Abstract
Pharmaceuticals are one of the chemical groups largely used in health care, diagnosis aids, cure, treatment, and prevention of disease. Increasing production and use of pharmaceutical products has led to the entry of these products into the environment and eventual pollution. Several processes have been studied including the use of ultrasound for the removal of these pollutants from the aquatic environment. This review summarizes recent research advances dealing with the development of sonochemical technologies for the degradation of pharmaceutical organic pollutants. The review also includes the mechanism of sonochemical processes, the characteristics of irradiation sources, and the types of reactors used. Moreover, the important factors affecting the sonochemical oxidation efficiency are discussed, including the electrical power, frequency, and temperature. Finally, this paper discusses the recent applications of sonochemical processes on the degradation of pharmaceutical organic pollutants and suggests new research directions for the development of this promising technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals belong to a group of compounds used by humans or administrated to animals to enhance growth or health of livestock. Depending on the medication, up to 90 % of these products pass through a human body unchanged and are rejected in the environment (Boxall et al. 2012; Fram and Belitz 2011; Gadipelly et al. 2014). Scientists demonstrated that organic pollutants, such as pharmaceuticals, may enter the receiving water source through excretion, improper disposal of pharmaceutical waste, and excess quantities used by human and animal (Heberer et al. 2002; Roberts and Thomas 2006). This results in a substantial amount of anthropogenic compounds being directly or indirectly released into the aquatic environment which is present in relatively low concentrations of the range of ng L−1 to µg L−1 (Andreozzi et al. 2003; Heberer et al. 2002). The recent development of new analytical techniques has allowed detection of these low concentrations in the environment (Siddiqui et al. 2013). This type of concentration of some pollutants has been detected in surface water in many countries, such as USA, Canada, UK, and several other countries in Europe. Table 1 illustrates several pharmaceuticals found in water and wastewater. Although they are often present in low concentration, various studies on effects on quality and ecological functioning of water systems show that these chemicals form a potential new problem (Langenhoff et al. 2013). Their estrogenic and carcinogenic toxicity will impact the quality of ecological life and possibly affect human life (Leal et al. 2010). The toxicological studies have reported that the exposure of fish and other aquatic organisms to pharmaceuticals causes adverse reproductive effects, such as reduced viability of eggs, endocrine disruption, and changes in sperm density (Belgiorno et al. 2007). A considerable effort has been spent on understanding the removal and the degradation efficiencies of pharmaceuticals in water resources and wastewater discharges. Conventional wastewater treatment plant typically possess biological degradation using the activated sludge process, whereas advanced plants may have tertiary treatment processes, such as UV disinfection, reverse osmosis, ozonation, and other advanced oxidation process. Several works have been reported on the fact that pharmaceuticals are difficult to be completely removed using conventional biological/physicochemical treatment processes (Ferrey 2011; Fram and Belitz 2011). Some pharmaceutical discharges are degraded within the sewage treatment plants, but low removal rate was recorded depending on these physical and chemical characteristics (White et al. 2006). The majority of pharmaceuticals are relatively hydrophobic and therefore less effectively removed by sorption to sludge (Vieno et al. 2007). Wu et al. (2008) found a removal rate typical of 12–80 % for chlortetracycline (CTC) using conventional biological/physicochemical treatment processes, while others found even lower than 10 % for CBZ (Bound and Voulvoulis 2006).
Advanced oxidation process is chemical oxidation with hydroxyl radicals, which are very reactive and short-lived oxidants (Illes et al. 2013; Riesz and Christman 1986). The radicals need to be produced on site, in a reactor where the radicals can contact the organics contaminant in the wastewater (Mahamuni and Adewuyi 2010). Hydroxyl radicals may be produced in systems using: ultraviolet radiation/hydrogen peroxide, ozone/hydrogen peroxide, ultraviolet radiation/ozone, and through other means. Advanced oxidation treatment processes (AOP), such as ozonation, electrochemical oxidation, can generally achieve higher removal rates for pharmaceuticals compared with conventional processes (Mahamuni and Adewuyi 2010). Ultrasound is one example of AOPs that transform or destroy organic contaminants (Bremner et al. 2011; Leong et al. 2011). When water is exposed to ultrasound, acoustic pressure waves are produced and lead to the formation of bubbles. When the sound intensity is greater than the cavitation threshold, within several cycles of growing and shrinking, bubbles will exponentially and eventually collapse. The collapse of bubbles causes extremely high temperatures and pressures within a microenvironment in the liquid leading to the breakdown of gaseous water molecules in the bubbles to form hydroxyl radicals. This radical is capable of oxidizing and removing great variety of organic contaminants from water (Gogate et al. 2001; Leong et al. 2011; Mason 2011, 2012).
Recently, the use of ultrasound alone or combining with other process is contributed important role in all technical field and also for water treatment (Abdelsalam and Birkin 2002; Agranonik Ia et al. 1990). Ultrasound comprises unique advantages compared to other technologies, including no addition of chemicals, ease of use or automation, and high efficiency (Birkin et al. 2001; Bremner et al. 2011). However, according to the knowledge of the authors, a review of recent advances in the sonochemical techniques for the degradation of pharmaceutical organic pollutants was never published. This review presents the mechanisms and principal parameters for the degradation of pharmaceutical pollutants and its future prospects.
Mechanism of the sonochemical process
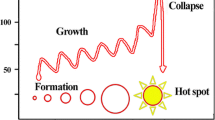
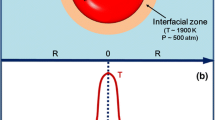
Ultrasound-induced cavitation is an extremely useful and versatile tool to carry out chemical reactions. Sonochemistry refers to the area of chemistry where chemical reactions are induced by sound (Mason 2012; Rooze et al. 2013). The extreme temperature conditions generated by a collapsing bubble can also lead to the formation of radical chemical species. Sonochemistry normally uses frequencies between 20 and 100 kHz as this is the range employed in common laboratory equipment (Klima 2011; Leong et al. 2011). However, since acoustic cavitation in liquids can be generated well above these frequencies, recent research into sonochemistry uses wide range up to 1 MHz (Bremner et al. 2011). Ultrasound consists of cycles of compression and rarefaction. When ultrasound is introduced into liquid, it creates oscillating regions; compression cycles exert a positive pressure, while rarefaction cycles exert negative pressure (Bremner et al. 2011; Klima 2011). Correspondingly, the liquid molecules experience periodic compression and expansion cycles. When the pressure amplitude exceeds the tensile strength of liquid during the rarefaction of ultrasonic waves, cavitational bubbles are formed as microbubbles; they absorb energy from ultrasound waves and grow. However, it will reach a stage of unstable size where it can no longer efficiently absorb energy (Bringas et al. 2011; Gallego-Juarez 2010). Figure 1 presents the formation, growth, and implosion of a cavitation bubble with various reaction sites in a bubble. Without the energy input, the cavity can no longer sustain itself and implodes during the compression cycle of ultrasonic wave. It is this implosion of the cavity that creates an unusual environment for chemical reactions (Gogate 2007; Rooze et al. 2013). Localized hot spots are formed, which reach temperatures and pressures around 5000 K and 500 atm, respectively, depending on factors, such as ultrasonic power, frequency, hydrostatic pressure, temperature, solvent property, and dissolved gas (Gogate 2007; Gogate et al. 2001, 2003). Some authors (Mason 2012; Rooze et al. 2013; Sinisterra 1992) have reported that sonochemical reactions can occur at internal site or interface of a bubble. The first one is the bubble’s interior gas phase, and it is suggested as the dominant site for sonochemical reaction due to the intense temperatures attained during collapse (~5000 K) (Leong et al. ). In this site, the typical reaction can be decomposed of water with the formation of radical species and the production of oxygen gas. The second one is the liquid–gas