Abstract
In managed forests, partial harvest within riparian areas is one way to provide harvest opportunities and maintain some of the functions of riparian vegetation. We examined the response of coniferous seedlings to a 50 %-partial harvest in a second-growth, coniferous riparian forest in the Pacific Northwest. Partial canopy opening facilitated the establishment of Tsuga heterophylla and Thuja plicata, but Pseudotsuga menziesii did not show the same tendency. Results suggested that differences in seedling establishment were related to differences in light. Increased light availability also stimulated growth of Rubus spectabilis, especially in moist sites, and the dense cover precluded establishment of coniferous seedlings. Once R. spectabilis was established, continued stem recruitment maintained a dense stable cover, and even intense disturbance did not affect the stability of the populations. R. spectabilis would be a major competitor to constrain conifer seedlings to regenerate in riparian zones. Although Gaultheria shallon extended their cover, competition from dense G. shallon was not as severe as from R. spectabilis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The riparian area is the boundary between terrestrial and aquatic ecosystems, and riparian vegetation affects many aquatic ecosystem structures and functions (Naiman et al. 2005; Richardson et al. 2005). For instance, riparian vegetation modifies light and temperature regimes, alters water quality, provides food and habitat for aquatic and terrestrial consumers, stabilizes streambanks, and provides coarse wood to streams, which can alter fish habitat, channel morphology, and sediment routing (Kiffney et al. 2003). For many of the above reasons it has become common practice to retain some amount of riparian vegetation cover along streamsides (Richardson et al. 2012).
Partial harvest is one of the forest management tools to provide harvest opportunities and maintain some of the functions of riparian vegetation (Lecerf and Richardson 2010). Partial canopy retention is effective in retaining some biological elements, providing residual structure, refugia, and a local source of propagules for disturbance-sensitive species, and creating barriers to invasion of light-demanding and non-native species (Nelson and Halpern 2005). Because management history and condition of the surrounding managed stand determine the function of vegetation, riparian management practices would be desired to contribute to maintaining ecologically sound wood production. With knowledge of the effects of different harvest disturbances on riparian vegetation, forest managers would be able to choose silvicultural strategies that balance commodity production and conservation objectives.
Seed germination and seedling establishment is one of the most vulnerable transitions in a plant’s life cycle (Gómez-Aparicio et al. 2005), and seedling establishment is affected by many factors, such as climate (Gómez-Aparicio et al. 2005), canopy (Keyes et al. 2001) and groundcover (Sugita and Tani 2001). Environmental factors are highly heterogeneous in space and time, and suitable microsites for seedling growth differ among species. An examination of which microhabitats, under which environmental conditions constitute safe sites for seedling establishment, is essential to consideration of the establishment and trajectories of future forests (Gómez-Aparicio et al. 2005).
The forest floor is composed of various microsites (e.g., bare soil, leaf litter, coarse wood, mound, and pit). Coarse wood, such as fallen logs, large branches and stumps, is an important establishment site for tree regeneration, serving as a “nurse log” (Harmon and Franklin 1989; Kennedy and Quinn 2001). Because fallen logs are elevated above the ground surface, they have some advantages, i.e., seedlings growing on the logs can avoid competition with other plants, being buried in litter accumulation, or being consumed (Callaway 1995; Swanson et al. 2011).
The partial harvest of the overstory trees would bring some changes, and it could have significant effects on the understorey. First, some fallen logs and stumps resulting from the harvest would create favorable sites for tree regeneration. Second, resource availability and physical disturbance would increase with formation of canopy gaps. The increases in resource availability change interspecific competition, and promote germination from the seed bank and regeneration of suppressed trees (Narukawa and Yamamoto 2001), and this change promotes invasion of ruderal species, shade-intolerant species, and introduced species (Nelson and Halpern 2005; González-Alday et al. 2009).
