Dent Res 89:1175–1185. doi:10.1177/0022034510377794
OECD and Allianz (2008) Small sizes that matter: Opportunities and risks of nanotechnologies. Report in cooperation with the OECD International Futures Programme. http://www.oecd.org/science/nanosafety/37770473.pdf
Anand P, Isar J, Saran S, Saxena R (2006) Bioaccumulation of copper by Trichoderma viride. Bioresour Technol 97:1018–1025. doi:10.1016/j.biortech.2005.04.046
Arokiyaraj S, Saravanan M, Prakash N, Valan Arasu M, Vijayakumar B, Vincent S (2013) Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: an in vitro study. Mater Res Bull 48:3323–3327. doi:10.1016/j.materresbull.2013.05.059
Aruguete DM, Bojeong K, Michael FH, Yanjun M, Yingwen C, Andy H, Jie L, Amy P (2013) Antimicrobial nanotechnology: its potential for the effective management of microbial drug resistance and implications for research needs in microbial nanotoxicology. Environ Sci Process Impacts 15:93–102. doi:10.1039/C2EM30692A
Ashwood P, Thompson R, Powell J (2007) Fine particles that adsorb lipopolysaccharide via bridging calcium cations may mimic bacterial pathogenicity towards cells. Exp Biol Med 232(1):107–117
Auffan M, Rose J, Proux O, Borschneck D, Masion A, Chaurand P, Hazemann J, Haneac C, Jolivet J, Wiesner M, Van Geen A, Bottero J (2008) Enhanced adsorption of arsenic onto maghemites nanoparticles: As (III) as a probe of the surface structure and heterogeneity. Langmuir 24(7):3215–3222. doi:10.1021/la702998x
Auffan M, Rose J, Bottero J, Lowry G, Jolivet J, Wiesner M (2009) Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol 4(10):634–641. doi:10.1038/nnano.2009.242
Ayranci E, Duman O (2005) Adsorption behaviors of some phenolic compounds onto high specific area activated carbon cloth. J Hazard Mater 124:125–132. doi:10.1016/j.jhazmat.2005.04.020
Ayranci E, Duman O (2006) Adsorption of aromatic organic acids onto high area activated carbon cloth. J Hazard Mater 136:542–552. doi:10.1016/j.jhazmat.2005.12.029
Ayranci E, Duman O (2007) Removal of anionic surfactants from aqueous solutions by adsorption onto high area activated carbon cloth studied by in situ UV spectroscopy. J Hazard Mater 148:75–82. doi:10.1016/j.jhazmat.2007.02.006
Ayranci E, Duman O (2009) In-situ UV-visible spectroscopic study on the adsorption of some dyes onto activated carbon cloth. Sep Sci Technol 44:3735–3752. doi:10.1080/01496390903182891
Ayranci E, Duman O (2010) Structural effects on the interactions of benzene and naphthalene sulfonates with activated carbon cloth during adsorption from aqueous solutions. Chem Eng J 156:70–76. doi:10.1016/j.cej.2009.09.038
Baker S, Satish S (2015) Biosynthesis of gold nanoparticles by Pseudomonas veronii AS41G inhabiting Annona squamosa L. Spectrochim Acta A 150:691–695. doi:10.1016/j.saa.2015.05.080
Balestra G (2014) Starch-based nanoparticles in sustainable agriculture. In: Proceedings in workshop on “Nanotechnology for the agricultural sector: from research to the field”. JRC Scientific and Policy Reports. European Commission. doi:10.2791/80497
Baruah S, Khan M, Dutta J (2016) Perspectives and applications of nanotechnology in water treatment. Environ Chem Lett 14:1–14. doi:10.1007/s10311-015-0542-2
Baruwati B, Polshettiwar V, Varma R (2009) Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem 11:926–930. doi:10.1039/B902184A
Batley G, Kirby J, McLaughlin M (2012) Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res 46(3):854–862. doi:10.1021/ar2003368
Beard M, Midgett A, Hanna M, Luther J, Hughes B, Nozik A (2010) Comparing multiple exciton generation in quantum dots to impact ionization in bulk semiconductors: implications for enhancement of solar energy conversion. Nano Lett 10(8):3019–3027. doi:10.1021/nl101490z
Biener J, Stadermann M, Suss M, Worsley M, Biener M, Rose K, Baumann T (2011) Advanced carbon aerogels for energy applications. Energy Environ Sci 4:656–667. doi:10.1039/C0EE00627K
Bin Hussein MZ, Yahaya AH, Zainal Z, Kian LH (2005) Nanocomposite-based controlled release formulation of an herbicide, 2,4-dichlorophenoxyacetate encapsulated in zinc-aluminium-layered double hydroxide. Sci Technol Adv Mater 6(8):956–962. doi:10.1016/j.stam.2005.09.004
Bindhu M, Umadevi M (2014) Silver and gold nanoparticles for sensor and antibacterial applications. Spectrochim Acta A 128:37–45. doi:10.1016/j.saa.2014.02.119