interface surrounding the bubble, which can reach temperatures of up to 1900 K (Leong et al. 2011). Thi2011s region is represented by intermediate temperature/pressure. When the bubble collapses, the generation of shock waves/microstreamers/microjets in this region will lead to increased turbulence and shear forces which can facilitate mass transport (Leong et al. 2011; Mason and Lorimer 2003d). In addition to these two primary reaction sites on the bubble, solutes in bulk solution beyond the bubble itself can react with the radicals formed inside or on the surface of the collapsing bubble (Marken et al. 1998; Mason 2011). Any species dissolved in the solution is clearly going to be subject to chemical reaction in theses site. As a result of such processes, chemical reactions, by-products formation/degradation or cleavage and radical generation from small molecules, can result. In a typical treatment, organic contaminants may degrade by decomposition (by heat produced in the gas and interfacial regions of the cavitation bubbles) and degrade by oxidation (react with hydroxyl radical in the gas, interface, and bulk region of the cavitation bubbles) (Leong et al. 2011; Xiao et al. 2014b). The degradation pathway of each organic contaminant is strongly dependent on its physical/chemical properties. For example, the major degradation site for volatile contaminants is in the gas region; hydrophobic pollutants in the interface site; and hydrophilic contaminants in bulk site. It is worth noting that the hydroxyl radicals generated during cavitation can be used for the oxidative degradation of organic pollutants in an aqueous system. The heat produced in the cavitation process can be used to remove volatile pollutants by pyrolytic decomposition (Leong et al. 2011). Generally, the production of hydroxyl radical, oxygen gas, and hydrogen peroxide is followed the reaction (Mason and Lorimer 2003d):
The hydronium and hydroxyl radical formed in this reaction are highly reactive and rapidly interact with other radical or chemical species in solution. H· are highly reducing in nature, and ·OH radicals are highly oxidizing (Gogate 2007; Mason and Lorimer 2003d). A common product of this reaction in water is hydrogen peroxide (Birkin et al. 2001; Mark et al. 1998). The generation of H· and ·OH radicals, commonly referred to as primary radicals, has been confirmed and quantified by a number of experimental techniques. Several methods of monitoring the sonochemical efficiency are available including the use of electron spin resonance (ESR) spin traps or the reaction with chemicals, such as terephthalic acid. In an alkaline aqueous solution, terephthalic acid produces terephthalate anions that react with hydroxyl radicals to generate highly fluorescent 2-hydroxyterephthalate ions which can be assayed using spectroscopy (Mark et al. 1998; Riesz and Christman 1986). In another dosimeter method, Fricke dosimetry, Fe2+ is oxidized by OH radicals and hydrogen peroxide to form Fe3+, and Fe3+ is determined photometrically (Iida et al. 2005). One of the most simple methods used to quantify the amount of ·OH radicals formed in water is based on “Weissler” method. This method involves the oxidation of iodide ions (Birkin et al. 2001; Gogate et al. 2001; Leong et al. 2011). In this technique, iodine is added to water which is then sonicated and reacts with the hydrogen peroxide, and the reaction scheme for this method is:
The absorbance of I3 − at 355 nm with the molar absorptivity ɛ = 26,300 dm3 mol−1 cm−1 can be measured with the spectrophotometer. The dosimeters based on photometry, such as Weissler method, produced reliable and reproducible results, but the sensitivity is not enough for special applications, for example chemical monitoring of single-bubble cavitation (Iida et al. 2005).
Sonochemical reactors
Ultrasonic transducers
Ultrasonic transducers are designed to convert either mechanical or electrical energy into high-frequency sound, and there are three main types: gas driven, liquid driven, and electromechanical (Leong et al. 2011; Tudela et al. 2011). The most readily available experimental setup for carrying out sonochemical reactions is the electromechanical transducers, which is currently used not only at laboratory level but also on a larger scale (Compton et al. 1997). The two main types of electromechanical transducers are based on either the piezoelectric or the magnetostrictive effect. Magentostrictive transducer is based on ferromagnetic materials which change dimension when a magnetic field is applied. Rapid on/off application leads to vibrations and sound energy (Mason and Lorimer 2003a). These are devices which use an effect found in some materials, e.g., nickel which reduces in size when placed in a magnetic field and returns to normal dimensions when the field is removed. When the magnetic field is applied as a series of short pulses to a magnetostrictive material, it vibrates at the same frequency (Mason and Lorimer 2003b). The most commonly used ones are piezoelectric transducers, generally employed to power the bath- and probe-type sonicator systems (Mason and Lorimer 2003e). Piezoelectric transducer is the most common piezoelectric effect—instantaneous generation of electric charge between opposite faces of certain materials when a sudden pressure is applied across them; transducers use opposite of this effect by either expansion or contraction when an electric charge is applied (Gonzalez-Garcia et al. 2010b; Mason and Lorimer 2003e). Usually an alternating charge at 20 kHz is applied. Piezoelectric materials are ceramics dispersed with BaTiO3, PbNb2O6, and lead zirconate titanate (PZT). Although more expensive than mechanical transducers, still electromechanical transducers are by far the most versatile (Compton et al. 1997; Margulis 1985). The most common form is a disk with or without a central hole. Another form is the piezoelectric transducer within the converter, where it is changed to mechanical vibrations (Mason and Peters 2002). The vibrations from the converter are intensified by the horn (probe), creating pressure waves in the liquid. Normally, piezoelectric devices must be cooled if they are to be used for longer periods at high temperatures because the ceramic material will degrade under these conditions (Mason and Peters 2002). Such transducers are highly efficient (>95 %), and depending on dimensions, they can be used over the whole range of ultrasonic frequencies from 20 kHz to several MHz (Leong et al. 2011; Mason and Lorimer 2003c; Rooze et al. 2013).
Ultrasonic reactors
The reactor design plays an important role for the efficacy of sonochemical reaction. Table 2 presents typical sonochemical transducers system used for degradation of pharmaceuticals. Ultrasonic baths were mostly manufactured for cleaning purposes. Typical baths usually have the transducers (pieces of piezoelectric in form of disk or plate) attached to the bottom, although the transducers can be submersed in a conventional tank to obtain similar effects (Gogate 2007; Gogate et al. 2011). For this type of transducer, the size (or diameter) of immersion transducer relative to the reactor size is one of the most important parameters. This ratio mainly affects the level of turbulent dissipation of energy and the intensity of the ultrasound and hence would be more essential where the physical effects are needed (Gogate et al. 2011). Bath systems are widely used in sonochemical research because they are readily available and relatively inexpensive. The reaction vessel is typically immersed in the fluid contained in the bath (indirect sonication) (Grcic et al. 2012; Reisse et al. 1994). In addition, obtaining reproducible results may be difficult because the amount of power reaching the reaction mixture is highly dependent upon the placement of the sample in the bath (Gogate et al. 2011; Klima 2011). When indirect sonication is used, the ultrasonic power which reaches the reaction vessel is relatively low as compared to other ultrasonic systems, such as a probe (Andaluri et al. 2012; Gogate 2007). The probe systems, also called horn systems, in which the transducers can be submersed in the fluid contained in the reactor, are more frequently used for sonochemical research in the laboratory due to the fact that this type of system is capable of delivering large amounts of power directly to the reaction mixture which can be regulated by varying the amplitude delivered to the transducer (Grcic et al. 2012; Siddique et al. 2011). The position of immersion probe in an ultrasonic horn is an important parameter which may affect the extent of reflection of the incident sound waves from the liquid surface as well as the reactors walls (Gogate et al. 2011). A few novel reactor configurations based on continuous operation have been developed for food processing and other application (Gogate and Pandit 2015; Graff 2015). However, in the laboratory scale, the batch operation approach is commonly used due to the size and possibility of successful application at small operation.