Rubus spectabilis Pursh (Rosaceae) and Gaultheria shallon Pursh (Ericaceae) are common shrubs in the understory in coastal forests of the Pacific Northwest of North America (Naiman et al. 2000), and their dense undergrowth affects forest vegetation. Rubus spectabilis, a deciduous shrub, is common in moist sites, Alnus rubra stands and riparian stands (Pojar and MacKinnon 2004). Gaultheria shallon, an evergreen shrub, occurs on somewhat drier, more interior sites than R. spectabilis. A greater amount of light reaches the forest floor following disturbance to the overstory. G. shallon and R. spectabilis rapidly respond to such disturbances and can produce pure, dense (>20,000 stems ha−1) cover (Tappeiner et al. 1991; Mallik 1995; Tappeiner et al. 2001). These species also expand their cover from the seed bank. Tappeiner and Zasada (1993) documented that the emergence of G. shallon and R. spectabilis was greater on mineral soil than on undisturbed soil because of their small seed with relatively low stored energy.
Despite several studies of light intensity related changes to forest floor vegetation have been conducted (Mallik 1995; Tappeiner et al. 2001), influences of alteration of light availability caused by a partial harvest in riparian vegetation on the dynamics of shrubs that form dense cover is not studied. Also, there is insufficient knowledge of the competitive relationship between shrub cover and tree seedlings, and of the difference in the responses among species of tree seedlings there. The present study addresses this lack of knowledge by discussing the following questions: What are changes to forest floor vegetation, particularly shrub species, after 50 % partial harvest? What is the difference in distribution of the species along a stream to upland gradient? Finally, what is the potential difference in tree seedling establishment and growth with gap formation? We hypothesised that dense cover of G. shallon and R. spectabilis could be found in partial harvest sites and these shrub species could constrain the distribution of conifer seedlings. This may consequently affect the structure and species composition of forest stands (Fraser et al. 1995; Mallik 1995; Kennedy and Quinn 2001; Villarin et al. 2009). Based on these underlying assumptions, we examined how partial harvest affected G. shallon and R. spectabilis densities, the relationship between the differences of G. shallon and R. spectabilis densities, and the distribution of conifer seedlings.
Materials and methods
Study site
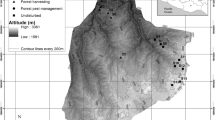
We conducted this study in the Malcolm Knapp Research Forest of the University of British Columbia (122°34′W, 49°16′N), Maple Ridge, BC, Canada (Fig. 1). The forest is in the foothills of the Coast Mountains, approximately 60 km east of Vancouver. The forest lies in the coastal western hemlock biogeoclimatic zone. The dominant forest species are Tsuga heterophylla (Raf.) Sarg. (Pinaceae), Thuja plicata Donn ex D. Don (Cupressaceae), and Pseudotsuga menziesii (Mirb.) Franco (Pinaceae) (Kiffney et al. 2003). The forest was logged before 1931 and the current stand resulted from a stand-replacing fire in 1931 (The University of British Columbia 2010), which is now dense second-growth forest (550–650 trees ha−1, average diameter at breast height 40 cm; average stand height 45 m; Kiffney et al. 2003). Soils are shallow and dominantly podzols formed in ablation until colluviums were overlying relatively impermeable basal till or granitic bedrock (Gomi et al. 2006). The forest receives much precipitation from ca. 2,200 mm per year at the southern end of the forest to ca. 3,000 mm per year at the north end (The University of British Columbia 2010). More than 70 % of the total annual precipitation falls between October and March, mostly in the form of rain (Kiffney et al. 2003).
The study site at Griffith Creek is part of a riparian management experiment at the Malcolm Knapp Research Forest (Richardson et al. 2010). Griffith Creek is a typical headwater stream. As a part of the riparian project to test the effectiveness of riparian management treatments, one treatment was a partial harvest (removal of 50 % of the stems based on basal area in the riparian and adjacent areas of the experimental streams), which was applied along Griffith Creek in 2004. The harvested trees were skidded from the site to load on trucks at the road, but some coarse woody debris (branches or small logs) remained. We chose this area as the study site to examine the effects of a partial harvest on regeneration of conifers. Multiplots should be required for replication, nonetheless, the present study was based on a single sample at a single location, because there was no site with similar conditions around the study site.