Boldyryeva H, Umeda N, Plaskin A, Takeda Y, Kishimoto N (2005) High-influence implantation of negative metal ions into polymers for surface modification and nanoparticle formation. Sur Coat Technol 196:373–377. doi:10.1016/j.surfcoat.2004.08.159
Brady-Estevez A, Schnoor M, Kang S, Elimelech M (2010) SWCNT-MWCNT hybrid filter attains high viral removal and bacterial inactivation. Langmuir 26(24):19153–19158. doi:10.1021/la103776y
Brunet L, Lyon D, Hotze E, Alvarez P, Wiesner M (2009) Comparative photoactivity and antibacterial properties of C60 fullerenes and titanium dioxide nanoparticles. Environ Sci Technol 43(12):4355–4360. doi:10.1021/es803093t
Brunner T, Piusmanser P, Spohn P, Grass R, Limbach L, Bruinink A, Stark W (2006) In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol 40:4374–4381. doi:10.1021/es052069i
Champion J, Mitragotri S (2006) Role of target geometry in phagocytosis. Proc Natl Acad Sci USA 103(13):4930–4934. doi:10.1073/pnas.0600997103
Chen F, Gerion D (2004) Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett 4:1827–1832. doi:10.1021/nl049170q
Chen H, Yada R (2011) Nanotechnologies in agriculture: new tools for sustainable development. Trends Food Sci Technol 22:585–594. doi:10.1016/j.tifs.2011.09.004
Chhipa H (2017) Nanofertilizers and nanopesticides for agriculture. Environ Chem Lett 15:12–22. doi:10.1007/s10311-016-0600-4
Chin T, Kok H, Yit T, Abdul R, Sharif H, Soon H (2012) Energy and environmental applications of carbon nanotubes. Environ Chem Lett 10:265–273. doi:10.1007/s10311-012-0356-4
Chinnamuthu C, Kokiladevi E (2007) Weed management through nanoherbicides. In: Chinnamuthu CR, Chandrasekaram B, Ramasamy C (eds) Application of nanotechnology in agriculture. Tamil Nadu Agricultural University, Coimbatore, India
Chompoosor A, Saha K, Ghosh P, Macarthy D, Miranda O, Zhu Z, Arcaro K, Rotello V (2010) The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small 6(20):2246–2249. doi:10.1002/smll.201000463
Choucair M, Mauron P (2015) Versatile preparation of graphene-based nanocomposites and their hydrogen adsorption. Int J Hydrogen Energy 40:6158–6164. doi:10.1016/j.ijhydene.2015.03.065
Chwalibog A, Sawosz E, Hotowy A, Szeliga J, Mitura S, Mitura K, Grodzik M, Orlowski P, Sokolowska A (2010) Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int J Nanomed 5:1085–1094. doi:10.2147/IJN.S13532
Dasgupta N, Ramalingam Ch (2016) Silver nanoparticle antimicrobial activity explained by membrane rupture and reactive oxygen generation. Environ Chem Lett 14:477–485. doi:10.1007/s10311-016-0583-1
Dhillon G, Brar S, Kaur S, Verma M (2012) Green approach for nanoparticle biosynthesis by fungi: current trends and applications. Crit Rev Biotechnol 32(1):49–73. doi:10.3109/07388551.2010.550568
Diallo M (2009) Water treatment by dendrimer-enhanced filtration (DEF): principles and applications in Nanotechnology. Applications for clean water. In: Savage N, Diallo M, Duncan J, Street A, Sustich R (ed) chapter 11. William Andrew Inc., Norwich. doi:10.1016/B978-0-8155-1578-4.50020-2
Ditta A (2012) How helpful is nanotechnology in agriculture? Adv Nat Sci: Nanosci Nanotechnol 3:10. doi:10.1088/2043-6262/3/3/033002
Dizaj S, Mennati A, Jafari S, Khezri K, Adibkia K (2015) Antimicrobial activity of carbon-based nanoparticles. Adv Pharm Bull 5(1):19–23. doi:10.5681/apb.2015.003
Du M, Zhan G, Yang X, Wang H, Lin W, Zhou Y, Zhu J, Lin L, Huang J, Sun D, Jia L, Li Q (2011) Ionic liquid-enhanced immobilization of biosynthesized Au nanoparticles on TS-1 toward efficient catalysts for propylene epoxidation. J Catal 283:192–201. doi:10.1016/j.jcat.2011.08.011
Duman O, Ayranci E (2005) Structural and ionization effects on the adsorption behaviors of some anilinic compounds from aqueous solution onto high-area carbon cloth. J Hazard Mater 120:173–181. doi:10.1016/j.jhazmat.2004.12.030
Duman O, Ayranci E (2006) Adsorption characteristics of benzaldehyde, sulphanilic acid and p-phenolsulfonate from water, acid or base solutions onto activated carbon cloth. Sep Sci Technol 41:3673–3692. doi:10.1080/01496390600915072
Duman O, Ayranci E (2010a) Adsorptive removal of cationic surfactants from aqueous solutions onto high-area activated carbon cloth monitored by in situ UV spectroscopy. J Hazard Mater 174:359–367. doi:10.1016/j.jhazmat.2009.09.058
Duman O, Ayranci E (2010b) Attachment of benzo crown ethers onto activated carbon cloth to enhance the removal of chromium, cobalt and nickel ions from aqueous solutions by adsorption. J Hazard Mater 176:231–238. doi:10.1016/j.jhazmat.2009.11.018