Parameters affecting the sonochemical process
Influence of the ultrasonic power
The power of ultrasound is the power delivered to the liquid divided by the surface area of the ultrasonic transducer (Gallego-Juárez and Graff 2015). The power can be characterized by two parameters: ultrasonic intensity, defined as the ratio between the power inputs to the irradiated medium to the transmitting area, or the power density, defined as the ratio between the power inputs to the irradiated medium to the sonication volume (Andaluri et al. 2012; Gallego-Juarez 2010). Higher acoustic pressure (amplitude of vibration), greater amounts of cavitational events, and more violent cavitational collapse happen at elevated power intensity of ultrasound. The relationship between the ultrasonic power intensity and the acoustic pressure may be expressed as Eq. 5 (Mason et al. 1990):
where I is the power intensity of a sound wave (W), P 0 is the acoustic pressure (Pa), ρ is the density of the liquid (kg m−3), C is the sound speed in the liquid (m s−1).
There exists a threshold value of intensity at which the beneficial effects of cavitation start to occur. Some authors have demonstrated that the increase in the yield with the power input is weak and reaches saturation, and attribute this effect to the coalescence of the bubbles, which would increase their size, leading to lower pressure pulses at the end of the collapse (Gallego-Juarez 2010; Pang et al. 2011). This saturation has been observed in several systems. Isariebel et al. (2009) investigates the influence of ultrasonic power on levodopa and paracetamol degradation carried out at the frequency of 574 kHz, and ultrasonic powers of 9, 17, 22 and 32 W were found that above a threshold power of about 9 W, the initial degradation rates increase linearly with the actual power for both products (with correlation coefficients of 0.9939 for levodopa and 0.9967 for paracetamol). This result is expected because by increasing the magnitude of power dissipation of the horn, there will be an increase in the number of cavities generated, and hence, the cumulative pressure pulse (number of cavities multiplied by the collapse pressure due to a single cavity) will also increase. After investigating the sonophotolytic degradation of synthetic pharmaceutical wastewater (composition of 4-aminophenol, paracetamol, phenol, chloramphenicol, benzoic acid, salicylic acid, diclofenac sodium, and nitrobenzene), Ghafoori et al. (2015) have found that by increasing the ultrasound power, higher TOC percent removal was achieved. The TOC removal was 60.22 % at 20 W compared to 75.26 % with power of 140 W. The elevated ultrasound power causes the higher rate of the breakage of H2O2 molecules in aqueous solution; consequently, the concentration of hydroxyl radicals was increased, and the produced radicals attack the organic matters. Furthermore, an increase in the ultrasonic power contributed to enhance mixing intensity due to the turbulence and microstreaming which is generated during the cavitational microbubble collapse (Ghafoori et al. 2015). Other authors have pointed out that lower intensities are more effective than higher intensities at the same total power, not only in experiments on water sonolysis but also in the degradation of organic compounds. Increasing intensity will raise the acoustic amplitude, resulting in a more violent cavitation bubble collapse. A study by Memarian and Farhadi (2008) to investigate the oxidation of dihydropyrimidinones by sonothermal process has shown that by increasing the ultrasound intensity, a decrease in the time of reaction is observed. The time of disappearance of dihydropyrimidinones was lowest (11 min) at highest power (460 W), due to the formation of oxidant compounds brought about by the sonochemical process. Similar phenomena have been observed by other authors (Mendez-Arriaga et al. 2008; Naddeo et al. 2009; Suri et al. 2007; Villegas-Guzman et al. 2015), while sonochemical process for degradation of estrogen hormones, ibuprofen, diclofenac, and dicloxacillin was applied; the degradation rates increased linearly with the power. This was confirmed when the hydrogen peroxide and hydroxyl radical formation is increased when ultrasound power increased (Villegas-Guzman et al. 2015). Lan et al. (2012) also observed that the extent of degradation increased with an increase in the ultrasonic power. The degradation efficiency of naproxen by combination of Fenton reagent and ultrasound irradiation after 33 min was only 66 % with power amplitude at 40 %.When power amplitude was set at 90 %, the degradation efficiency increased to almost 100 %. In the study of Thokchom et al. (2015) for the ultrasonically electrochemical oxidation of ibuprofen, the rate constants of ibuprofen were increased linearly with increasing ultrasonic power density. The degradation trend followed: 100 W L−1 > 80 W L−1 > 60 W L−1 > 40 W L−1. The number of active cavitation bubbles increases with an increase in the acoustic power leading to an increase in the corresponding amount of OH radicals generated. However, in another study the degradation of the antibiotic cephalexin in aqueous solution, Guo et al. (2010) have obtained that the optimal ultrasound power for cephalexin degradation in the system was 200 W (when compared to other ultrasonic powers 100, 300, 400, and 500 W). At higher power output, a large number of gas bubbles exist in the solution, which scatter the sound waves to the walls of the vessel or back to the transducer. Thus, less energy is dissipated in the liquid due to cavitational activity, although the vessel is exposed to higher power. Bubble cloud formation may occur at the surface of the horn or transducer if the power is too high, resulting in attenuation of the sound wave (Guo et al. 2010). Also, due to the higher number of cavities per unit volume of liquid, there is a coalescence of the cavities resulting in the formation of a larger cavity (the collapse pressure is inversely proportional to the size of the cavity). Sutar and Rathod (2015) also reported this phenomenon using ultrasound assisted enzyme catalyzed degradation of cetirizine dihydrochloride. Effect of irradiation power on degradation was investigated by varying the power in the range of 30–150 W and keeping constant frequency of 25 kHz. The degradation was less at lower irradiation power of 30 and 50 W (~50 %), as compared to higher irradiation power (~90 % at 100 W and ~80 % at 150 W). At highest power, more bubbles were formed with further increase in irradiation power, hampering the propagation of shock waves so that degradation gets decreased. Also at higher irradiation power, the bubbles coalesce to form bigger bubbles causing weak implosion so that degradation was reduced (Sutar and Rathod 2015). It is interesting that some of the authors have argued that the trend in energy consumption and its efficiency, higher power gave the best efficiency from the view point of degradation; however, the application of lower power is recommended for scale-up purposes (Gogate and Pandit 2015; Mason 2012; Thokchom et al. 2015). Summary of effect of the ultrasonic power on the degradation of pharmaceutical compounds is presented in Table 3.