We established three sites; harvested, border, and control sites (unreplicated), along the creek (Fig. 1). The intersite distance was approximately 100 m at ca. 400 m asl. The harvested site is the partial-harvested area in 2004. We chose an unharvested area as the control site that was adjacent to and the same age and same vegetation (Richardson et al. 2010) as the partial-harvested area because pre-harvest data were unavailable. The border site is set along the edge of the harvested site (upper end of the cutblock), and we established the border site where harvest effects might be indirect, or some changes such as edge effects would affect seedlings. These three sites were set on the left bank of the creek and their mean slope angles were 8.4 % (the harvested site), 13.9 % (the border site), and 11.8 % (the control site), respectively.
Sampling design
We established a 1 m × 45 m transect along the environmental gradient from the creek to the upper slope on each site. Small plots (1 m × 1 m) were established along each transect for counting seedlings and other major understorey plants. The first small plot was established immediately adjacent to the creek and successive plots were established at 4-m intervals, for a total of ten small plots in each transect. All seedlings encountered on each small plot were counted, and species, height, and their substrate types as a microsite for their growth were recorded. Seedlings were defined roughly as individuals <25 cm in height (ground to apical tip; Hughes et al. 2009). The substrate type consisted of leaf litter, fallen log, or stump. Major understorey plant covers were estimated visually as the percentage of each small plot’s ground area covered by the plants. Percentage of canopy openness was quantified at four points (10, 20, 30, and 40 m from the creek) on each transect based on a spherical densiometer (Model-A, Forest Densiometers, USA). Then we calculated percentage of mean canopy openness of each transect.
There were many patches of G. shallon and R. spectabilis in the harvest site. In order to evaluate the potential influence of G. shallon and R. spectabilis on seedlings, we recognized patches in which either one shrub species or the other was observed. We also classified patches of the shrub species based on percent cover. The classification into five cover classes was as follows: patches with <10 % total cover of both species (open); patches with 10–80 % cover of G. shallon or R. spectabilis (SG, sparse G. shallon; SR, sparse R. spectabilis); and patches with ≥80 % cover of G. shallon or R. spectabilis (DG, dense G. shallon; DR, dense R. spectabilis). Ten additional small plots (1 m × 1 m) were placed randomly on the patches with each cover class (open, SG, SR, DG, DR) within the harvested site (50 small plots in all, not on the transects). We recorded species and height of seedlings established in each small plot. We measured soil water content with an auto-analyzer (Hydrosense CD620 + CS620, Campbell Scientific, Australia) in the 50 small plots. Samples of the soil were collected from each small plot for analysis of soil chemistry. The samples were dried and sieved with a 2 mm mesh sieve in the laboratory. The samples of 20 g (dry mass) were analyzed for ammonia–N, nitrate–N, and phosphate–P in the Applied Environmental Research Laboratories at Vancouver Island University, in Nanaimo, BC.
Statistical analysis
We tested the difference in the height of each coniferous seedling established on the harvested, border, and control sites, and also the difference in the total number of seedlings and the number of each coniferous seedling established in three different cover classes of G. shallon (open, SG, DG) and R. spectabilis (open, SR, DR), respectively, using the Steel-Dwass test, which is a nonparametric multiple comparison test. We also tested the differences in the soil chemistry between three different cover classes of G. shallon and R. spectabilis, using the Tukey-Kramer test, which is a parametric multiple comparison test. All statistical analysis was conducted with KyPlot (version 5.0, Kyenslab Inc., Tokyo, Japan).
Results
The highest mean canopy openness was found in the harvested site (37.05 ± SD 5.80 %), and it was lower in the border (25.09 ± SD 7.58 %) and control (22.83 ± SD 3.98 %) sites.