Duman O, Tunc S, Polat TG (2015) Adsorptive removal of triarylmethane dye (Basic Red 9) from aqueous solution by sepiolite as effective and low-cost adsorbent. Micropor Mesopor Mater 210:176–184. doi:10.1016/j.micromeso.2015.02.040
Duman O, Tunc S, Polat T, Bozoglan B (2016a) Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr Polym 147:79–88. doi:10.1016/j.carbpol.2016.03.099
Duman O, Tunc S, Bozoglan B, Polat T (2016b) Removal of triphenylmethane and reactive azo dyes from aqueous solution by magnetic carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite. J Alloys Compd 687:370–383. doi:10.1016/j.jallcom.2016.06.160
Durgun E, Ciraci S, Yildirim T (2008) Functionalization of carbon based nanostructures with light transition-metal atoms for hydrogen storage. Phys Rev B 77:085405. doi:10.1103/PhysRevB.77.085405
Elumalai K, Velmurugan S (2015) Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl Surf Sci 345:329–336. doi:10.1016/j.apsusc.2015.03.176
European Commission (2014) Guidance on the protection of the health and safety of workers from the potential risks related to nanomaterials at work. http://ec.europa.eu/progress
Ferk G, Stergar J, Makovec D, Hamler A, Jagli Z, Drofenik M, Ban I (2015) Synthesis and characterization of Ni-Cu alloy nanoparticles with a tunable Curie temperature. J Alloys Compd 648:53–58. doi:10.1016/j.jallcom.2015.06.067
Fujishima A, Zhang T, Tryk D (2008) TiO2 photocatalysis and related surface phenomena. Surf Sci Rep 63(12):515–582. doi:10.1016/j.surfrep.2008.10.001
Garnett M, Kallinteri P (2006) Nanomedicines and nanotoxicology: some physiological principles. Occup Med 56:307–311. doi:10.1093/occmed/kql052
Gaya UI, Abdullah Abdul H (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem PhotobiolC Photochem Rev 9(1):1–12. doi:10.1016/j.jphotochemrev.2007.12.003
Gogos A, Knauer K, Bucheli T (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem 60(39):9781–9792. doi:10.1021/jf302154y
Goodman C, McCusker C, Yilmaz T, Rotello V (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chem 15:897–900. doi:10.1021/bc049951i
Gruère G, Narrod C, Abboot L (2011) Agriculture, food and water technologies for the poor opportunities and constrains policy Brief 19, June 2011. International Food Policy Research Institute (IFPRI). http://www.ifpri.org/sites/default/files/publications/bp019.pdf
Guang Lu et al (2012) Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat Chem 4:310–316. doi:10.1038/nchem.1272
Gurr J, Wang A, Chen C, Jan K (2005) Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 213:66–73. doi:10.1016/j.tox.2005.05.007
Gurunathan S, Han JW, Dayem A, Eppakayala V, Kim JH (2012) Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomed 7:5901–5914. doi:10.2147/IJN.S37397
Hajipour M, Fromm K, Ashkarran A, Aberasturi D, Larramendi I, Rojo T, Serpooshan V, Parak W, Mahmoudi M (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30(10):499–511. doi:10.1016/j.tibtech.2012.06.004
Helmut Kaiser Consultancy Group (2015) Study: nanotechnology in Food and Food processing Industry 2008–2010–2015. http://www.hkc22.com/nanofood.html
Hernandez-Delgadillo R, Velasco-Arias D, Diaz D, Arevalo-Niño K, Garza-Enriquez M, De la Garza-Ramos MA, Cabral-Romero C (2012) Zerovalent bismuth nanoparticles inhibit Streptococcus mutans growth and formation of biofilm. Int J Nanomed 7:2109–2113. doi:10.2147/IJN.S29854
Hochella MF, Lower SK, Maurice PA (2008) Nanominerals, mineral nanoparticles, and earth systems. Science 319:1631–1635. doi:10.1126/science.1141134
Hoek E, Ghosh A (2009) Nanotechnology-based membranes for water purification in “Nanotechnology Applications for Clean Water”. In: Savage N, Diallo M, Duncan J, Street A, Sustich R (eds) chapter 4. William Andrew Inc., Norwich, NY. p 47. doi:10.1016/B978-0-8155-1578-4.50013-5
Hoshino A, Fujioka K, Oku T, Suga M, Sasaki Y, Ohta T, Yasuhara M, Suzuki K, Yamamoto K (2004) Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett 4:2163–2169. doi:10.1021/nl048715d
Huang J, Zhan G, Zheng B, Sun D, Lu F, Lin Y, Chen H, Zheng Z, Zheng Y, Li Q (2011) Biogenic silver nanoparticles by Cacumen Platycladi extract: synthesis, formation mechanism, and antibacterial activity. Eng Chem Res 50:9095–9106. doi:10.1021/ie200858y
Hussain S, Hess K, Gearhart J, Geiss K, Schlager J (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro 19:975–983. doi:10.1016/j.tiv.2005.06.034
