Abstract
l-Leucine is an essential amino acid that has wide and expanding applications in the industry. It is currently fast-growing market demand that provides a powerful impetus to further increase its bioconversion productivity and production stability. In this study, we rationally engineered the metabolic flux from pyruvate to l-leucine synthesis in Corynebacterium glutamicum to enhance both pyruvate availability and l-leucine synthesis. First, the pyc (encoding pyruvate carboxylase) and avtA (encoding alanine-valine aminotransferase) genes were deleted to weaken the metabolic flux of the tricarboxylic acid cycle and reduce the competitive consumption of pyruvate. Next, the transcriptional level of the alaT gene (encoding alanine aminotransferase) was down regulated by inserting a terminator to balance l-leucine production and cell growth. Subsequently, the genes involved in l-leucine biosynthesis were overexpressed by replacing the native promoters PleuA and PilvBNC of the leuA gene and ilvBNC operon, respectively, with the promoter Ptuf of eftu (encoding elongation factor Tu) and using a shuttle expression vector. The resulting strain WL-14 produced 28.47 ± 0.36 g/L l-leucine in shake flask fermentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Leucine, one of the branched-chain amino acids (BCAAs), is an essential amino acid that plays important roles in a variety of biological processes [1]. It is currently widely used in industries as a flavor enhancer, an animal feed additive, an ingredient in cosmetics, and a specialty nutrient in pharmaceutical and medical fields [1, 2]. It has recently attracted much research attention because of its potential in improving human health and its fast-growing market demand [3]. Traditional extraction of l-leucine from protein hydrolysate and enzymatic synthesis has been replaced by microbial production in the industry [1]. Corynebacterium glutamicum has been recognized as a generally regarded as safe (GRAS) strain for the production of most amino acids, particularly l-glutamate, l-lysine, and BCAAs [4]. Due to the long biosynthetic pathway of l-leucine and the complex regulatory mechanism, the yield and conversion rate of l-leucine are still low in microbial strains [5]. Therefore, the requirement is to culture strains with high production capacity, high conversion rate, and stable production performance.
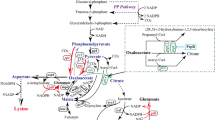
The biosynthetic pathway of BCAAs has been relatively clearly established; in C. glutamicum, their biosynthesis is controlled at the transcriptional level through a complex regulatory cascade [6,7,8]. The four common enzymes that use pyruvate as a precursor and are involved in parallel reactions are acetohydroxyacid synthase (AHAS), acetohydroxyacid isomeroreductase (AHAIR), dihydroxyacid dehydratase (DHAD), and branched-chain amino acid transaminase (BCAT) (Fig. 1). AHAS is the key enzyme of BCAA biosynthetic pathways. l-Leucine is derived from α-ketoisovalerate, a direct precursor of l-valine, in a specific series of four reactions [9]. Additionally, α-isopropylmalate synthase (IPMS), as a rate-limiting enzyme, catalyzes the conversion of α-ketoisovalerate and acetyl-CoA to α-isopropylmalate. Development of bacterial strains for l-leucine production has not yet been successful, primarily because they possess complicated regulatory mechanisms for BCAA synthesis [10, 11].
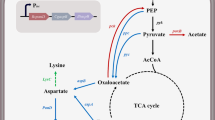
The biosynthetic pathways of l-leucine in C. glutamicum and the strategies for constructing l-leucine-producing strains. The blue boxes represented the genes that were upregulated by promoter replacement in the genome. The brown box represented the gene that was downregulated by terminator insertion in the genome. The cross marks represented the genes that were deleted. The ilvBNC and leuA genes were overexpressed using the expression vector pEC-XK99E. TCA tricarboxylic acid cycle, PEP phosphoenolpyruvate, OAA oxaloacetate, PEPC phosphoenolpyruvate carboxylase, PC pyruvate carboxylase
Pyruvate is the end product of glycolysis, and it enters into the tricarboxylic acid (TCA) cycle [12]. Several studies have revealed that pyruvate availability is critical for pyruvate-amino acid overproduction [13, 14]. However, an active TCA cycle is not conducive to the efficient synthesis of pyruvate-family amino acids, because it leads to a waste of carbon [15]. Hence, there may be a trade-off between the TCA cycle and product synthesis. Additionally, the phosphoenolpyruvate (PEP)–pyruvate–oxaloacetate (OAA) node is one of the most important links between glycolysis and the TCA cycle [16]. A series of reactions at this node could direct the carbon flux into appropriate directions, making it a highly relevant switch point for carbon flux distribution within the central metabolism [16, 17]. In this node, phosphoenolpyruvate carboxylase (PEPC) catalyzes the conversion of PEP to OAA, and pyruvate carboxylase (PC) catalyzes the conversion of pyruvate to OAA [17]. PEPC and PC are two important anaplerotic enzymes for supplying OAA in C. glutamicum, and OAA supply determines the flux of the TCA cycle [18]. The metabolic flow of the PEP–pyruvate–OAA node may be significantly changed by regulating the TCA cycle supplementation pathway that PEPC and PC participate in [7, 19].