Influence of the frequency
Frequency is the number of occurrences of a repeating event per unit time. The frequency is one of the most important operational variables in a sonochemical process. The frequency of ultrasound directly affects the generation, oscillation, resonant size, and final collapse of cavitational bubbles in terms of the amount of collapse and the violence of collapse. At lower frequency, more violent cavitation will be produced, resulting in higher localized temperature and pressure. High frequency will reduce cavitational effect because the negative pressure produced by rarefaction cycle is insufficient in duration and/or intensity to initiate cavitation, or compression cycle occurs faster than the time for microbubbles to collapse (Gonzalez-Garcia et al. 2010a; Leong et al. 2011). Figure 2 shows the frequencies range diagram and its effects on sonochemistry. Generally, at low frequency, ultrasound has stronger sonophysical effects, and at high frequency, it increases the production of hydroxyl radicals (Chen et al. 2011; Mason et al. 1990). Thokchom et al. (2015) have reported that high frequencies in the range of a few hundred to thousand kilohertz are recommended for the efficient degradation of contaminants pollutants in wastewater treatment, and lower frequencies are more typically employed in biotechnological applications, textile processing, solid–liquid extractions. At very high frequencies, the cavitation activity is reduced because either the rarefaction cycle of the sound wave produces a negative pressure which is insufficient in its duration and intensity to initiate cavitation or the compression cycle occurs faster than the time required for the bubbles to collapse (Gonzalez-Garcia et al. 2010a; Mason 2011). For example, at 20 kHz, 20,000 implosions per second could occur, whereas at 300 kHz, approximately 100,000 cavitational events occur per second (Chen et al. 2011). In a study by Rahmani et al. (2014), the application of the frequencies over 120 kHz does not have a noticeable effect on efficiency removal of tinidazole from aqueous solution by sonolysis in the presence of hydrogen peroxide. The efficiency degradation was about 40 % at 40 kHz and 60 % at 80 kHz. At higher frequencies, the efficiency was 74 % at 120 kHz and 75 % at 180 kHz. The author argued that by increasing the frequency, the number of acoustic cycles and bubble collapse increased. The resulting bubbles released less energy than the low-frequency ones for one single pulsation. Thus, applying higher frequency may compensate for the lower energy released in the single-bubble explosion. In the study of sonoelectrochemical oxidation of ibuprofen (IBP), Thokchom et al. (2015) have found that the kinetic rate constants for frequencies from 35 to 1000 kHz were found to increase from 0.014 (~19 % IBP removal) to 0.027 min−1 (~58 % IBP removal) and 1000 kHz shown to be the best for the oxidation of IBP. Author suggested that increased frequency decreases the lifetime of bubbles, thereby increasing bubble collapse per unit time producing more OH radicals, which in turn facilitates the transport activities across the interface (Thokchom et al. 2015). Besides, at a higher frequency, a shorter lifetime and quick collapse favor the ejection of hydroxyl radicals before they are able to recombine (Vajnhandl and Le Marechal 2007). For relatively low frequency, the number of cavitational events is increased, and bubbles have relatively more time to grow and collapse, which results in maximum size and violent collapse. However, for relatively high frequency, a large number of oscillations (the pulsation and collapse of the bubble occurs more quickly) might still be attributed to a larger fraction of hydroxyl radical escaped from the bubbles and lead to a higher frequency resulting in increase in the number of free radicals in the system. These results were confirmed by Im et al. (2013) during degradation of acetaminophen and naproxen by using ultrasound in the presence of single-walled carbon nanotubes. The authors found that the efficiency degradation of acetaminophen was 27.1 % at 28 kHz and 86.1 % at 1000 kHz. Similar results have also been reported in the case of naproxen, and the degradation efficiency was 52.5 % at 28 kHz and more than 99 % at 1000 kHz. After applying ultrasound assisted enzyme catalyzed degradation of cetirizine dihydrochloride, Sutar and Rathod (2015) showed higher degradation at 25 kHz (~80 %) than 40 kHz (~30 %). At higher frequency, the cavitation decreases because smaller and less energetic bubbles are formed which lead to decreased cavitation zone violence. Also it has been observed that at same energy dissipation in the reactor: less scattering and attenuation of sound waves as well as easy cavitation at lower frequency (Sutar and Rathod 2015). The optimal frequency for the degradation of an organic compound also depends on its properties, such as low or high solubility, volatility. For the low volatility (similar to properties of most of the pharmaceutical products), the degradation rate was better at the frequency at which the formation of hydroxyl radical or hydrogen peroxide was higher. Isariebel et al. (2009) showed that the degradation efficiency of the levodopa and the paracetamol in aqueous solution by the ultrasound at various frequencies (574, 860, and 1134 kHz) is maximum at 574 kHz, which was strongly linked to the reaction, whereby hydroxyl radicals and hydrogen peroxide were formed. Guyer and Ince (2011) indicated that a relatively high degradation rate was achieved at a frequency of 861 kHz during degradation of diclofenac in water by sonolysis. After 60 min of treatment, maximum rates were attained at 861 kHz due to a larger number of active bubbles and oscillations and ultimately efficiency of hydroxyl radical release into the bulk solution. Some other authors (Nejumal et al. 2014; Xiao et al. 2014a) have reported the same phenomena that faster degradation of pharmaceutical organic compounds at frequencies ranging from 350 to 600 kHz. The summary effect of the various frequencies on the sonochemical degradation of pharmaceutical organic compounds is shown in Table 4.
Influence of the temperature
It is known from the literature that cavitation-induced reactions are optimal with respect to operating temperature (Mason 2012; Rooze et al. 2013). Most of the ultrasonic experiments were carried out in temperature-controlled system to ensure that the isothermal conditions are maintained (Mason and Lorimer 2003c). With increasing temperature, the vapor pressure of water increased leading to an easier generation of cavitation bubbles and decreasing temperatures and pressures during the collapse-phase (collapse intensity) (Leong et al. 2011; Margulis and Margulis 2002). With the increasing vapor pressure, more cavitation bubbles are generated, collapsing with lower temperatures resulting in decreased hydroxyl radical concentration and therefore observed decrease in contaminant degradation. Thokchom et al. (2015) mentioned that the degradation trend followed: 40 °C > 30 °C > 20 °C > 10 °C when applied ultrasound to enhanced electrochemical oxidation of ibuprofen. An increase in temperature leads to the increased diffusion rate caused by the decreased viscosity of the medium with increasing temperature. Furthermore, less dissolved gas is present at higher temperatures and higher intensities, and more cavitation bubbles may be formed. Braeutigam et al. (2012), while investigating the degradation of carbamazepine in water by hydrodynamic-acoustic-cavitation, have found that the rate constant depends on the reaction temperature, and thus, an increase in temperature should lead to increased conversion. Reactions below the value of 25 °C resulted in lower formation of cavitation bubbles, lower bubble density, higher collapse intensity, and lower rate constants. For carbamazepine, an optimal temperature of 25 °C was detected which showed 90 % degradation of CBZ. With low solubility of the organic compound, the solubility was improved at higher temperature of the test samples from a heated stock solution (40 °C), as shown by Ziylan et al. (2013), and degradation of diclofenac at pH 3.0 by ultrasound contributed to higher degradation rate. However, an increase in solution temperature was shown to have a negative effect on the formation of cavitation, leading to a linear decrease in the collapse pressure. High temperatures during sonication likely facilitate bubble formation by increasing the equilibrium vapor pressure, and bubbles contain more vapors that cushion bubble implosion and decrease the temperature which was achieved upon bubble collapse, reducing the cavitation effects (Manousaki et al. 2004). Moreover, high temperatures can cause degassing of the liquid phase reducing the number of microsite available for cavitation. The degradation rate of organic compounds was shown to be directly proportional to temperature as organic molecules migrated from the bulk solution to the gas–liquid interface region where the temperature and hydroxyl radical concentration are high (Gallego-Juárez and Graff 2015; Neis 2015; Pétrier 2015). This phenomenon was observed by Im et al. (2013) while investigating ultrasound degradation of acetaminophen and naproxen in the presence of single-walled carbon nanotubes. The degradation rates of the acetaminophen increased gradually from 15 to 25 °C or 35 °C; however, temperatures higher than 35◦C resulted in a slight decrease in acetaminophen degradation at 1000 kHz. Similar results clearly show that the increase in temperature is not responsible for a faster mineralization of antipyrine in aqueous solution by sonophotocatalytic (Duran et al. 2013). Another report of Sutar and Rathod (2015) for the degradation of cetirizine dihydrochloride also shows that at lower temperature (30–50 °C), the extent of degradation is lower as compared to higher temperature range (60–70 °C). With an increase in reaction temperature from 40 to 50 °C, the degradation obtained after 7 h was increased from 58 to 84 %. The cavitation effect starts decreasing as the temperature was further increased higher, and only ~70 % of cetirizine dihydrochloride was removed at 60 °C and even lower at 70 °C (~20 %). Table 5 summarizes the effect of temperature on removal of different types of pharmaceutical organic pollutants in solution using sonochemical processes.