INSHT (Instituto Nacional de Seguridad e Higiene en el Trabajo) (2015) Seguridad y Salud en el trabajo con nanomaterials
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650. doi:10.1039/c1gc15386b
Iyakutti K, Kawazoe Y, Rajarajeswari M, Surya V (2009) Aluminum hydride coated single-walled carbon nanotube as a hydrogen storage medium. Int J Hydrogen Energy 34:370–375. doi:10.1016/j.ijhydene.2008.09.086
Ji L, Chen W, Duan L, Zhu D (2009) Mechanisms for strong adsorption of tetracycline to carbon nanotubes: a comparative study using activated carbon and graphite as adsorbents. Environ Sci Technol 43(7):2322–2327. doi:10.1021/es803268b
Johnston C (2010) Probing the nanoscale architecture of clay minerals. Clay Miner 45:245–279. doi:10.1180/claymin.2010.045.3.245
Kharissova O, Dias R, Kharisov B, Olvera Pérez B, Jiménez Pérez V (2013) The greener synthesis of nanoparticles. Trends Biotechnol 31(4):240–248. doi:10.1016/j.tibtech.2013.01.003
Khodakovskaya M, Dervishi E, Mahmood M, Yang X, Zhongrui L, Watanabe F, Biris A (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3:3221–3227. doi:10.1021/nn900887m
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64–70. doi:10.1016/j.cropro.2012.01.007
Kim H, Karkamkar A, Autrey T, Chupas P, Proffen T (2009) Determination of structure and phase transition of light element nanocomposites in mesoporous silica: case study of NH3BH3 MCM-41. J Am Chem Soc 131(38):13749–13755. doi:10.1021/ja904901d
Kim J, Lee C, Choi W (2010) Platinized WO3 as an environmental photocatalyst that generates OH radicals under visible light. Environ Sci Technol 44(17):6849–6854. doi:10.1021/es101981r
Kirchner C, Liedl T, Kudera S, Pellegrino T, Javier A, Gaub H, Stozle S, Fertig N, Parak W (2005) Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett 5:331–338. doi:10.1021/nl047996m
Kitching M, Ramani M, Marsili E (2014) Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microb Biotechnol. doi:10.1111/1751-7915.12151
Koeppenkastrop D, Decarlo E (1993) Uptake of rare-earth elements from solution by metal-oxides. Environ Sci Technol 27(9):1796–1802. doi:10.1021/es00046a006
Kruk T, Szczepanowicz K, Stefanska J, Socha R, Warszynski P (2015) Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloid Surf B 128:17–22. doi:10.1016/j.colsurfb.2015.02.009
Laborie MPG (2009) Bacterial cellulose and its polymeric nanocomposites. In: Lucia LA, Rojas OJ (eds) The nanoscience and technology of renewable biomaterials (chapter 9). Wiley, Chichester
Lamsal K, Kim SW, Jung JH, Kim YS, Kim KS, Lee YS (2011) Inhibition effects of silver nanoparticles against powdery mildews on cucumber and pumpkin. Mycobiology 39:26–32. doi:10.4489/MYCO.2011.39.1.026
Lee J, Mackeyev Y, Cho M, Wilson L, Kim J, Alvarez P (2010) C(60) aminofullerene immobilized on silica as a visible light-activated photocatalyst. Environ Sci Technol 44(24):9488–9495. doi:10.1021/es1028475
Leistritz FL, Hodur N, Senechal D, Stowers M, McCalla D, Saffron C (2007) Biorefineries using agricultural residue feedstock in the great plains. AAE Report 07001 working paper, Agricultural Experiment Station, North Dakota State University, Department of Agribusiness and Applied Economics. https://ideas.repec.org/p/ags/nddssr/7323.html
Leroueil P, Hong S, Mecke A, Baker J, Orr B, Banaszak M (2007) Nanoparticle interaction with biological membranes: does nanotechnology present a Janus face? Acc Chem Res 40:335–342. doi:10.1021/ar600012y
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4:26–49. doi:10.1002/smll.200700595
Li Z, Chen JF, Liu F (2007) Study of UV-shielding properties of novel porous hollow silica nanoparticle carriers for avermectin. Pest Manag Sci 63(3):241–246. doi:10.1002/ps.1301
Lim K, Kazemian H, Yaakob Z, Daud W (2010) Solid-state materials and methods for hydrogen storage: a critical review. Chem Eng Technol 33:213–226. doi:10.1002/ceat.200900376
Limbach L, Wick P, Manser P, Grass R, Bruinink A, Stark W (2007) Exposure of engineered nanoparticles to human lung epithelial cells: influence of chemical composition and catalytic activity on oxidative stress. Environ Sci Technol 41:4158–4163. doi:10.1021/es062629t
Liu F, Wen L-X, Li Z-Z, Yu W, Sun H-Y, Chen J-F (2006) Porous hollow silica nanoparticles as controlled delivery system for water-soluble pesticide. Mater Res Bull 41(12):2268–2275. doi:10.1016/j.materresbull.2006.04.014