Gaultheria shallon and R. spectabilis were the most common species, appearing in all sites. G. shallon had the highest cover in the border and control sites, but R. spectabilis showed the highest cover in the harvested site (Table 1). Vaccinium spp. were also observed in all sites, but the cover was not high. We found the mean community height of R. spectabilis (74.59 ± SD 47.32 cm) with upright stems was much taller than G. shallon (28.35 ± SD 12.90 cm) with sprawling stems in our study site.
Cover of G. shallon varied markedly between small plots on all sites regardless of the distance from the creek (Fig. 2). R. spectabilis was found within 20 m from the creek (small plot # 4), and the highest cover (90 %) was found at 15 m point from the creek in the harvested site (Fig. 2). In addition, the cover decreased with proximity to the upper slope from the creek in all sites.
The greatest number of seedlings could be observed in the harvested site, 81 individuals·10 m−2 of T. heterophylla, 146 individuals·10 m−2 of T. plicata, and 45 individuals·10 m−2 of P. menziesii were found (Fig. 3). There also were many T. heterophylla seedlings (83 individuals·10 m−2) in the border site. Seedlings of T. plicata and P. menziesii were rarely found in the border site, and no seedling in the control site. There were almost all T. plicata and P. menziesii seedlings on leaf litter, and only one individual of both species was found on fallen logs. T. heterophylla seedlings were also found on leaf litter commonly, and 15 % of the seedlings were also found on woody substrates in the border site.
The mean height of T. heterophylla in the harvested site was significantly higher than in the border site (4.8 ± SD 3.0 cm, 2.4 ± SD 2.5 cm, P < 0.001, Steel–Dwass test), and the mean height of T. plicata in the harvested site was also significantly higher than in the border site (2.9 ± SD 2.5 cm, 1.3 ± SD 1.0 cm, P < 0.05, Steel–Dwass test), but the mean height of P. menziesii showed no significant difference between sites (5.4 ± SD 3.8 cm, 3.4 ± SD 1.9 cm for the harvested and border sites, respectively, Steel–Dwass test). The distribution of all species in the harvested site showed an almost inverse-J shape (Fig. 4). In the border site, a weak inverse-J shaped distribution of sizes can be found for T. heterophylla but not for the other two species.
Height class (cm) distribution of the three coniferous species in the Harvested and Border sites. As no coniferous seedlings were found in the Control site (Fig. 2), bars were not depicted in the figure
Tsuga heterophylla and T. plicata seedlings showed an almost inverse-J shaped distribution in the open, SG, and SR cover class, but the number of P. menziesii seedlings in <5 cm height class was fewer than the other two species (Fig. 5). While a number of seedlings were observed in the DG cover class, almost no seedlings could be found in the DR cover class. Significant differences were found in T. heterophylla and T. plicata with <5 cm height class between the SR (10.4 ± SD 12.6 and 14.9 ± SD 14.5) and DR (0.2 ± SD 0.6 and 0.4 ± SD 1.3) cover classes, and open (14.3 ± SD 25.6 and 9.3 ± SD 13.6) and DR (0.2 ± SD 0.6 and 0.4 ± SD 1.3) classes (P < 0.01 in T. heterophylla, P < 0.05 in T. plicata, Steel–Dwass test). In the case of all seedlings of three coniferous species together, significant differences were also found between SR (32.7 ± SD 25.1) and DR (2.3 ± SD 3.3) cover classes, and open (36.5 ± SD 43.6) and DR (2.3 ± SD 3.3) classes (P < 0.05, Steel–Dwass test).
