Abstract
There are excessive by-products in the biocatalysis process of this whole-cell biocatalytic production of melibiose from raffinose with current Saccharomyces cerevisiae strains. To solve this problem, we constructed engineered strains based on a liquor yeast (S. cerevisiae) via gene deletion (mel1 gene), heterologous integration (fsy1 or/and ffzi1 gene from Candida magnoliae), and gene overexpression (gcr1 gene). Functional verification showed that deletion of the mel1 gene led to elimination of the reactions catalyzed by α-galactosidase, as well as elimination of the degradation of melibiose and the formation of galactose by-product. Insertion of the fsy1 or/and ffzi1 gene and overexpression of the gcr1 gene could contribute to fructose transport for enhancing the biopurification rate of the fructose by-product. Compared with the wild-type strain, the optimal engineered strain of MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) had improved about 30% on yield, 31% on productivity, and 36% on purity of the melibiose product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melibiose (α-d-Gal-(1 → 6)-α-d-Glc), an important natural oligosaccharides with moderate sweetness, is widely used as an additive in manufactured foods, beverages, and cosmetics [13]. In particular, increasing evidence indicates that melibiose can be used as a main ingredient in human functional foods, animal-feed supplements, and pharmaceutical formulations. For instance, because of its prebiotic properties, melibiose can promote proliferation of the intestinal probiotic group such as Bifidobacteria and Lactobacillus [29]. Melibiose can adjust and maintain the balance of the human immune system to relieve allergic dermatitis symptoms [26, 27]. Additionally, it has been shown that melibiose is a potential therapeutic drug for polyQ-mediated neurodegenerative diseases such as Alzheimer’s disease [21]. Therefore, melibiose possesses potential applications for disease treatment, assisted food therapy, and animal disease prevention and treatment, which should expand the market for melibiose greatly over traditional sweeteners.
Melibiose was first identified in honey and later in many plants. Despite the higher content of melibiose in cottonseed, sugar beet, and mallow than in other plant sources, it is arduous to extract melibiose from these plants on a commercial scale because of the high cost of the purification process [8, 14, 25]. However, in some by-products of the agricultural industry, such as cottonseed and bean pulp, the content of raffinose (α-d-Gal-(1 → 6)-α-d-Glc-(1 → 2)-β-d-Fru), a trisaccharide composed of one melibiose and one fructose, is high enough for economical extraction [11, 25]. Hence, melibiose can be produced from raffinose by enzyme hydrolysis with invertase or by whole-cell biocatalysis with Saccharomyces cerevisiae [16, 24, 30]. Compared with enzyme hydrolysis, whole-cell biocatalysis has several advantages such as more flexible reaction conditions and convenient cell-cycle utilization for production cost control without needing the expensive enzyme. However, our previous study shown that whole-cell biocatalytic production of melibiose by S. cerevisiae strains with raffinose as the reaction substrate has the drawback of producing excessive by-products in this reaction system, such as sucrose, galactose, glucose, and fructose [30].
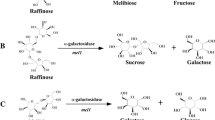
To solve this problem, it is essential to inhibit unwanted reactions induced by the α-galactosidase (labeled with red× in Fig. 1). Furthermore, since the α-galactosidase is encoded by the mel1 gene [22], the screening of wild-type S. cerevisiae strains that lack the mel1 gene and the deletion of the mel1 gene in a S. cerevisiae strain are effective strategies to prevent such reactions. Consequently, only the target product (melibiose) and one by-product (fructose) should be produced in this reaction system after implementation of this strategy.