Liu C, Li F, Ma L, Cheng H (2010) Advanced materials for energy storage. Adv Mater 22:E28–E62. doi:10.1002/adma.200903328
Liu S, Zeng T, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y (2011) Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5(9):6971–6980. doi:10.1021/nn202451x
Liu J, Notarianni M, Ll Rintou, Motta N (2014) Encapsulation of nanoparticles into single-crystal ZnO nanorods and microrods. Beilstein J Nanotechnol 5:485–493. doi:10.3762/bjnano.5.56
Lochan R, Head-Gordon M (2006) Computational studies of molecular hydrogen binding affinities: the role of dispersion forces, electrostatics, and orbital interactions. Phys Chem Chem Phys 8:1357–1370. doi:10.1039/B515409J
Lok CN, Ho CM, Chen R, He QY, Yu W, Sun H, Tam P, Chiu J, Che C (2006) Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 5:916–924. doi:10.1021/pr0504079
Lu S, Chiu H, Liu CT (2006) Removal of zinc (II) from aqueous solution by purified carbon nanotubes: kinetics and equilibrium studies. Ind Eng Chem Res 45(8):2850–2855. doi:10.1021/ie051206h
Machado S, Pinto S, Grosso J, Nouws H, Albergaria J, Delerue-Matos C (2013) Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci Total Environ 445–446:1–8. doi:10.1016/j.scitotenv.2012.12.033
Mahmood M (2011) Enhanced visible light photocatalysis by manganese doping or rapid crystallization with ZnO nanoparticles. Mater Chem Phys 30(1–2):531–535. doi:10.1016/j.matchemphys.2011.07.018
Mahmoudi M, Serpooshan V (2011) Large protein absorptions from small changes on the surface of nanoparticles. J Phys Chem C 115:18275–18283. doi:10.1021/jp2056255
Mathew AP, Laborie M, Oksman K (2009) Cross-linked chitosan-chitin whiskers nanocomposites with improved permeation selectivity and pH stability. Biomacromol 10(6):1627–1632. doi:10.1021/bm9002199
McNicholas P, Wang A, O’Neill K, Anderson R, Stadie N, Kleinhammes A, Parilla P, Simpson L, Ahn C, Wang Y, Wu Y, Liu J (2010) H2 storage in microporous carbons from PEEK precursors. J Phys Chem C 114:13902–13908. doi:10.1021/jp102178z
Meena M, Jacob J, Philip D (2015) Green synthesis and applications of Au–Ag bimetallic nanoparticles. Spectrochim Acta A 137:185–192. doi:10.1016/j.saa.2014.08.079
Milani N, McLaughlin M, Stacey SP (2012) Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. J Agric Food Chem 60(16):3991–3998. doi:10.1021/jf205191y
Moaveni P, Talebi R, Farahani H, Maroufi K, Maroufi K (2011) Study of TiO2 nano particles spraying effect on the some physiological parameters in Barley (Hordem Vulgare L.). Adv Environ Biol 5(7):1663–1667
Murphy K (2008) Nanotechnology: agriculture’s next “Industrial” revolution, 3-5. Financial Partner, Spring pp 3–5
Namazi H, Adeli M, Zarnegar Z Jafari, Dadkhah S, Shukla A (2007) Encapsulation of nanoparticles using linear–dendritic macromolecules. Colloid Polym Sci 285(14):1527–1533. doi:10.1007/s00396-007-1717-6
Nanotechnology in Agriculture: Scope and Current Relevance (2013). National Academy of Agricultural Sciences, New Delhi, December 2013. Policy Paper. https://es.scribd.com/document/244339453/Nano-in-agriculture-scope-current-relevance-pdf
Narayanan K, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface 156:1–13. doi:10.1016/j.cis.2010.02.001
Nel A, Xia T, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627. doi:10.1126/science.1114397
Niskanen J, Shan J, Tenhu H, Jiang H, Kauppinen E, Barranco V, Pico F, Yliniemi K, Kontturi K (2010) Synthesis of copolymer stabilized silver nanoparticles for coating materials. Colloid Polym Sci 288:543–553. doi:10.1007/s00396-009-2178-x
Nozik J (2008) Multiple exciton generation in semiconductor quantum dots. Chem Phys Lett 457:3–11. doi:10.1016/j.cplett.2008.03.094
Owolade O, Ogunleti D (2008) Effects of titanium dioxide on the diseases, development and yield of edible cowpea. J Plant Prot Res 48(3):329–336
Papp T, Schiffmannp D, Weiss D, Castranova V, Vallyathan V, Rahman Q (2008) Human health implications of nanomaterial exposure. Nanotoxicology 2:9–27. doi:10.1080/17435390701847935
Park T, Lee K, Lee S (2016) Advances in microbial biosynthesis of metal nanoparticles. Appl Microbiol Biotechnol 100:521–534. doi:10.1007/s00253-015-6904-7
Parsons J, Peralta-Videa J, Gardea-Torresdey J (2007) Use of plants in biotechnology: synthesis of metal nanoparticles by inactivated plant tissues, plant extracts, and living plants. Environ Sci 5:463–485. doi:10.1016/S1474-8177(07)05021-8
Pendergast M, Hoek E (2011) A review of water treatment membrane nanotechnologies. Energy Environ Sci 4(6):1946–1971. doi:10.1039/c0ee00541j