Frequency distribution of height (cm) of seedlings associated with shrub cover, open, SG, DG, SR, DR sites (each with n = 10). White, dark gray and black bars show T. heterophylla, T. plicata, and P. menziesii, respectively. Abbreviations for each cover class are SG sparse G. shallon, DG dense G. shallon, SR sparse R. spectabilis and DR dense R. spectabilis. Sparse and dense were defined as 10–80 % cover and ≥80 % cover, respectively, and open was defined as <10 % cover of both species. Same letters on bars indicate significant differences (a and c, P < 0.01; b and d, P < 0.05, Tukey–Kramer test)
The soil properties did not significantly differ among the five sites, except for Nitrate–N among SR (4.25 ± SD 7.51 mg kg−1) and DR (29.48 ± SD 32.68 mg kg−1) cover classes, and open (4.42 ± SD 9.14 mg kg−1) and DR (29.48 ± SD 32.68 mg kg−1) cover classes (P < 0.05, Tukey–Kramer test) (Table 2). Water content, Ammonia–N and Nitrate–N, were relatively high in the DG and DR cover classes compared with the open SG and SR cover classes but not with significant differences (P > 0.05).
Discussion
The comparison of total number of seedlings and their height observed in the three sites showed that the light availability might promote seedling establishment and growth. Canopy openness is a good surrogate of light availability (Rich et al. 1993; Easter and Spies 1994; Machado and Reich 1999); therefore, the highest mean canopy openness in the harvested sites and lower in the other sites show that light availability to the understory layer increased through the manipulation of the harvest. The overstory vegetation inhibited regeneration of seedlings (Keyes et al. 2001), and high-light conditions created by gap formation or harvest promoted seedling establishment and growth (Narukawa and Yamamoto 2001; Boudreau and Lawes 2005). We found that only 15 % of variation in canopy openness affected distribution of conifer seedlings (Fig. 3).
The different distribution pattern among the tree species to the partial harvest could be due to different tolerance to water and light. Previous studies (Minore and Weatherly 1994; Villarin et al. 2009) conducted in riparian areas in the Pacific Northwest documented that the absence of P. menziesii seedlings might be attributed to intolerance of flooding and shallow water tables and preference to dry sites. In addition, P. menziesii has the lowest shade-tolerance of the three species (Harrington 2006). Higher canopy openness brought by the 50 % partial harvest in our study may cause increased light availability (Machado and Reich 1999). It was relative enough to enhance of P. menziesii seedlings, but the small size seedlings (<5 cm) were not found to be as many as others. Thus, the partial harvest promoted an increase of especially T. heterophylla and T. plicata seedlings. It is known that light requirements for survival and growth is in order from P. menziesii to T. plicata to T. heterophylla (Harrington 2006).
Leaf litter was favorable for growth of T. heterophylla, T. plicata, and P. menziesii. Many studies have documented the importance of rotten wood for seedling establishment (Christy and Mack 1984; Harmon and Franklin 1989; Minore and Weatherly 1994; Hibbs and Giordano 1996; Beach and Halpern 2001). The forest floor condition would be one of the important factors that determine suitable substrates for seedling growth. Indeed, fallen logs were not suitable because many logs felled by the harvest were still fresh, and they were not valuable as the nurse logs in our study sites. Leaf litter (Hovstad and Ohlson 2009) and moss (Harmon and Franklin 1989; Nakamura 1992; Katsumata et al. 2008) have both facilitative and inhibitory effects on seedling establishment. The effect of leaf litter on seedling establishment depends on edaphic conditions, climate, and type and quantity of litter (Hovstad and Ohlson 2009). In our study site, litter accumulation was formed by small needles of T. heterophylla, and the litter layer was not thick. Moss cover was small and not thick in our study site. Therefore, the litter layer would not cause inhibitory effects such as physical obstruction and dark condition for germination.
When light levels increase, not only will tree seedlings grow well, but other plant species will also accrue. After retention harvest and gap formation in the Pacific Northwest, observations generally describe a decrease in forest herb and shrub richness, and an increase in early seral species and introduced species, which would be competitors with tree seedlings (Nelson and Halpern 2005; Fahey and Puettmann 2007; Ares et al. 2009). We recognized great cover of R. spectabilis in the harvested site. As Minore and Weatherly (1994) and Villarin et al. (2009) showed, R. spectabilis develops dense cover with increased proximity to the creek. In addition, G. shallon often forms dense cover with a wide distribution in our study site. Establishment of the seedlings appeared to be restricted by the competition with these shrubs.