In C. glutamicum, l-alanine, one of the main by-products of l-leucine production, is biosynthesized from pyruvate via two distinct pathways [20]. Alanine aminotransferase (AlaT) converts pyruvate to l-alanine in an l-glutamate-dependent manner, and alanine-valine aminotransferase (AvtA) converts pyruvate to l-alanine in an l-valine-dependent reaction [21, 22]. C. glutamicum with a deletion of either of the corresponding genes does not exhibit an obvious growth deficiency, but a double mutant is auxotrophic for l-alanine; this suggests that AlaT and AvtA are the only aminotransferases involved in l-alanine biosynthesis and they cannot be deleted simultaneously [23]. Although the addition of l-alanine to the medium might recover cell growth, it would likely incur additional costs. Thus, the attenuation of alaT expression is a potential strategy for balancing l-leucine yield and cell growth. Transcription is the first stage of gene expression, and the terminator is an important element of the transcription process and thus crucial for protein expression [24, 25]. Terminators may be used as a tuning knob to down regulate alaT expression and improve l-leucine production.
Alteration of the expression of some important genes via promoter replacement is a widely used approach to enhance production. At the same time, it should be noted that in addition to increasing the expression of certain genes, the precise control of gene expression is a critical step, which can influence the amount of key pathway enzymes available for maximizing the production of target products [26, 27]. This objective can be achieved using promoters with different strengths. Promoters Ptac and Ptuf with different strengths are widely used to improve the expression level of enzymes in C. glutamicum [1, 20, 22, 28, 29].
A few of the current strain development strategies, including elimination of the feedback inhibition of enzymes [4, 22], strengthening carbon influx by overexpressing the key genes and weakening the competing branches [1, 22], optimizing the aminotransferases to increase enzyme specificity [2], and altering the redox balance [6], have yielded notably improved l-leucine-producing strains. Additionally, high production efficiency is a crucial constraint for any economically viable industrial production process [30, 31]. In this study, the pyc gene (encoding PC) was deleted to regulate the TCA cycle supplementation pathway and increase the pyruvate availability. Subsequently, the avtA gene was deleted and the alaT expression was downregulated to weaken the metabolic flux from pyruvate to l-alanine. Considering the importance of ilvBNC and leuA in the l-leucine biosynthetic pathway, two promoters with differing strengths, viz., Ptuf and Ptac, were selected to fine-tune the expression of ilvBNC and leuA, respectively.
Materials and methods
Bacterial strains, plasmids, and culture conditions
The strains and plasmids used in this study are listed in Table 1. The l-leucine-producing strain C. glutamicum XQ-9-∆ltbR, which was derived from wild-type C. glutamicum ATCC 13,032 and stored in our laboratory, was used as the working and parent strain [6]. This strain was auxotrophic for l-isoleucine and l-methionine. Many amino acid exchanges occur in C. glutamicum XQ-9-∆ltbR with respect to the corresponding sequence from the wild-type strain ATCC 13,032 [6]. Especially, there are five amino acid exchanges related to the leuA gene, suggesting that the working strain XQ-9-∆ltbR with feedback-resistant enzymes could accumulate l-leucine, whereas the wild-type strain typically would not Escherichia. coli JM109 and BL21 (DE3) were grown in LB medium (5-g/L yeast extract, 10-g/L tryptone, and 10-g/L NaCl) at 37 °C, and C. glutamicum strains were grown at 30 °C in LBG (LB medium supplemented with 5-g/L glucose). LBHIS medium (5-g/L tryptone, 5-g/L NaCl, 2.5-g/L yeast extract, 18.5-g/L Brain Heart Infusion powder, and 91-g/L sorbitol) was used for the transformation of the mutant gene into C. glutamicum cells. Where appropriate, kanamycin and isopropyl β-d-thiogalactoside at final concentrations of 50 μg/mL and 1 mmol/L, respectively, were added to the medium.
For l-leucine production in shake flask cultivation, the strains were grown in 500-mL Erlenmeyer flasks containing 50-mL seed medium for 14–18 h [6]. The fermentation medium was inoculated with a seed solution of 10% inoculum. All strains were cultured at 30 ℃ under continuous shaking at 100 rpm. The fermentation lasted for 72 h. The medium used for seed culture contained 30 g/L glucose, 35 g/L corn steep liquor, 5 g/L (NH4)2SO4, 1.3 g/L KH2PO4·3H2O, 0.4 g/L MgSO4·7H2O, 0.01 g/L MnSO4·H2O, 10 g/L sodium citrate, 10 g/L yeast extract, 2 g/L urea, 0.4 g/L l-methionine, 0.0002 g/L biotin, 0.0003 g/L thiamine, and 20 g/L CaCO3. The fermentation medium contained 130 g/L glucose, 25 g/L corn steep liquor, 15 g/L (NH4)2SO4, 15 g/L CH3COONH4, 1.3 g/L KH2PO4·3H2O, 0.5 g/L MgSO4·7H2O, 0.01 g/L MnSO4·H2O, 2 g/L sodium citrate, 2 g/L urea, 0.8 g/L l-methionine, 0.06 g/L l-isoleucine, 0.5 g/L l-glutamate, 0.00008 g/L biotin, 0.0002 g/L thiamine, and 30 g/L CaCO3. Both media were adjusted to pH 7.2 with NaOH.