Pendergast M, Nygaard J, Ghosh K, Hoek E (2010) Using nanocomposite materials technology to understand and control reverse osmosis membrane compaction. Desalination 261(3):255–263. doi:10.1016/j.desal.2010.06.008
Petersen E, Nelson B (2010) Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal Bioanal Chem 398:613–650. doi:10.1007/s00216-010-3881-7
Peter-Varbanets M, Zurbrugg C, Swartz C, Pronk W (2009) Decentralized systems for potable water and the potential of membrane technology. Water Res 43(2):245–265. doi:10.1016/j.watres.2008.10.030
Pişkin S, Palantöken A, Yılmaz M (2013) Antimicrobial activity of synthesized TiO2 nanoparticles. In: International conference on emerging trends in engineering and technology (ICETET’2013) Dec 7–8, 2013 Patong Beach, Phuket (Thailand)
Prucek R, Tucek J, Kilianová M, Panácek A, Kvítek L, Filip J, Kolár M, Tománková Katerina, Zboril R (2011) The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles. Biomaterials 32:4704–4713. doi:10.1016/j.biomaterials.2011.03.039
Pumera M (2011) Graphene-based nanomaterials for energy storage. Energy Environ Sci 4:668–674. doi:10.1039/C0EE00295J
Rahaman M, Vecitis C, Elimelech M (2012) Electrochemical carbon-nanotube filter performance toward virus removal and inactivation in the presence of natural organic matter. Environ Sci Technol 46(3):1556–1564. doi:10.1021/es203607d
Rai MK, Deshmukh SD, Ingle AP, Gade AK (2012) Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol 112(5):841–852. doi:10.1111/j.1365-2672.2012.05253.x
Ranjan S, Ramalingan Ch (2016) Titanium dioxide nanoparticles induce bacterial membrane rupture by reactive oxygen generation. Environ Chem Lett 14:487–494. doi:10.1007/s10311-016-0586-y
Rao G, Lu C, Su F (2007) Sorption of divalent metal ions from aqueous solution by carbon nanotubes: a review. Sep Purif Technol 58(1):224–231. doi:10.1016/j.seppur.2006.12.006
Ravindran A, Chandran P, Khan S (2013) Biofunctionalized silver nanoparticles: advances and prospects. Colloid Surface B 105:342–352. doi:10.1016/j.colsurfb.2012.07.036
Regulatory Considerations for Nanopesticides and Veterinary Nanomedicines (2014) A draft APVMA report. Australian Governement. https://apvma.gov.au/sites/default/files/docs/report-draft-regulatory-considerations-nanopesticides-veterinary-nanomedicines.pdf
Robertson C, Mokaya R (2013) Microporous activated carbon aerogels via a simple subcritical drying route for CO2 capture and hydrogen storage. Micropor Mesopor Mater 179:151–156. doi:10.1016/j.micromeso.2013.05.025
Sanchez-Mendieta V, Vilchis-Nestor A (2012) Green synthesis of noble metal (Au, Ag, Pt) nanoparticles, assisted by plant-extracts. In: Yen-Hsun S (ed) Noble metals, INTECH, pp 391–408. doi:10.5772/34335
Sangeetha G, Rajeshwari S, Venckatesh R (2011) Green synthesis of zinc oxide nanoparticles by Aloe barbadensis miller leaf extract: structure and optical properties. Mater Res Bull 46:2560–2566. doi:10.1016/j.materresbull.2011.07.046
Sangeetha G, Rajeshwari S, Venckatesh R (2012) Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: optical properties. Spectrochim Acta A 97:1140–1144. doi:10.1016/j.saa.2012.07.096
Santhoshkumar T, Rahuman A, Jayaseelan Ch, Rajakumar G, Marimuthu S, Kirthi A, Velayutham K, Thomas J, Venkatesan J, Kim S (2014) Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac J Trop Dis. doi:10.1016/S1995-7645(14)60171-1
Santos C, Albuquerque R, Sampaio F, Keyson D (2013) Nanomaterials with antimicrobial properties: applications in health sciences. In: Méndez-Vilas A (ed) Microbial pathogens and strategies for combating them: science, technology and education. http://www.formatex.info/microbiology4/vol1/143-154.pdf
Sassolas A, Blum L, Leca-Bouvier B (2012) Immobilization strategies to develop enzymatic biosensors. Biotechnol Adv 30(3):489–511. doi:10.1016/j.biotechadv.2011.09.003
Saxena R, Williams W, Mcgee J, Daniels M, Boykin E, Gilmour I (2007) Enhanced in vitro and in vivo toxicity of poly-dispersed acid-functionalized single-wall carbon nanotubes. Nanotoxicology 1:291–300. doi:10.1080/17435390701803110
Schneider J (2007) Can microparticles contribute to inflammatory bowel disease: innocuous or inflammatory? Exp Biol Med 232:1–2
Scott N, Chen H (2003) Nanoscale science and engineering on agriculture and food systems. In: Roadmap report of national planning workshop. Washington DC, November 18–19, 2002. http://www.nseafs.cornell.edu/web.roadmap.pdf