The shrub species, particularly R. spectabilis cover decreased along the creek to upland, and seedlings could rarely be found in the higher cover patches of the shrubs. This indicated that the dense shrub cover may interfere with conifer regeneration near the creek. The possible causes of the poor establishment of coniferous seedlings under dense shrub would be their competition for resources, such as light conditions. Dosono and Nyland (2006) described that Rubus spp. have interference effects on tree regeneration because they shade the ground, intercept and transpire water, and reduce the rate of litter decomposition and nutrient cycling. Other studies have indicated that the inhibitory effect was primarily due to competition for nutrients (Fraser et al. 1995; Mallik and Prescott 2001; Bennett et al. 2003), but soil chemical properties did not differ significantly between three sites, i.e., neither sparse nor dense cover of G. shallon and R. spectabilis have a potential to affect soil nutrient availability in the present study. This result suggested that a competitive influence of these shrubs on coniferous seedlings was to shade ground (Dosono and Nyland 2006). We have gained new insights that increasing light availability has not always facilitated growth of coniferous seedlings, such as P. menziesii as confirmed in this study, if anything, it may accelerate expansion of shrub cover rather than the regeneration of tree seedlings so that P. menziesii seedlings, especially the shorter individuals, have not been observed in the dense shrub cover caused by their competition demanding light resources.
As observed previously by Minore and Weatherly (1994), competition from dense G. shallon cover did not seem to be more severe than dense R. spectabilis cover. R. spectabilis could strongly affect tree seedlings establishment due to two factors. First, differences in seed dormancy would affect their growth after disturbance and inhibitory effects. G. shallon seeds have little or no dormancy, but R. spectabilis seeds have deep dormancy and remain in a buried seed bank for many years (Tappeiner and Zasada 1993). When light levels are increased by a disturbance, R. spectabilis would regenerate more quickly, and the effect can result in its persistence as the dominant understory species for long periods of time. Second, the ability of R. spectabilis to shade seedlings would be stronger than G. shallon. Thick cover of R. spectabilis might strongly reduce the access of seedlings to light as compared with G. shallon. Both factors may be responsible for the inhibitory effect.
Dosono and Nyland (2006) reported that interference effects of Rubus spp. decreased at 5–7 years, so seedlings could grow through the Rubus spp. However, they also pointed out that very poorly drained sites would likely prove difficult for regenerating tree seedlings. In our study site, we found that R. spectabilis developed densely along the creek side. Thus, shading by shrub species is considered a possible reason for limiting the regeneration of conifer species; however, further experiments are needed to test this.
We recognized that dense cover of G. shallon and R. spectabilis was found in the harvested site, and also there were lesser numbers of conifer seedlings in the dense shrub cover. These results supported out hypothesis that dense cover of shrubs could be found in partial harvest sites, and they could constrain the distribution of conifer seedlings. In the dense R. spectabilis cover observed near creek side, we have considered that distribution of conifer seedlings was restricted by competition with especially exuberant foliage of R. spectabilis for light conditions. This suggests that harvesting near creek side should be constrained, at least 30 m from a stream, in riparian management.