Sonochemical degradation of contaminants in association with different methods
The sonochemical processes for the oxidation of organic compounds have been developed as a powerful tool for treatment of effluents (Compton et al. 1997; Gogate and Pandit 2004; Mason 2012). Thus, Fenton reaction is well known in the degradation of organic material by extra generation of hydroxyl radicals. Hydrogen peroxide generated through cavitation action (ultrasound) of molecular oxygen is highly active toward the destruction of an organic species. Fenton reagent system can be applied to circumvent this problem that enabled the maximum amount of free radicals (specifically hydroxyl radicals) to be generated. This was achieved by the addition of Fe2+ to the solution that is known to catalyze the destruction of organic material through the generation of extra hydroxyl radicals, according to a Fenton-type mechanism. Lan et al. (2012) presented a method for degradation of naproxen by combination of Fenton reagent and ultrasound irradiation. Optimum dosage of Fenton reagent for naproxen removal comprised hydrogen peroxide at 9.98 mmol L−1, ferrous ion at 4.83 mg L−1 while naproxen at 20 mg L−1. The degradation of naproxen by ultrasound alone was very slight (only 30 %); however, when combined with Fenton, a degradation efficiency of 100 % was achieved within 10 min under sonolysis (Lan et al. 2012). In the Fenton process, Fe3+ reacted with H2O2 and produced a complex intermediate (Fe–O2H2+). Under ultrasound irradiation, the decomposition rate of Fe–O2H2 + could be greatly enhanced. Once the Fe2+ was isolated, it reacted with H2O2 and produced ·OH again, and then, a cycle mechanism was established (Sun et al. 2007) following the reaction:
Due to that, more ·OH can be formed rapidly in the process of US/Fenton, and so, the best degradation result of naproxen was achieved. On the other hand, there are other methods associated with sonochemical processes, such as in the presence of ozone or hydrogen peroxide and in conjunction with photocatalysis. Duran et al. (2013) reported the degradation of antipyrine in aqueous solution using an innovative homogeneous sono-photocatalytic oxidation process. At the selected operation conditions [H2O2] = 1500 ppm, [Fe2+] = 12 ppm, pH = 2.7, ultrasound amplitude = 100 %, pulse length (cycles) = 0.3 for 15 min and later 1 min, 92 % of TOC was removed after 50 min resulting in an aqueous solution containing 50 ppm of antipyrine. An important synergistic effect between sonolysis and photoFenton (UV/H2O2/Fe) of 45.4 % was quantified using the first-order rate constants for TOC (Duran et al. 2013). Ghafoori et al. (2015) have investigated the use of ultrasound to enhance degradation of synthetic pharmaceutical wastewater (a mixture of 4-aminophenol, paracetamol, phenol, chloramphenicol, benzoic acid, salicylic acid, diclofenac sodium, and nitrobenzene) by applying different processes including sonolysis (US alone), photolysis (UV alone), and combination of both UV and US. Total organic carbon (TOC) measurements were used to determine the effectiveness of oxidation process. After 120 min treatment time, it was found that removal of TOC from US is only about 3.1 % higher with photolytic (8 % reduction in the TOC was observed). However, combining UV and US did not enhance the degradation. The TOC reduction in the US/UV process was 4 % which is lower than that of the single process alone. The lower in reported results could be explained by considering the nature of components being studied which is a multicomponent wastewater more complicated than that of single component. Also, the output power of the US instrument and the power of the UV light could be influential in the degradation efficiency (Ghafoori et al. 2015). In another study, Naddeo et al. (2009) reported a method for the removal of diclofenac in aqueous solutions using ultrasound treatment with ozone addition. Under the conditions applied (31 g h−1 O3 flow, at 400 W L−1 ultrasonic power density), ozone, ultrasound, and combination of both proved effective in inducing diclofenac oxidation, leading to 22 % of mineralization for O3 and 36 % for US after 40 min of treatment. The synergy observed in the combined schemes, mainly due to the effects of US in enhancing the O3 decomposition, led to higher mineralization (about 39 %) for 40 min treatment and to a significantly higher mineralization level for shorter treatment duration. The molecular ozone in aqueous solutions is one of the most active oxidizing agents. It also can interact with water, giving oxygen, hydroxyl ion, and radical. Unlike the ozone, Rahmani et al. (2014) used hydrogen peroxide and ultrasonics assisted in enhancement of the degradation efficiency for the tinidazole removal from aqueous solution. The maximum removal efficiency of 75 % was achieved under the optimum operating conditions (pH 3, 120 kHz frequency, 333 mM L−1 of H2O2 and 150 min of operating time). It has been confirmed that the ultrasound waves improve agitation and also play a significant role in increasing the reaction rate, which could be due to the enhancement of the contact area between the hydroxyl radicals and the pollutants. The results also revealed that no harmful intermediate compounds were observed. Another approach to the combination, Secondes et al. (2014) have investigated the removal of emerging contaminants (diclofenac, carbamazepine, and amoxicillin) by simultaneous application of membrane ultrafiltration (Me), activated carbon adsorption (A), and ultrasound (US). It is interesting that the addition of ultrasound in the membrane filtration increased removal rate from an average of 92 % in the A–Me process to over 99 % in the US–A–Me process. This enhancement is a result of a fluid flow effect called microstreaming, and intense shear stress is produced as the flow velocities change which allows the contaminants to penetrate the porous structure. Biological is an alternative method for treatment of organic contaminant and has now been investigated along with ultrasound irradiation (Serna-Galvis et al. 2015). This technique uses aerobic microorganisms, and ultrasound was found to be able to remove the pharmaceutical fluoxetine. Biological degradation test of the sonicated and nonsonicated effluents has shown ~70 % of TOC removal within 360 min of sonication compared to ~10 % of TOC removal without sonication. The improvement was due to the transformation of the fluoxetine into more biodegradable substances under ultrasound irradiation (the BOD5/COD ratio changes from 0.05 to 0.41), and therefore, they could be more easily eliminated in a biological process (Serna-Galvis et al. 2015). The use of enzyme catalyzed in conjunction with ultrasound for degradation of cetirizine dihydrochloride has been also shown to be more effective as compared to conventional enzymatic degradation technique (Sutar and Rathod 2015). Using enzyme catalyzed alone, cetirizine dihydrochloride was degraded less about 13 % only in 24 h. On the combination, the maximum degradation of 91 % has been achieved at optimized experimental parameters (0.02 % enzyme loading (w v−1), 50 °C, power 100 W, frequency 25 kHz and 50 % duty cycle with agitation speed of 200 rpm). It is observed that enzymatic degradation of cetirizine dihydrochloride under the influence of ultrasound irradiation not only enhances the degradation but also reduces the time of degradation. This is due to the fact that more interaction between enzyme and substrate particles enhances the degradation percentage. Recently, the study of using ultrasound, along with electrochemical, has increased considerably for degradation of pharmaceutics contaminants (Thokchom et al. 2015; Tran et al. 2015). The beneficial results from exposing electrochemical cells to the effects of power ultrasound include the enhancement of mass transport, the increase in current efficiencies, and the continuous activation of the electrode surface (Birkin and Silva-Martinez 1997). These effects can be ascribed to the rapid generation and collapse of microbubbles within the electrolyte medium or nearby the electrode surface. This cleaning effect has been reported to improve electron exchanges by peeling out passivation films on surface electrode or piercing them by microholes (Compton et al. 1997). Tran et al. (2015) have described the sonolytic degradation of pharmaceutics pollutants and found that the carbamazepine concentration (10 mg L−1) could be optimally diminished up to 90 % by applying a current intensity of 4.86 A for a 177-min reaction period and by imposing an ultrasound power of 38.29 W. The optimal conditions were subsequently applied for tertiary treatment of municipal wastewater effluent contaminated with 10 µg CBZ L−1. The removal efficiencies of CBZ, TOC, COD, and color recorded were 93, 60, 93, and 86 %, respectively. Likewise, the toxicity was completely removed (bacterium Vibrio fischeri) from municipal wastewater effluent. The synergistic effect is defined as an effect arising between two or more agents, entities, factors, and substances that produce an effect greater than the sum of their individual effects. The degree of synergy, S, which can be calculated via kinetics constant k by the equation:
From this equation, the author calculated the degree of synergy was 11.11 % indicating that the CBZ removal is higher when both processes, ultrasound and electrochemical, are present than individually. In this combination, ultrasounds enhance the mass transfer between the electrolyte and the electrodes (Tran et al. 2015). Other studies performed by Thokchom et al. (2015) on the sonoelectrochemical degradation of Ibuprofen reported that among the methods examined (US, EC, and US/EC), the hybrid method US/EC resulted 89.32, 81.85, and 88.7 % degradations using NaOH, H2SO4, and deionized water, respectively, with a constant electrical voltage of 30 V, an ultrasound frequency of 1000 kHz, and a power density of 100 W L−1 at 25 °C in 1-h treatment time. When compared to single process alone, only 73.81 % (NaOH), 50.54 % (H2SO4), and 21.46 % (deionized water) removals of IBP were achieved by electrolysis. The positive synergic process may be attributed to various mechanisms such as acoustic streaming induced by the ultrasonic transducer, enhancing mass transport to the electrode, electrode surface activation by hindering passive layer formation due to cavitational collapse at the solid–liquid interface; a highly reactive ·OH radicals produced by violent collapse of ultrasonic cavitation (Thokchom et al. 2015). Table 6 summarizes the removal of different types of pharmaceutical organic pollutants in solution using sonochemical combination process.