Recombinant DNA work for plasmid and strain construction
All DNA primers were synthesized by General Biosystems Co. Ltd. (Anhui, China), and are listed in Table S1. The shuttle vector pEC-XK99E was used for gene transfer between E. coli and C. glutamicum and for gene expressions in C. glutamicum [6]. The vector pK18mobsacB was used for in-frame deletions and integrations of DNA sequences via two-step homologous recombination in C. glutamicum [22]. The upstream and downstream fragments of the pyc gene were amplified from the genome of C. glutamicum XQ-9-∆ltbR, and then these fragments were overlapped together by PCR, resulting in pK18mobsacB-∆pyc. The upstream and downstream fragments of the ppc gene (encoding PEPC) were also amplified from the genome of C. glutamicum XQ-9-∆ltbR and then assembled with the EcoRI/HindIII-linearized pK18mobsacB fragment using a seamless cloning kit, resulting in pK18mobsacB-∆ppc. The same method was applied to construct pK18mobsacB-∆avtA and pK18mobsacB-∆alaT using corresponding primer pairs. Moreover, for inserting the terminator in front of the alaT gene, four terminator sequences with strengths of 10.94 a.u., 29.97 a.u., 40.39 a.u., and 93.18 a.u., discovered and measured by Chen et al. [25], were added between the upstream and downstream primer sequences. Subsequently, the PCR products were assembled with the EcoRI/BamHI-linearized pK18mobsacB fragment using a seamless cloning kit. In addition, for promoter replacement in the genome, the upstream and downstream fragments and the promoter sequence fragment were assembled into plasmid pK18mobsacB. Promoter Ptac and Ptuf sequences were amplified using pDXW-8 and C. glutamicum ATCC 13,032 genome as template, respectively. Finally, as per the method described in a previous study [6], the leuA and ilvBNC genes were cloned into plasmid pEC-XK99E. For strain construction, plasmids were transformed into C. glutamicum by electroporation. All constructed plasmids including chromosomal deletions and integrations in the engineered strains were verified by DNA sequencing.
RT-PCR for mRNA quantification
Total RNA was isolated from all bacterial strains using the EZ-10 Total RNA Mini-Preps Kit (Sangon Biotech, Shanghai, China) and was then used as the template to synthesize cDNA following the AMV reverse transcriptase-based protocol (Sangon Biotech, Shanghai, China). The cDNA targets were quantified with the PrimeScript™ One Step RT-PCR Kit II (Takara, Tokyo, Japan) using 16S RNA as the internal control [32]. PCR primers used in RT-PCR are listed in Table S1.
Analytical methods
Samples were taken every 12 h to determine various parameters. Cell growth was monitored by measuring the optical density of the culture at 562 nm (OD562) using a spectrophotometer (721 N, Shanghai, China) after diluting 0.2 mL of the samples with 5 mL of 0.25 M HCl to dissolve CaCO3. The dry cell weight (DCW) per liter was calculated using the formula: DCW (g/L) = 0.57 × OD562 + 0.23, which was experimentally determined in a previous study [21]. Glucose and glutamate concentrations were analyzed using the SBA-40E immobilized enzyme biosensor (Biology Institute of Shandong Academy of Sciences, Jinan, China). Pyruvate compounds (l-leucine, l-valine, and l-alanine) were analyzed by high-pressure liquid chromatography (HPLC) on an Agilent 1200 system (Agilent Technologies, Santa Clara, CA, USA) [6].