References
Ares A, Berryman SD, Puettmann KJ (2009) Understory vegetation response to thinning disturbance of varying complexity in coniferous stands. Appl Veg Sci 12:472–487
Beach EW, Halpern CB (2001) Controls on conifer regeneration in managed riparian forests: effects of seed source, substrate, and vegetation. Can J For Res 31:471–482
Bennett JN, Blevins LL, Barker JE, Blevins DP, Prescott CE (2003) Increases in tree growth and nutrient supply still apparent 10 to 13 years following fertilization and vegetation control of salal-dominated cedar-hemlock stands on Vancouver Island. Can J For Res 33:1516–1524
Boudreau S, Lawes MJ (2005) Small understorey gaps created by subsistence harvesters do not adversely affect the maintenance of tree diversity in a sub-tropical forest. Biol Conserv 126:279–286
Callaway RM (1995) The positive interactions among plants. Bot Rev 61:306–349
Christy EJ, Mack RN (1984) Variation in demography of juvenile Tsuga heterophylla across the substratum mosaic. J Ecol 72:75–91
Dosono PJ, Nyland RD (2006) Interference to hardwood regeneration in northeastern North America: the effects of raspberry (Rubus spp.) following clearcutting and shelterwood methods. North J Appl For 23:288–296
Easter MJ, Spies TA (1994) Using hemispherical photography for estimating photosynthetic photon flux-density under canopies and in gaps in Douglas-fir forests of the Pacific-Northwest. Can J For Res 24:2050–2058
Fahey RT, Puettmann KJ (2007) Ground-layer disturbance and initial conditions influence gap partitioning of understory vegetation. J Ecol 95:1098–1109
Fraser LH, Chanway CP, Turkington R (1995) The competitive role of Gaultheria shallon on planted western hemlock and western red cedar saplings on northern Vancouver Island. For Ecol Manag 75:27–39
Gómez-Aparicio L, Gómez JM, Regino Z (2005) Microhabitats shift rank in suitability for seedling establishment depending on habitat type and climate. J Ecol 93:1194–1202
Gomi T, Moore RD, Dhakal AS (2006) Headwater stream temperature response to clear-cut harvesting with different riparian treatments, coastal British Columbia, Canada. Water Resour Res 42:11
González-Alday J, Martínez-Ruiz C, Bravo F (2009) Evaluating different harvest intensities over understory plant diversity and pine seedlings, in a Pinus pinaster Ait. Natural stand of Spain. Plant Ecol 201:211–220
Harmon ME, Franklin JF (1989) Tree seedlings on logs in Picea-Tsuga forests of Oregon and Washington. Ecology 70:48–59
Harrington TB (2006) Five-year growth responses of Douglas-fir, western hemlock, and western redcedar seedlings to manipulated levels of overstory and understory competition. Can J For Res 36:2439–2453
Hibbs DE, Giordano PA (1996) Vegetation characteristics of alder-dominated riparian buffer strips in the Oregon coast range. Northwest Sci 70:213–222
Hovstad KA, Ohlson M (2009) Conspecific versus heterospecific litter effects on seedling establishment. Plant Ecol 204:33–42
Hughes NM, Johnson DM, Akhalkatsi M, Abdaladze O (2009) Characterizing Betula Litwinowii seedling microsites at the alpine-treeline ecotone, central Greater Caucasus Mountains, Georgia. Arct Antarct Alp Res 41:112–118
Katsumata N, Okitsu S, Minami Y (2008) Influence of feather mossmat on the occurrence and growth of Abies veitchii seedlings in subalpine forest, Mt. Fuji, Japan. Lindbergia 33:50–57
Kennedy PG, Quinn T (2001) Understory plant establishment on old-growth stumps and the forest floor in western Washington. For Ecol Manag 154:193–200
Keyes CR, Acker SA, Greene SE (2001) Overstory and shrub influences on seedling recruitment patterns in an old-growth Ponderosa pine stand. Northwest Sci 75:204–210
Kiffney PM, Richardson JS, Bull JP (2003) Responses of periphyton and insects to experimental manipulation of riparian buffer width along forest streams. J Appl Ecol 40:1060–1076
Lecerf A, Richardson JS (2010) Litter decomposition can detect effects of high and moderate levels of forest disturbance on stream condition. For Ecol Manag 259:2433–2443