Current, future prospects, and challenges of sonochemical processes
Ultrasound-induced cavitation is an effective tool for application of sonochemistry in the field of wastewater treatment. This tool has been recently exploited rapidly due to the availability of industrial transducers and ultrasonic reactors. The hydroxyl radicals generated during cavitation can be used for the oxidative degradation of organic pollutants in an aqueous system. Recently reported studies have shown that sonochemical degradation of various pharmaceutical organic pollutants could be achieved by both oxidative and pyrolysis mechanisms. Ultrasound has shown that they have been often used in conjunction with conventional techniques, such as ultra filtration, biological, UV, Fenton, ozonation, electrochemical, that can increase effectiveness and also demanded lower requirements for chemical or energy usage. In combination with ozone or hydrogen peroxide, ultrasound can be used to add excessive oxidizing power which provides faster degradation rates. Ultrasound can also combine with ultraviolet light for the destruction of chemical pollutants. The improvement is the result of mechanical effects of ultrasound to increase mass transport in the system. However, effect of ultrasound on the synergistic effect (lower or higher efficiency), mass transport (increase or decrease in the transport of species in the bulk), especially effect of ultrasound on the cleaning electrodes surfaces (in sono-electrochemical or sono-photo-electrochemical), is still not cleared and is under development. More over many of these techniques based on ultrasound are frequently unclear because by-products have been unidentified or even more persistent and toxic than original contaminants. It is well known that the beneficial effect of ultrasound for cleaning is widely used, and during the electrochemical treatment, the organic compounds and/or sample constituents have usually strong interactions with the surface of electrodes thus inhibiting their usage in practical. The future study should be focus on the association of ultrasound with other methods and the synergistic phenomena. The energy consumption, efficiency, and another important factor such as reactor design, scale-up also need to be considered. The reactor design in terms of the energy requirement, the size of the transducer, ratio of the transducer to the reactor diameter, also size of reactor, and position of the transducers should be considered as to play an important role in the specific application. Electrochemical processes can be a powerful tool for the degradation of contaminant, since the ultrasound is responsible for the increase in mass transport, activation of electrodes surfaces using the electrochemical direct/indirect oxidized contaminants. Ultrasound is particularly effective due to its cavitational effect, and it can reach electrode surface or mixing the system which is not easily reached by conventional methods. The main drawback of sonochemical processes is its energy cost and its application at large scale. The important scale-up consideration is to set up the optimum parameters for the treatment in terms of the operating cost/design/efficiency factor that influence cavitation. However, the application of ultrasound on an electrolytic solution is beneficial so that it reduces the resistant, activation electrode surface, thereby allowing discharge at lower applied voltage, which could contribute to lower consumption of energy for total system. Also, the design and produced materials should be planned to overcome application on large scale to provide economically viable treatments.
Conclusion
Ultrasonic cavitation, which is an AOP, has been proposed as an attractive alternative method for the treatment of pharmaceutical contaminants due to its advantages of being nonselective and without generating secondary pollutants. Different theories are usually used to explain sonochemical effect, but hot-spot theory is usually used to explain the process, in which microbubbles are produced to generate heat and different reactive species. Ultrasonic cavitation is known to generate reactive species which are able to oxidize toxic contaminants present in the environment. The mechanisms of ultrasound make it unique when compared with other AOPs. Ultrasound can effectively decompose pharmaceutical compounds in aqueous solution, and the extent of degradation depends strongly on the operating conditions, such as ultrasound power, ultrasound frequency, and temperature of the medium. However, the degradation rate is slow if only the ultrasonic treatment is used. Therefore, some efforts have been made to increase the degradation efficiency by applying hybrid techniques, such as sonobiological, sonofiltration, sonoelectrochemical, sonophotocatalytic, sono/Fenton/ozonation. The combination has shown that the effectiveness of this application can be increased. This proves that sonochemical processes is an advanced technology and is gaining importance for the purification of contaminated water, especially for pharmaceutical pollutants.
References
Abdelsalam ME, Birkin PR (2002) A study investigating the sonoelectrochemical degradation of an organic compound employing Fenton’s reagent. Phys Chem Chem Phys 4:5340–5345
Agranonik Ia R, Karpukhin VF, Faingo’l’d ZL (1990). [Sonochemical destruction of pollution of industrial waste waters]. Antibiotiki i khimioterapiia = Antibiotics and chemoterapy [sic]/Ministerstvo meditsinskoi i mikrobiologicheskoi promyshlennosti SSSR 35:48–51
Andaluri G, Rokhina EV, Suri RPS (2012) Evaluation of relative importance of ultrasound reactor parameters for the removal of estrogen hormones in water. Ultrason Sonochem 19:953–958
Andreozzi R, Marotta R, Paxeus N (2003) Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 50:1319–1330
Belgiorno V, Rizzo L, Fatta D, Della Rocca C, Lofrano G, Nikolaou A, Naddeo V, Meric S (2007) Review on endocrine disrupting-emerging compounds in urban wastewater: occurrence and removal by photocatalysis and ultrasonic irradiation for wastewater reuse. Desalination 215:166–176
Birkin PR, Silva-Martinez S (1997) A study on the effect of ultrasound on electrochemical phenomena. Ultrason Sonochem 4:121–122
Birkin PR, Power JF, Leighton TG (2001) Electrochemical evidence of H· produced by ultrasound. Chem Commun 21:2230–2231
Bound JP, Voulvoulis N (2006) Predicted and measured concentrations for selected pharmaceuticals in UK rivers: implications for risk assessment. Water Res 40:2885–2892
Boxall ABA, Monteiro SC, Fussell R, Williams RJ, Bruemer J, Greenwood R, Bersuder P (2012) Targeted monitoring for human pharmaceuticals in vulnerable source and final waters. Drinking Water Inspectorate project WD0805, (DWI 70/2/231), London
Braeutigam P, Franke M, Schneider RJ, Lehmann A, Stolle A, Ondruschka B (2012) Degradation of carbamazepine in environmentally relevant concentrations in water by Hydrodynamic-Acoustic-Cavitation (HAC). Water Res 46:2469–2477
Bremner DH, Burgess AE, Chand R (2011) The chemistry of ultrasonic degradation of organic compounds. Curr Org Chem 15:168–177