Sculley J, Yuan D, Zhou H (2011) The current status of hydrogen storage in metal–organic frameworks—updated. Energy Environ Sci 4:2721–2735. doi:10.1039/C1EE01240A
Sharifi S, Behzadi S, Laurent S, Laird Forrest M, Stroeve P, Mahmoudi M (2012) Toxicity of nanomaterials. Chem Soc Rev 41:2323–2343. doi:10.1039/c1cs15188f
Sharma Y, Srivastava V, Singh V, Kaul S, Weng C (2009) Nano-adsorbents for the removal of metallic pollutants from water and waste water. Environ Technol 30(6):583–609. doi:10.1080/09593330902838080
Singh S, Kumar B, Yadav S, Gupta K (2015) Applications of nanotechnology in agricultural and their role in disease management. Res J Nanosci Nanotechnol 5(1):1–5. doi:10.3923/rjnn.2015.1.5
Smuleac V, Varmab R, Sikdarb S, Bhattacharyya D (2011) Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J Membr Sci 379:131–137. doi:10.1016/j.memsci.2011.05.054
Song Y, Li X, Du X (2009) Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J 34:559–567. doi:10.1183/09031936.00178308
Stebounova L, Guio E, Grassian V (2011) Silver nanoparticles in simulated biological media: a study of aggregation, sedimentation and dissolution. J Nanopart Res 13(1):233–244. doi:10.1007/s11051-010-0022-3
Sugunan A, Warad H, Thanachayamont C, Dutta J and Hoffmann H (2005) Zinc oxides nanowires on non-epitaxial substrates from colloidal processing for gas sensing applications. In: Vaseashta A, Dimova-Malinovska D, Marshall J (eds) Proceedings of NATO advanced study institute on nanostructured and advanced materials for applications in sensors, optoelectronic and photovoltaic technology, (NATO Science Series II: Mathematics, Physics and Chemistry, vol 204) XI, Springer, Berlin, p 425
Sukla AK, Iravani S (2017) Metallic nanoparticles: green synthesis and spectroscopic characterization. Environ Chem Lett. doi:10.1007/s10311-017-0618-2
Surya V, Iyakutti K, Rajarajeswari M, Kawazoe Y (2009) Functionalization of single-walled carbon nanotube with borane for hydrogen storage. Physica E 41:1340–1346. doi:10.1016/j.physe.2009.03.007
Surya V, Iyakutti K, Venkataramanan N, Mizuseki H, Kawazoe Y (2010) The role of Li and Ni metals in the adsorbate complex and their effect on the hydrogen storage capacity of single walled carbon nanotubes coated with metal hydrides, LiH and NiH2. Int J Hydrog Energy 35:2368–2376. doi:10.1016/j.ijhydene.2010.01.001
Sweet J, Chesser A, Singleton I (2012) Review: metal-based nanoparticles; size, function, and areas for advancement in applied microbiology. Adv Appl Microbiol 80:13–42. doi:10.1016/B978-0-12-394381-1.00005-2
Tarafdar JC, Agrawal A, Raliya R, Kumar P, Burman U, Kaul R (2012a) ZnO nanoparticles induced synthesis of polysaccharides and phosphatases by Aspergillus fungi. Adv Sci Eng Med 4:1–5. doi:10.1166/asem.2012.1160
Tarafdar JC, Raliya R, Rathore I (2012b) Microbial synthesis of phosphorus nanoparticles from Tri-calcium phosphate using Aspergillus tubingensis TFR-5. J Bionanosci 6:84–89. doi:10.1166/jbns.2012.1077
Tegos P, Demidova N, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin M (2005) Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol 12(10):1127–1135. doi:10.1016/j.chembiol.2005.08.014
Tetreault N, Arsenault E, Heiniger L, Soheilnia N, Brillet J, Moehl S, Zakeeruddin G, Ozin A, Grätzel M (2011) High-efficiency dye-sensitized solar cell with three-dimensional photoanode. Nano Lett 11:4579–4584. doi:10.1021/nl201792r
Tripathi S, Sonkar SK, Sarker S (2011) Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 3(3):1176–1181. doi:10.1039/c0nr00722f
Trivedi P, Axe L (2000) Modelling Cd and Zn sorption to hydrous metal oxides. Environ Sci Technol 34(11):2215–2223. doi:10.1021/es991110c
Varma S (2012) Greener approach to nanomaterials and their sustainable applications. Curr Opin Chem Eng 1:123–128. doi:10.1016/j.coche.2011.12.002
Vecitis C, Zodrow K, Kang S, Elimelech M (2010) Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 4(9):5471–5479. doi:10.1021/nn101558x
Vecitis C, Schnoor H, Rahaman S, Schiffman J, Elimelech M (2011) Electrochemical multiwalled carbon nanotube filter for viral and bacterial removal and inactivation. Environ Sci Technol 45(8):3672–3679. doi:10.1021/es2000062
Vörösmarty C, McIntyre P, Gessner M, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn S, Sullivan C, Liermann C, Davies P (2010) Global threats to human water security and river biodiversity. Nature. doi:10.1038/nature09440
Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gai Y, Li B, Sun J, Li Y, Jiao F, Zhan Y, Chai Z (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Let 168(2):176–185. doi:10.1016/j.toxlet.2006.12.001
Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel A (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 23:2121–2134. doi:10.1021/nn800511k
Xiaolei Q, Alvarez J, Qilin L (2013) Applications of nanotechnology in water and waste water treatment. Water Res 47:3931–3946. doi:10.1016/j.watres.2012.09.058
Xiong J, Wang Y, Xue Q, Wu X (2011) Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem 13:900–904. doi:10.1039/c0gc00772b
Xiu Z, Ma J, Alvarez P (2011) Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol 45(20):9003–9008. doi:10.1021/es201918f
Xiu Z, Zhang Q, Puppala H, Colvin V, Alvarez P (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12(8):4271–4275. doi:10.1021/nl301934w
Xue X, Cheng R, Shi L, Zhong M, Zheng X (2017) Nanomaterials for water pollution monitoring and remediation. Environ Chem Lett 15:23–27. doi:10.1007/s10311-016-0595-x
Yadav S, Tam J, Veer Singh Ch (2015) A first principles study of hydrogen storage on lithium decorated two dimensional carbon allotropes. Int J Hydrog Energy 40:6128–6136. doi:10.1016/j.ijhydene.2015.03.038
Yallappa S, Manjanna J, Dhananjaya B (2015) Phytosynthesis of stable Au, Ag and Au–Ag alloy nanoparticles using J. Sambac leaves extract, and their enhanced antimicrobial activity in presence of organic antimicrobials. Spectrochim Acta A137:236–243. doi:10.1016/j.saa.2014.08.030
Yang K, Xing B (2010) Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem Rev 110(10):5989–6008. doi:10.1021/cr100059s
Yang K, Wu W, Jing Q, Zhu L (2008) Aqueous adsorption of aniline, phenol, and their substitutes by multi-walled carbon nanotubes. Environ Sci Technol 42(21):7931–7936. doi:10.1021/es801463v
Yao K, Li S, Tzeng T, Cheng C, Chang C (2009) Fluorescence silica nanoprobe as a biomarker for rapid detection of plant pathogens. Adv Mater Res 79–82:513–516. doi:10.4028/www.scientific.net/AMR.79-82.513
Yavuz C, Mayo J, Yu W, Prakash A, Falkner C, Yean S, Cong L, Shipley H, Kan A, Tomson M, Natelson D, Colvin V (2006) Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 314(5801):964–967. doi:10.1126/science.1131475
Yong J, Kwon E, Soo B (2010) Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst Eng 33:159–164. doi:10.1007/s00449-009-0373-2
Zhan G, Huang J, Du M, Sun D, Abdul-Rauf I, Lin W, Hong Y, Li Q (2012) Liquid phase oxidation of benzyl alcohol to benzaldehyde with novel uncalcined bioreduction Au catalysts: high activity and durability. Chem Eng J 187:232–238. doi:10.1016/j.cej.2012.01.051
Zhang Y, Jiang G, Wong Ka W, Zheng Z (2010) Green synthesis of indium oxide hollow spheres with specific sensing activities for flammable organic vapors. Sensor Lett 8:355–361. doi:10.1166/sl.2010.1277
Zhang Q, Yodyingyong S, Xi J, Myers D, Cao G (2012) Oxide nanowires for solar cell applications. Nanoscale 4:436–1445. doi:10.1039/C2NR11595F
Zhang Q, Uchaker E, Candelaria S, Cao G (2013) Nanomaterials for energy conversion and storage. Chem Soc Rev 42:3127–3171. doi:10.1039/c3cs00009e
Zhao M, Xia Q, Feng X, Zhu X, Mao Z, Jiand L, Wang K (2010) Synthesis, biocompatibility and cell labeling of l-arginine-functional β-cyclodextrin-modified quantum dot probes. Biomaterials 31:4401–4408. doi:10.1016/j.biomaterials.2010.01.114
Zhou Y, Lin W, Huang J, Wang W, Gao Y, Lin L, Li Q, Lin L, Du M (2010) Biosynthesis of gold nanoparticles by foliar broths: roles of biocompounds and other attributes of the extracts. Nanoscale Res Lett 5:1351–1359. doi:10.1007/s11671-010-9652-8
Zhu Z, Wang H, Yan B, Zheng H, Jiang Y, Miranda O, Rotello V, Xing B, Vachet R (2012) Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ Sci Technol 46(22):12391–12398. doi:10.1021/es301977w
Acknowledgements
The Spanish Ministry of Economy and Competitiveness (MINECO) and JJCC Castilla-La Mancha are gratefully acknowledged for funding this work with Grants CTQ2016-78793-P and JCCM PEIC- 2014-001-P, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villaseñor, M.J., Ríos, Á. Nanomaterials for water cleaning and desalination, energy production, disinfection, agriculture and green chemistry. Environ Chem Lett 16, 11–34 (2018). https://doi.org/10.1007/s10311-017-0656-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-017-0656-9