Machado JL, Reich PB (1999) Evaluation of several measures of canopy openness as predictors of photosynthetic photon flux density in deeply shaded conifer-dominated forest understory. Can J For Res 29:1438–1444
Mallik AU (1995) Conservation of temperate forests into heaths: role of ecosystem disturbance and ericaceous plants. Environ Manag 19:675–684
Mallik AU, Prescott CE (2001) Growth inhibitory effects of salal on western hemlock and western red cedar. Agron J 93:85–92
Minore D, Weatherly HG (1994) Riparian trees, shrubs, and forest regeneration in the coastal mountains of Oregon. New For 8:249–263
Naiman RJ, Bilby RE, Bisson PA (2000) Riparian ecology and management in the Pacific Coastal rain forest. Bioscience 50:996–1011
Naiman RJ, Decamps H, McClain ME (2005) Riparia: ecology conservation and management of streamside communities. Elsevier Academic Press, San Diego
Nakamura T (1992) Effect of bryophytes on survival of conifer seedlings in subalpine forests of central Japan. Ecol Res 7:155–162
Narukawa Y, Yamamoto S (2001) Gap formation, microsite variation and the conifer seedling occurrence in a subalpine old-growth forest, central Japan. Ecol Res 16:617–625
Nelson CR, Halpern CB (2005) Edge-related responses of understory plants to aggregated relation harvest in the Pacific Northwest. Ecol Appl 15:196–209
Pojar J, MacKinnon A (2004) Plants of coastal British Columbia : including Washington, Oregon & Alaska. Lone Pine Publishing, Vancouver
Rich PM, Clark DB, Clark DA, Oberbauer SF (1993) Long-term study of solar radiation regimes in a tropical wet forest using quantum sensors and hemispherical photography. Agric For Meteorol 65:107–127
Richardson JS, Naiman RJ, Swanson FJ, Hibbs DE (2005) Riparian communities associated with Pacific Northwest headwater streams: assemblages, processes, and uniqueness. J Am Water Resour Assoc 41:935–947
Richardson JS, Feller MC, Kiffney PM, Moore RD, Mitchell S, Hinch SG (2010) Riparian management of small streams: an experimental trial at the Malcolm Knapp Research Forest. Streamline 13:1–16
Richardson JS, Naiman RJ, Bisson PA (2012) How did fixed-width buffers become standard practice for protecting freshwaters and their riparian areas from forest harvest practices? Freshw Sci 31:232–238
Sugita H, Tani M (2001) Difference in microhabitat-related regeneration patterns between two subalpine conifers, Tsuga diversifolia and Abies mariesii, on mount Hayachine, northern Honshu, Japan. Ecol Res 16:423–433
Swanson ME, Franklin JF, Beschta RL, Crisafulli CM, DellaSala DA, Hutto RL, Lindenmayer DB, Swanson FJ (2011) The forgotten stage of forest succession: early successional ecosystems on forest sites. Front Ecol Environ 9:117–125
Tappeiner JC, Zasada JC (1993) Establishment of salmonberry, salal, vine maple, and bigleaf maple seedlings in the coastal forests of Oregon. Can J For Res 23:1775–1780
Tappeiner JC, Zasada JC, Ryan P, Newton M (1991) Salmonberry clonal and population structure: the basis for a persistent cover. Ecology 72:609–618
Tappeiner JC II, Zasada JC, Huffman DW, Ganio LM (2001) Salmonberry and salal annual aerial stem production: the maintenance of shrub cover in forest stands. Can J For Res 31:1629–1638
The University of British Columbia (2010) Malcolm Knapp Research Forest. http://www.mkrf.ubc.ca/. Accessed 20 Oct 2010
Villarin LA, Chapin DM, Jones JE (2009) Riparian forest structure and succession in second-growth stands of the central Cascade Mountains, Washington, USA. For Ecol Manag 257:1375–1385
Acknowledgments
We thank Mr. Ionut Aron of the Malcolm Knapp Research Forest, The University of British Columbia, for his helpful coordination enabling us to carry on our research work in the forest, and the staff of the analytical facility at the Applied Environmental Research Laboratories at Vancouver Island University for soil analysis.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Minami, Y., Oba, M., Kojima, S. et al. Distribution pattern of coniferous seedlings after a partial harvest along a creek in a Canadian Pacific northwest forest. J For Res 20, 328–336 (2015). https://doi.org/10.1007/s10310-015-0479-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10310-015-0479-0