Bringas E, Saiz J, Ortiz I (2011) Kinetics of ultrasound-enhanced electrochemical oxidation of diuron on boron-doped diamond electrodes. Chem Eng J 172:1016–1022
Buser HR, Poiger T, Muller MD (1998) Occurrence and fate of the pharmaceutical drug diclofenac in surface waters: rapid photodegradation in a lake. Environ Sci Technol 32:3449–3456
Chen D, Sharma SK, Mudhoo A (2011) Handbook on applications of ultrasound: sonochemistry for sustainability. Taylor & Francis, London
Compton RG, Eklund JC, Marken F (1997) Sonoelectrochemical processes: a review. Electroanalysis 9:509–522
Duran A, Monteagudo JM, Sanmartin I, Garcia-Diaz A (2013) Sonophotocatalytic mineralization of antipyrine in aqueous solution. Appl Catal B Environ 138:318–325
Ferrey M (2011) Wastewater treatment plant endocrine disrupting chemical monitoring study. Minnesota Pollution Control Agency
Fram MS, Belitz K (2011) Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci Total Environ 409:3409–3417
Gadipelly C, Perez-Gonzalez A, Yadav GD, Ortiz I, Ibanez R, Rathod VK, Marathe KV (2014) Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Ind Eng Chem Res 53:11571–11592
Gallego-Juarez JA (2010) High-power ultrasonic processing: recent developments and prospective advances. Phys Proc 3:35–47
Gallego-Juárez JA, Graff KF (2015) 1: Introduction to power ultrasonics. In: Gallego-Juárez JA, Graff KF (eds) Power ultrasonics. Woodhead Publishing, Oxford, pp 1–6
Ghafoori S, Mowla A, Jahani R, Mehrvar M, Chan PK (2015) Sonophotolytic degradation of synthetic pharmaceutical wastewater: statistical experimental design and modeling. J Environ Manag 150:128–137
Gogate PR (2007) Application of cavitational reactors for water disinfection: current status and path forward. J Environ Manag 85:801–815
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8:553–597
Gogate PR, Pandit AB (2015) 24: Design and scale-up of sonochemical reactors for food processing and other applications. In: Gallego-Juárez JA, Graff KF (eds) Power ultrasonics. Woodhead Publishing, Oxford, pp 725–755
Gogate PR, Shirgaonkar IZ, Sivakumar M, Senthilkumar P, Vichare NP, Pandit AB (2001) Cavitation reactors: efficiency assessment using a model reaction. AIChE J 47:2526–2538
Gogate PR, Mujumdar S, Pandit AB (2003) Sonochemical reactors for waste water treatment: comparison using formic acid degradation as a model reaction. Adv Environ Res 7:283–299
Gogate PR, Sutkar VS, Pandit AB (2011) Sonochemical reactors: important design and scale up considerations with a special emphasis on heterogeneous systems. Chem Eng J 166:1066–1082
Gonzalez-Garcia J, Esclapez MD, Bonete P, Hernandez YV, Garreton LG, Saez V (2010a) Current topics on sonoelectrochemistry. Ultrasonics 50:318–322
Gonzalez-Garcia J, Saez V, Esclapez MD, Bonete P, Vargas Y, Gaete L (2010b) Relevant developments and new insights on sonoelectrochemistry. Phys Proc 3:117–124
Graff KF (2015) 6: Power ultrasonic transducers: principles and design. In: Gallego-Juárez JA, Graff KF (eds) Power ultrasonics. Woodhead Publishing, Oxford, pp 127–158
Grcic I, Sipic A, Koprivanac N, Vrsaljko D (2012) Global parameter of ultrasound exploitation (GPUE) in the reactors for wastewater treatment by sono-Fenton oxidation. Ultrason Sonochem 19:270–279
Guo WL, Wang HZ, Shi YH, Zhang GY (2010) Sonochemical degradation of the antibiotic cephalexin in aqueous solution. Water Sa 36:651–654
Guyer GT, Ince NH (2011) Degradation of diclofenac in water by homogeneous and heterogeneous sonolysis. Ultrason Sonochem 18:114–119
Hapeshi E, Achilleos A, Papaioannou A, Valanidou L, Xekoukoulotakis NP, Mantzavinos D, Fatta-Kassinos D (2010) Sonochemical degradation of ofloxacin in aqueous solutions. Water Sci Technol J Int Assoc Water Pollut Res 61:3141–3146
Heberer T, Reddersen K, Mechlinski A (2002) From municipal sewage to drinking water: fate and removal of pharmaceutical residues in the aquatic environment in urban areas. Water Sci Technol 46:81–88
Hirsch R, Ternes T, Haberer K, Kratz KL (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225:109–118
Iida Y, Yasui K, Tuziuti T, Sivakumar M (2005) Sonochemistry and its dosimetry. Microchem J 80:159–164
Illes E, Takacs E, Dombi A, Gajda-Schrantz K, Racz G, Gonter K, Wojnarovits L (2013) Hydroxyl radical induced degradation of ibuprofen. Sci Total Environ 447:286–292
Im JK, Heo J, Boateng LK, Her N, Flora JRV, Yoon J, Zoh KD, Yoon Y (2013) Ultrasonic degradation of acetaminophen and naproxen in the presence of single-walled carbon nanotubes. J Hazard Mater 254:284–292
Isariebel QP, Carine JL, Ulises-Javier JH, Anne-Marie W, Henri D (2009) Sonolysis of levodopa and paracetamol in aqueous solutions. Ultrason Sonochem 16:610–616
Jelic A, Michael I, Achilleos A, Hapeshi E, Lambropoulou D, Perez S, Petrovic M, Fatta-Kassinos D, Barcelo D (2013) Transformation products and reaction pathways of carbamazepine during photocatalytic and sonophotocatalytic treatment. J Hazard Mater 263:177–186
Klima J (2011) Application of ultrasound in electrochemistry: an overview of mechanisms and design of experimental arrangement. Ultrasonics 51:202–209
Lan RJ, Li JT, Sun HW, Su WB (2012) Degradation of naproxen by combination of Fenton reagent and ultrasound irradiation: optimization using response surface methodology. Water Sci Technol 66:2695–2701
Langenhoff A, Inderfurth N, Veuskens T, Schraa G, Blokland M, Kujawa-Roeleveld K, Rijnaarts H (2013) Microbial removal of the pharmaceutical compounds Ibuprofen and diclofenac from wastewater. BioMed Res Int 2013:325806
Leal LH, Vieno N, Temmink H, Zeeman G, Buisman CJ (2010) Occurrence of xenobiotics in gray water and removal in three biological treatment systems. Environ Sci Technol 44:6835–6842
Leong T, Ashokkumar M, Kentish S (2011) The fundamentals of power ultrasound: a Review. Acoust Aust 39:54–63
Mahamuni NN, Adewuyi YG (2010) Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: a review with emphasis on cost estimation. Ultrason Sonochem 17:990–1003
Manousaki E, Psillakis E, Kalogerakis N, Mantzavinos D (2004) Degradation of sodium dodecylbenzene sulfonate in water by ultrasonic irradiation. Water Res 38:3751–3759
Margulis MA (1985) Sonoluminescence and sonochemical reactions in cavitation fields: a review. Ultrasonics 23:157–169
Margulis MA, Margulis IM (2002) Contemporary review on nature of sonoluminescence and sonochemical reactions. Ultrason Sonochem 9:1–10
Mark G, Tauber A, Laupert R, Schuchmann H-P, Schulz D, Mues A, von Sonntag C (1998) OH-radical formation by ultrasound in aqueous solution—part II: terephthalate and Fricke dosimetry and the influence of various conditions on the sonolytic yield. Ultrason Sonochem 5:41–52
Marken F, Goldfarb DL, Compton RG (1998) Sonoelectrochemistry in highly resistive media: mass transport effects. Electroanalysis 10:562–566
Mason TJ (2011) Therapeutic ultrasound an overview. Ultrason Sonochem 18:847–852
Mason TJ (2012) Trends in sonochemistry and ultrasonic processing. AIP Conf Proc 1433:21–26
Mason TJ, Lorimer JP (2003a) General principles, applied sonochemistry. Wiley-VCH Verlag GmbH & Co. KGaA, New York, pp 25–74
Mason TJ, Lorimer JP (2003b) Introduction to applied ultrasonics, applied sonochemistry. Wiley-VCH Verlag GmbH & Co. KGaA, New York, pp 1–24