Results and discussion
Increasing cytoplasmic pyruvate availability by deletion of the pyc gene
Recently, precursor supplementation has emerged as an attractive strategy to improve the production of the corresponding metabolite [33]. l-leucine is produced from pyruvate, and the pyruvate node comprises a series of metabolic pathways, including l-glutamate, l-alanine, and other BCAA biosynthetic pathways, that compete for carbon fluxes with l-leucine synthesis. Thus, pyruvate availability is already recognized as a major bottleneck for l-leucine production [33]. It is well known that the PEP–pyruvate–OAA node plays an important role in amino acid synthesis [7]. Therefore, deletion of the genes pyc (encoding PC) and/or ppc (encoding PEPC) involved in the anaplerotic pathway would be expected to further regulate the carbon flux distribution at the pyruvate node so as to decrease the production of by-products involved in the TCA cycle. As shown in Fig. 2a, the PC-deficient strain WL-1 (pyc gene deletion) produced 16.86 ± 0.50 g/L of l-leucine, which was approximately 7% higher than the amount of l-leucine produced by the original strain ∆LtbR (15.72 ± 0.72 g/L). PEPC is dispensable. Several studies have revealed that the PC-catalyzed anaplerotic reaction plays an important role in glutamate production, and PEPC activity in C. glutamicum is distinctly enhanced under PC deficiency [18, 19]. Enhanced l-leucine production in the WL-1 strain in our study suggested that the supplementation of OAA, which is catalyzed only by PEPC, could not maintain the original metabolic flux of the TCA cycle, and instead, the flux from pyruvate to α-acetolactate was enhanced. In addition, l-valine production by the WL-1 strain was also increased (from 5.93 ± 0.27–6.74 ± 0.19 g/L) (Fig. 2d). We consider this as a consequence of shared enzymes and intermediates in the synthesis of BCAAs. However, the PEPC-deficient strain WL-2 (ppc gene deletion) produced only 8.13 ± 0.39 g/L of l-leucine but led to l-glutamate accumulation of up to 4.24 ± 0.22 g/L, which was much higher than that by the ∆LtbR strain (Fig. 2a, b). It was probable that the supplementation of OAA, which is believed to form a precursor of l-glutamate, was increased, because PC activity could be enhanced under the PEPC-deficient condition. The intracellular concentration of pyruvate decreased because of the improved PC activity, which in turn decreased the conversion of pyruvate to other metabolites such as BCAAs. Interestingly, the cell growth remained almost unaffected, whereas glucose consumption was slightly increased by the inactivation of PC or PEPC (Fig. 2b, c). These results indicate that PC disruption had a positive impact on l-leucine production, whereas PEPC disruption was conductive to l-glutamate synthesis. Thus, we conclude that adequate OAA supplementation in the WL-1 strain was mainly provided by the PC reaction, and the pyc gene deletion could downregulate the metabolic flux of the TCA cycle, which facilitated l-leucine synthesis. This conclusion is consistent with previous studies showing that improving the intracellular availability of precursor reduces carbon flux to undesirable by-products and increases the yield of the target products [34, 35]. Furthermore, cutting off either PEPC or PC did not affect the growth of C. glutamicum. This result was consistent with a previous study showing that these two enzymes can compensate for the loss of the other [36]. Notably, double disruption of PEPC and PC (i.e., the WL-3 strain) led to an explicit growth deficiency and almost no l-leucine yield (Fig. 2a, c).
Comparison of different C. glutamicum strains during shake flask cultivation in fermentation medium to test the effects of pyc and ppc deletion. al-Leucine production, b glucose concentration, c cell growth, and d by-product accumulation. All strains were tested in triplicate. The error bar denotes standard deviation of the mean
Decreasing l-alanine accumulation by deletion of avtA gene and attenuation of alaT expression
The above-mentioned results showed that l-valine and l-alanine were the main by-products of l-leucine production by fermentation, which had a negative impact on the yield and downstream processing [22, 37]. Due to the tightly coordinated regulatory mechanism of BCAA biosynthesis, it is infeasible to directly delete ilvE to block l-valine biosynthesis by traditional methods [2, 38]. Moreover, in C. glutamicum, l-alanine biosynthesis occurs via two pathways, one catalyzed by AlaT and another by AvtA, with pyruvate used as the direct precursor [39], and the former pathway is the major contributor [40]. To decrease the competitive consumption of pyruvate and the conversion of l-valine to l-alanine, avtA was first knocked out in C. glutamicum WL-1, resulting in the WL-4 (WL-1-∆avtA) strain. Compared with the WL-1 strain, the WL-4 strain showed slightly higher l-valine production, but no significant change in l-leucine production (Figs. 2d, 3). Additionally, alanine accumulation by WL-4 was slightly lower than that by WL-1 (4.63 ± 0.13 g/L vs. 5.27 ± 0.35 g/L), showing that l-alanine accumulation is achieved only by the alaT-catalyzed reaction. These results also indicate that the avtA gene knockout was effective for decreasing pyruvate consumption and l-alanine accumulation. However, compared with the WL-4 strain, the l-leucine production and cell growth rate of the double mutant WL-5 strain were both dramatically reduced (Fig. 3). These results are consistent with a previous study reporting that the double mutant produces some undesired results, including extra nutritional requirements and growth deficiency [21]. With the aim of further decreasing l-alanine production in the WL-4 strain, attenuation of alaT expression was a potential strategy that also balanced cell growth and l-leucine production [41]. Thus, four terminator sequences, discovered