Mason TJ, Lorimer JP (2003c) Sonochemistry in environmental protection and remediation, applied sonochemistry. Wiley-VCH Verlag GmbH & Co. KGaA, New York, pp 131–156
Mason TJ, Lorimer JP (2003d) Sonoelectrochemistry, applied sonochemistry. Wiley-VCH Verlag GmbH & Co. KGaA, New York, pp 225–266
Mason TJ, Lorimer JP (2003e) Ultrasonic equipment and chemical reactor design, applied sonochemistry. Wiley-VCH Verlag GmbH & Co. KGaA, New York, pp 267–293
Mason T, Peters D (2002) 1: An introduction to the uses of power ultrasound in chemistry. In: Peters TM (ed) Practical sonochemistry, 2nd edn. Woodhead Publishing, Oxford, pp 1–48
Mason TJ, Lorimer JP, Walton DJ (1990) Sonoelectrochemistry. Ultrasonics 28:333–337
McArdell CS, Molnar E, Suter MJF, Giger W (2003) Occurrence and fate of macrolide antibiotics in wastewater treatment plants and in the Glatt Valley Watershed, Switzerland. Environ Sci Technol 37:5479–5486
Memarian HR, Farhadi A (2008) Sono-thermal oxidation of dihydropyrimidinones. Ultrason Sonochem 15:1015–1018
Mendez-Arriaga F, Torres-Palma RA, Petrier C, Esplugas S, Gimenez J, Pulgarin C (2008) Ultrasonic treatment of water contaminated with ibuprofen. Water Res 42:4243–4248
Nachiappan S, Muthukumar K (2013) Treatment of pharmaceutical effluent by ultrasound coupled with dual oxidant system. Environ Technol 34:209–217
Naddeo V, Belgiorno V, Ricco D, Kassinos D (2009) Degradation of diclofenac during sonolysis, ozonation and their simultaneous application. Ultrason Sonochem 16:790–794
Neis U (2015) 32: The use of power ultrasound for wastewater and biomass treatment. In: Gallego-Juárez JA, Graff KF (eds) Power ultrasonics. Woodhead Publishing, Oxford, pp 973–996
Nejumal KK, Manoj PR, Aravind UK, Aravindakumar CT (2014) Sonochemical degradation of a pharmaceutical waste, atenolol, in aqueous medium. Environ Sci Pollut Res Int 21:4297–4308
Pang YL, Abdullah AZ, Bhatia S (2011) Review on sonochemical methods in the presence of catalysts and chemical additives for treatment of organic pollutants in wastewater. Desalination 277:1–14
Pétrier C (2015) 31: The use of power ultrasound for water treatment. In: Gallego-Juárez JA, Graff KF (eds) Power ultrasonics. Woodhead Publishing, Oxford, pp 939–972
Rahmani H, Gholami M, Mahvi AH, Alimohammadi M, Azarian G, Esrafili A, Rahmani K, Farzadkia M (2014) Tinidazole removal from aqueous solution by sonolysis in the presence of hydrogen peroxide. Bull Environ Contam Toxicol 92:341–346
Reisse J, Francois H, Vandercammen J, Fabre O, Kirschdemesmaeker A, Maerschalk C, Delplancke JL (1994) Sonoelectrochemistry in aqueous-electrolyte: a new-type of sonoelectroreactor. Electrochim Acta 39:37–39
Richardson SD (2007) Water analysis: emerging contaminants and current issues. Anal Chem 79:4295–4323
Riesz P, Christman CL (1986) Sonochemical free radical formation in aqueous solutions. Fed Proc 45:2485–2492
Roberts PH, Thomas KV (2006) The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci Total Environ 356:143–153
Rooze J, Rebrov EV, Schouten JC, Keurentjes JTF (2013) Dissolved gas and ultrasonic cavitation: a review. Ultrason Sonochem 20:1–11
Secondes MFN, Naddeo V, Belgiorno V, Ballesteros F (2014) Removal of emerging contaminants by simultaneous application of membrane ultrafiltration, activated carbon adsorption, and ultrasound irradiation. J Hazard Mater 264:342–349
Serna-Galvis EA, Silva-Agredo J, Giraldo-Aguirre AL, Torres-Palma RA (2015) Sonochemical degradation of the pharmaceutical fluoxetine: effect of parameters, organic and inorganic additives and combination with a biological system. Sci Total Environ 524–525C:354–360
Siddique M, Farooq R, Khan ZM, Khan Z, Shaukat SF (2011) Enhanced decomposition of reactive blue 19 dye in ultrasound assisted electrochemical reactor. Ultrason Sonochem 18:190–196
Siddiqui MR, AlOthman ZA, Rahman N (2013) Analytical techniques in pharmaceutical analysis: a review. Arab J Chem. doi:10.1016/j.arabjc.2013.04.016
Sinisterra JV (1992) Application of ultrasound to biotechnology: an overview. Ultrasonics 30:180–185
Sun JH, Sun SP, Sun JY, Sun RX, Qiao LP, Guo HQ, Fan MH (2007) Degradation of azo dye Acid black 1 using low concentration iron of Fenton process facilitated by ultrasonic irradiation. Ultrason Sonochem 14:761–766
Suri RPS, Nayak M, Devaiah U, Helmig E (2007) Ultrasound assisted destruction of estrogen hormones in aqueous solution: effect of power density, power intensity and reactor configuration. J Hazard Mater 146:472–478
Sutar RS, Rathod VK (2015) Ultrasound assisted enzyme catalyzed degradation of Cetirizine dihydrochloride. Ultrason Sonochem 24:80–86
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32:3245–3260
Thokchom B, Kim K, Park J, Khim J (2015) Ultrasonically enhanced electrochemical oxidation of ibuprofen. Ultrason Sonochem 22:429–436
Tran N, Drogui P, Brar SK (2015) Sonoelectrochemical oxidation of carbamazepine in waters: optimization using response surface methodology. J Chem Technol Biotechnol 90:921–929
Tudela I, Saez V, Esclapez MD, Bonete P, Harzali H, Baillon F, Gonzalez-Garcia J, Louisnard O (2011) Study of the influence of transducer-electrode and electrode-wall gaps on the acoustic field inside a sonoelectrochemical reactor by FEM simulations. Chem Eng J 171:81–91
Vajnhandl S, Le Marechal AM (2007) Case study of the sonochemical decolouration of textile azo dye Reactive Black 5. J Hazard Mater 141:329–335
Vieno NM, Harkki H, Tuhkanen T, Kronberg L (2007) Occurrence of pharmaceuticals in river water and their elimination a pilot-scale drinking water treatment plant. Environ Sci Technol 41:5077–5084
Villegas-Guzman P, Silva-Agredo J, Giraldo-Aguirre AL, Florez-Acosta O, Petrier C, Torres-Palma RA (2015) Enhancement and inhibition effects of water matrices during the sonochemical degradation of the antibiotic dicloxacillin. Ultrason Sonochem 22:211–219
White JR, Belmont MA, Metcalfe CD (2006) Pharmaceutical compounds in wastewater: wetland treatment as a potential solution. Sci World J 6:1731–1736
Wu CX, Spongberg AL, Witter JD (2008) Determination of the persistence of pharmaceuticals in biosolids using liquid-chromatography tandem mass spectrometry. Chemosphere 73:511–518
Xiao R, He Z, Diaz-Rivera D, Pee GY, Weavers LK (2014a) Sonochemical degradation of ciprofloxacin and ibuprofen in the presence of matrix organic compounds. Ultrason Sonochem 21:428–435
Xiao R, Wei Z, Chen D, Weavers LK (2014b) Kinetics and mechanism of sonochemical degradation of pharmaceuticals in municipal wastewater. Environ Sci Technol 48:9675–9683
Ziylan A, Koltypin Y, Gedanken A, Ince NH (2013) More on sonolytic and sonocatalytic decomposition of Diclofenac using zero-valent iron. Ultrason Sonochem 20:580–586
Acknowledgments
Sincere thanks are extended to the National Sciences and Engineering Research Council of Canada for their financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tran, N., Drogui, P. & Brar, S.K. Sonochemical techniques to degrade pharmaceutical organic pollutants. Environ Chem Lett 13, 251–268 (2015). https://doi.org/10.1007/s10311-015-0512-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-015-0512-8