by Chen et al. [25] (Table 2), with strengths of 10.94 a.u., 29.97 a.u., 40.39 a.u., and 93.18 a.u., were selected and inserted in front of the alaT gene in the C. glutamicum genome, resulting in WL-6, WL-7, WL-8, and WL-9 mutants, respectively. Fermentation experiments were then performed to evaluate the effects of these modifications on cell growth and amino acid production. As shown in (Fig. 3), these four recombinant strains with different terminator strengths had different l-leucine yields as compared with the WL-4 strain. The l-leucine yield of the WL-8 strain was 18.13 ± 0.34 g/L, which was 6% higher than that of the WL-4 strain (17.08 ± 0.53 g/L). Furthermore, l-alanine accumulation by the WL-8 strain was 3.48 ± 0.21 g/L, which was 25% lower than that by the WL-4 strain (4.63 ± 0.13 g/L). As shown in Fig. 3, there was a remarkable improvement in the cell growth of the WL-6, WL-7, and WL-8 strains compared with the control strain WL-5. These results suggested that alaT expression was downregulated by inserting a terminator, resulting in the slight improvement of l-leucine production. Compared with the WL-4 strain; however, l-leucine production of the WL-9 strain decreased by 43% (Fig. 3). These results suggest that the insertion of a terminator with higher strength also affected the cell growth. Thus, in consideration of both cell growth and l-leucine production, the terminator with the strength of 40.39 a.u. was considered to be optimal for downregulating alaT expression. The optimal strain WL-8 relieved the cell growth disturbance and weakened l-alanine accumulation. Consequently, C. glutamicum WL-8 was used for the subsequent experiments.
Balancing the ilvBNC and leuA expression levels by inserting promoters with different strengths to improve l-leucine accumulation
The ultimate aim of this study was to maximize l-leucine production in C. glutamicum. AHAS catalyzes the key reaction in BCAA biosynthesis, and the ilvBNC operon plays a vital role in the BCAA biosynthetic pathways [11, 42]. α-Ketoisovalerate, the last intermediate of l-valine synthesis, is also the precursor for l-leucine catalyzed by IPMS [43]. Many reports have confirmed that ilvBNC and leuA overexpression promote l-leucine accumulation [6, 44]. Promoters are important elements in the transcriptional regulation of cells, and precisely regulating gene transcription by engineering promoters has become a very important metabolic engineering strategy [28, 34, 45]. The replacement of original promoters with promoters of various strengths is an effective strategy to fine-tune gene expression. Hence, for precisely regulating gene transcription and achieving a balance of carbon metabolism, two promoters with differing strengths, viz., Ptac and Ptuf, were used to rationally replace the native promoters of leuA and ilvBNC in the ∆LtbR strain, resulting in WL-10, WL-11, and WL-12 strains (Fig. 4f). In the WL-10 strain, the native promoters of ilvBNC and leuA in the genome were replaced by the strong Ptac promoter to enhance l-leucine biosynthesis. Gene expressions of ilvB, ilvN, ilvC, and leuA at 16 h during fermentation were tested by qRT-PCR, and the results showed that the promoter replacement strategy increased the translational levels of ilvB, ilvN, ilvC, and leuA (Fig. 4e). Data analysis indicated that compared with the WL-8 strain, the translational level of leuA in the WL-10 strain was increased by two times and that of ilvB, ilvN, and ilvC was increased by > 1.5 times. After fermentation assay, WL-10 produced 22.38 ± 0.53 g/L of l-leucine, which was 23% higher than the l-leucine yield of WL-8 (18.13 ± 0.34 g/L) (Fig. 4a). Moreover, glucose consumption and cell growth of WL-10 were improved slightly (Fig. 4b, c). Additionally, the accumulation of main by-products by WL-10 was remarkably lower than that by WL-8. These findings indicate that promoter replacement with the strong Ptac promoter enhanced the expression of genes involved in l-leucine synthesis, and more pyruvate was thus available for use in l-leucine synthesis. In addition, the production of by-products decreased. Similar results were also obtained with the other recombinant strains WL-11 and WL-12. Notably, leuA expression increased by 2.6-fold in the WL-11 strain (native promoters of ilvBNC and leuA replaced with Ptac promoter and Ptuf promoter, respectively) compared with that in the WL-8 strain, resulting in 24.73 ± 0.34-g/L yield of l-leucine. Meanwhile, l-valine accumulation by the WL-11 strain reduced to 3.26 ± 0.19 g/L, which was approximately 40% lower than that by the WL-10 strain (Fig. 4a, d). Therefore, leuA overexpression by the insertion of Ptuf promoter more effectively increased the carbon flux into the l-leucine biosynthetic pathway and reduced l-valine accumulation. This finding is consistent with the previous result reported by Vogt et al. [22]. Notably, the WL-12 strain (native promoters of both ilvBNC and leuA replaced with Ptuf promoter) provided the highest l-leucine yield (26.83 ± 0.42 g/L) among the three recombinant strains (WL-10, WL-11, and WL-12). The transcriptional levels of ilvB, ilvN, and ilvC in WL-12 were upregulated by > 1.9-fold compared with those in WL-8 (Fig. 4a, e). Furthermore, l-valine production by WL-12 was 4.58 ± 0.25 g/L, which was higher than that by WL-11 (Fig. 4d). This finding is attributable to the fact that leucine synthesis competes with the synthesis of other BCAAs for common precursors and the same enzymes [6]. Moreover, the insertion of Ptuf promoter in front of ilvBNC and leuA in the WL-12 strain more effectively increased carbon flux into l-leucine synthesis, in addition to increasing l-valine production. In summary, the WL-11 strain provided the lowest l-valine yield (3.26 ± 0.19 g/L) among the three recombinant strains, whereas the WL-12 strain provided the highest l-leucine yield (26.83 ± 0.42 g/L).
Comparison of different C. glutamicum strains during shake flask cultivation in fermentation medium to test the effect of balancing ilvBNC and leuA expression levels by inserting promoters of differing strengths. al-Leucine production, b glucose concentration, c cell growth, d by-product accumulation, e transcriptional levels of l-leucine biosynthesis genes, and f combination of strong promoter replacement
Further enhancing ilvBNC and leuA overexpression using a shuttle expression vector to improve l-leucine accumulation
To further enhance l-leucine synthesis, the key genes involved in l-leucine synthesis were overexpressed using the shuttle expression vector pEC-XK99E in the WL-12 strain. The WL-12 strain carrying an empty plasmid was used as the control (i.e., the WL-13 strain). As expected, the l-leucine yield reached 28.47 ± 0.36 g/L and 27.13 ± 0.48 g/L in the WL-14 with leuA expression and WL-15 with ilvBNC expression strains, respectively. l-valine accumulation in the WL-14 strain decreased to 3.85 ± 0.11 g/L (Fig. 5). These results further indicate that increasing leuA expression was a very efficient approach to redirect the metabolic flux from α-ketoisovalerate to l-leucine synthesis rather than l-valine synthesis. Notably, the affinity of IPMS for α-ketoisovalerate is tenfold greater than that of BCAT [9]. Hence, leuA overexpression was the most significant step to increase l-leucine production and reduce l-valine accumulation in C. glutamicum. One possible reason for l-valine accumulation and inadequate l-leucine yield could be the shortage of the second IPMS substrate acetyl-CoA.
However, it important to note that cell growth decreased slightly in all of the recombinant strains (Fig. 5). This observation is consistent with previous studies reporting that plasmid replication increases the metabolic burden of recombinant C. glutamicum [46]. The enhancement of pyruvate availability and enzyme activities is an efficient approach to redirect the metabolic flux to l-leucine synthesis. Our future work will focus on the metabolic engineering of strains with acetyl-CoA- and glucose-uptake systems, which have been successfully constructed for l-lysine production [47].
Conclusion
In this study, various efforts were made to increase the l-leucine yield of l-leucine-producing C. glutamicum strains. Increasing the precursor availability and engineering the biosynthetic pathways were shown to be critical factors for enhancing L-leucine production. The pyruvate availability was improved by deleting pyc and avtA genes and downregulating alaT expression. Given the importance of ilvBNC and leuA genes in l-leucine synthesis, their expression was upregulated by the insertion of strong Ptuf promoter and a shuttle expression vector, which enhanced carbon flux from pyruvate to l-leucine synthesis. Among all the strains constructed in this study, the WL-14 strain exhibited the highest l-leucine yield (i.e., 28.47 ± 0.36 g/L).
References
Wang YY, Xu JZ, Zhang WG (2019) Metabolic engineering of l-leucine production in Escherichia coli and Corynebacterium glutamicum: a review. Crit Rev Biotechnol 39(5):633–647
Feng LY, Xu JZ, Zhang WG (2018) Improved l-leucine production in Corynebacterium glutamicum by optimizing the aminotransferases. Molecules 23(9):2102
Zhang S, Zeng X, Ren M, Mao X, Qiao S (2017) Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol 8(1):10
Tsuchida T, Momose H (1986) Improvement of an l-leucine-producing mutant of Brevibacterium lactofermentum 2256 by genetically desensitizing it to α-acetohydroxy acid synthetase. Appl Environ Microbiol 51(5):1024–1027
Salmon KA, Yang CR, Hatfield GW (2006) Biosynthesis and regulation of the branched-chain amino acids. EcoSal Plus 2(1):1
Wang YY, Zhang F, Xu JZ, Zhang WG, Chen XL, Liu LM (2019) Improvement of l-leucine production in Corynebacterium glutamicum by altering the redox flux. Int J Mol Sci 20(8):220
Westbrook AW, Ren X, Moo-Young M, Chou CP (2018) Metabolic engineering of Bacillus subtilis for l-valine overproduction. Biotechnol Bioeng 115(11):2778–2792
Wang X, Zhang H, Quinn PJ (2018) Production of l-valine from metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 102(10):1–12
Yamamoto K, Tsuchisaka A, Yukawa H (2016) Branched-chain amino acids. Adv Biochem Eng Biotechnol 159:103–128
Oldiges M, Eikmanns BJ, Blombach B (2014) Application of metabolic engineering for the biotechnological production of l-valine. Appl Microbiol Biotechnol 98(13):5859–5870
Park JH, Lee SY (2010) Fermentative production of branched chain amino acids: a focus on metabolic engineering. Appl Microbiol Biotechnol 85(3):491–506
Kind S, Becker J, Wittmann C (2013) Increased lysine production by flux coupling of the tricarboxylic acid cycle and the lysine biosynthetic pathway metabolic engineering of the availability of succinyl-CoA in Corynebacterium glutamicum. Metab Eng 15:184–195
Eikmanns BJ, Blombach B (2014) The pyruvate dehydrogenase complex of Corynebacterium glutamicum: an attractive target for metabolic engineering. J Biotechnol 192:339–345
Wieschalka S, Blombach B, Eikmanns BJ (2012) Engineering Corynebacterium glutamicum for the production of pyruvate. Appl Microbiol Biotechnol 94(2):449–459
Bott M (2007) Offering surprises: TCA cycle regulation in Corynebacterium glutamicum. Trends Microbiol 15(9):417–425
Chen Z, Bommareddy RR, Frank D, Rappert S, Zeng AP (2014) Deregulation of feedback inhibition of phosphoenolpyruvate carboxylase for improved lysine production in Corynebacterium glutamicum. Appl Environ Microbiol 80(4):1388–1393
Buchholz J, Schwentner A, Brunnenkan B, Gabris C, Grimm S, Gerstmeir R, Takors R, Eikmanns BJ, Blombach B (2013) Platform engineering of Corynebacterium glutamicum with reduced pyruvate dehydrogenase complex activity for improved production of l-lysine, l-valine, and 2-ketoisovalerate. Appl Environ Microbiol 79(18):5566–5575
Peters-Wendisch PG, Schiel B, Wendisch VF, Katsoulidis E, Eikmanns BJ (2001) Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J Mol Microbiol Biotechnol 3(2):295–300
Yao W, Deng X, Zhong H, Liu M, Zheng P, Sun Z, Zhang Y (2009) Double deletion of dtsR1 and pyc induce efficient l-glutamate overproduction in Corynebacterium glutamicum. J Ind Microbiol Biotechnol 36(7):911–921
Chen C, Li Y, Hu J, Dong X, Wang X (2015) Metabolic engineering of Corynebacterium glutamicum ATCC13869 for l-valine production. Metab Eng 29:66–75
Hou X, Chen X, Zhang Y, Qian H, Zhang W (2012) l-valine production with minimization of by-products’ synthesis in Corynebacterium glutamicum and Brevibacterium flavum. Amino Acids 43(6):2301–2311
Vogt M, Haas S, Klaffl S, Polen T, Eggeling L, van Ooyen J, Bott M (2014) Pushing product formation to its limit: metabolic engineering of Corynebacterium glutamicum for l-leucine overproduction. Metab Eng 22:40–52
Hou X, Ge X, Wu D, Qian H, Zhang W (2012) Improvement of l-valine production at high temperature in Brevibacterium flavum by overexpressing ilvEBNrC genes. J Ind Microbiol Biotechnol 39(1):63–72
Nakamura M, Suzuki A, Akada J, Tomiyoshi K, Hoshida H, Akada R (2015) End joining-mediated gene expression in mammalian cells using PCR-amplified DNA constructs that contain terminator in front of promoter. Mol Biotechnol 57(11–12):1018–1029
Chen YJ, Liu P, Nielsen AA, Brophy JA, Clancy K, Peterson T, Voigt CA (2013) Characterization of 582 natural and synthetic terminators and quantification of their design constraints. Nat Methods 10(7):659–664
Zhang B, Zhang XM, Wang W, Liu ZQ, Zheng YG (2019) Metabolic engineering of Escherichia coli for d-pantothenic acid production. Food Chem 294:267–275
Pátek M, Nešvera J, Guyonvarch A, Reyes O, Leblon G (2003) Promoters of Corynebacterium glutamicum. J Biotechnol 104(1–3):311–323
Zhang B, Gao G, Chu XH, Ye BC (2019) Metabolic engineering of Corynebacterium glutamicum S9114 to enhance the production of l-ornithine driven by glucose and xylose. Bioresource Technol 284:204–213
Chen X, Gao C, Guo L, Hu G, Luo Q, Liu J, Nielsen J, Chen J, Liu L (2017) DCEO biotechnology: tools to design, construct, evaluate, and optimize the metabolic pathway for biosynthesis of chemicals. Chem Rev 118(1):4–72
Becker J, Wittmann C (2012) Bio-based production of chemicals, materials and fuels Corynebacterium glutamicum as versatile cell factory. Curr Opin Biotechnol 23(4):631–640
Becker J, Wittmann C (2015) Advanced biotechnology: metabolically engineered cells for the bio-based production of chemicals and fuels, materials, and health-care products. Angew Chem 54(11):3328–3350
Man Z, Xu M, Rao Z, Guo J, Yang T, Zhang X, Xu Z (2016) Systems pathway engineering of Corynebacterium crenatum for improved l-arginine production. Sci Rep UK 6:28629
Ma Y, Cui Y, Du L, Liu X, Xie X, Chen N (2018) Identification and application of a growth-regulated promoter for improving l-valine production in Corynebacterium glutamicum. Microb Cell Fact 17(1):185
Holatko J, Elisakova V, Prouza M, Sobotka M, Nesvera J, Patek M (2009) Metabolic engineering of the l-valine biosynthesis pathway in Corynebacterium glutamicum using promoter activity modulation. J Biotechnol 139(3):203–210
Zhang X, Xu G, Shi J, Koffas MAG, Xu Z (2018) Microbial production of l-serine from renewable feedstocks. Trends Biotechnol 36(7):700–712
Nagano-Shoji M, Hamamoto Y, Mizuno Y, Yamada A, Kikuchi M, Shirouzu M, Umehara T, Yoshida M, Nishiyama M, Kosono S (2017) Characterization of lysine acetylation of a phosphoenolpyruvate carboxylase involved in glutamate overproduction in Corynebacterium glutamicum. Mol Microbiol 104(4):677–689
Blombach B, Schreiner ME, Bartek T et al (2008) Corynebacterium glutamicum tailored for high-yield l-valine production. Appl Microbiol Biotechnol 79(3):471–479
Michael V, Haas S, Polen T, van Ooyen J, Bott M (2015) Production of 2-ketoisocaproate with Corynebacterium glutamicum strains devoid of plasmids and heterologous genes. Microbial Biotechnol 8(2):351–360
Hasegawa S, Suda M, Uematsu K, Natsuma Y, Hiraga K, Jojima T, Inui M, Yukawa H (2013) Engineering of Corynebacterium glutamicum for high-yield l-valine production under oxygen deprivation conditions. Appl Environ Microbiol 79(4):1250–1257
Marienhagen J, Eggeling L (2008) Metabolic function of Corynebacterium glutamicum aminotransferases AlaT and AvtA and impact on l-valine production. Appl Environ Microbiol 74(24):7457–7462
Zhang B, Yu M, Zhou Y, Ye BC (2018) Improvement of l-ornithine production by attenuation of argF in engineered Corynebacterium glutamicum S9114. AMB Express 8(1):26
Park JH, Lee KH, Kim TY, Lee SY (2007) Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci USA 104(19):7797–7802
Pátek M, Krumbach K, Eggeling L, Sahm H (1994) Leucine synthesis in Corynebacterium glutamicum: enzyme activities, structure of leuA, and effect of leuA inactivation on lysine synthesis. Appl Environ Microbiol 60(1):133–140
Huang Q, Liang L, Wu W, Wu S, Huang J (2017) Metabolic engineering of Corynebacterium glutamicum to enhance l-leucine production. Afr J Biotechnol 16(18):1048–1060
Zhang B, Yu M, Wei WP, Ye BC (2018) Optimization of l-ornithine production in recombinant Corynebacterium glutamicum S9114 by cg3035 overexpression and manipulating the central metabolic pathway. Microbial Cell Fact 17(1):91
Vogt M, Krumbach K, Bang W-G, van Ooyen J, Noack S, Klein B, Bott M, Eggeling L (2015) The contest for precursors: channelling l-isoleucine synthesis in Corynebacterium glutamicum without byproduct formation. Appl Microbiol Biotechnol 99(2):791–800
Xu JZ, Yu HB, Han M, Liu LM, Zhang WG (2019) Metabolic engineering of glucose uptake systems in Corynebacterium glutamicum for improving the efficiency of l-lysine production. J Ind Microbiol Biotechnol 46(7):937–949
Acknowledgements
This work was financially supported by Top-Notch Academic Programs Project of Jiangsu Higher Education Institutions, the National Natural Science Foundation of China (No. 31601459), and National First-class Discipline Program of Light Industry Technology and Engineering (No. LITE2018-07).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, YY., Shi, K., Chen, P. et al. Rational modification of the carbon metabolism of Corynebacterium glutamicum to enhance l-leucine production. J Ind Microbiol Biotechnol 47, 485–495 (2020). https://doi.org/10.1007/s10295-020-02282-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-020-02282-8