Raffinose is whole-cell biocatalyzed by Saccharomyces cerevisiae and the strategies are presented for reducing by-products in this whole-cell biocatalysis. a Raffinose catalyzed by invertase (encoded by the suc2 gene) secreted from S. cerevisiae cell to product melibiose (target product) and fructose (by-product). b Further, melibiose is catalyzed by another secreted enzyme, α-galactosidase (encoded by the mel1 gene), to product galactose (by-product) and glucose (by-product). c Raffinose also can be hydrolyzed by α-galactosidase to product galactose and sucrose. d The sucrose is then hydrolyzed by the invertase to product glucose and fructose. In order to reduce the formation of by-products, such as sucrose, galactose, glucose, and fructose, it is essential for the hydrolytic reaction induced by α-galactosidase to be inhibited (labeled with red cross). Consequently, only the target product (melibiose) and one by-product (fructose) should be produced in this reaction system. e Finally, the fructose-specific symporter(s) (such as encoded by the fsy1or/and ffzi1 gene(s)) was/were heterologous expressed in this S. cerevisiae strain for improvement of the fructose’s trans-membrane transport to reduce the by-product fructose (color figure online)
Further, to obtain higher purity melibiose, it is necessary to remove the by-product fructose by a low cost and highly efficient method. One strategy for removing fructose is to promote efficient trans-membrane transport of fructose at the same time as the whole-cell biocatalysis, which depends on a high-affinity fructose-specific symporter operating efficiently in the chosen S. cerevisiae strain. Previously, high-affinity fructose-specific symporters were identified in some fructophilic yeasts, such as in Saccharomyces carlsbergensis [10], Kluyveromyces lactis [7], Saccharomyces bayanus [6], Zygosaccharomyces bailii [23], wine yeast S. cerevisiae [9], Zygosaccharomyces rouxii [19], and Candida magnoliae [20]. Hence, the biological purification for the fructose (by-product) could be feasible through expression of the heterologous fructose-specific symporters (encoded by the fsy1or/and ffzi1 gene) in this S. cerevisiae strain for improvement of fructose’s trans-membrane transport (Fig. 1e).
In addition, the uptake and consumption of hexose (such as glucose or fructose) is a complex physiological process involving not only the trans-membrane transport of hexose but also the glycolysis. In S. cerevisiae, the efficient transcription of glycolytic genes and ribosomal protein (RP) requires the RAP1p/GCR1p regulatory complex [5]. Especially, over-expression of gcr1 gene (GCR1p) solely in S. cerevisiae can improve the uptake and consumption of glucose, via up-regulation of the expression levels of some RP genes (glucose-responsive target genes such as rpl11a, rps18b and rpl30)and some glycolytic genes (such as hxt1 and adh1) [18]. Since the glycolysis of fructose is similar as glucose, over-expression of gcr1 gene in the S. cerevisiae strain should contribute to the fructose transport and consumption for enhancing the biopurification rate of the fructose by-product in this whole-cell biocatalysis.
Based on the above strategy, in this study we constructed a series of engineered S. cerevisiae strains based on the chosen liquor yeast [30], involving deletion of the mel1 gene, insertion of the fsy1 or/and ffzi1 gene(s) from C. magnoliae, and overexpression of the gcr1 gene, both individually and combined. We implemented functional verification of the engineered S. cerevisiae strains and determined the optimal strain. Furthermore, we systematically evaluated an improvement strategy for construction of engineered S. cerevisiae strain to produce melibiose via whole-cell biocatalysis of raffinose. This study could contribute to improve the technology of melibiose production and provide a model for the development of such similar whole-cell biocatalysis to obtain product with higher yield, productivity and purity.
Materials and methods
Chemicals and materials
Raffinose and fructose were obtained from China National Sugar & Alcohol Group Co., Ltd (Beijing, China). The standards of raffinose, sucrose, melibiose, galactose, glucose, and fructose for HPLC analysis were purchased from China National Pharmaceutical Group Co., Ltd (Beijing, China).
Yeast genomic extract kit and PCR polymerase Q5 were purchased from OMEGA Co., Ltd (Manchester, United Kingdom) and New England Biolabs (Beijing, China), respectively. ClonExpress® Entry One Step Cloning Kit and ClonExpress® MultiS One Step Cloning Kit were purchased from Vazyme Biotech Co.,Ltd (Nanjing, China).
Strains, plasmids, and media
The strains and the plasmids used in this study are described in Table 1. E. coli DH5α cells were used as hosts in the construction of plasmids (pMP2 to pMP8). C. magnoliae (CGMCC 2.1919) was obtained from China General Microbiological Culture Collection Center (CGMCC; Beijing, China), and its genome was used as the PCR template for cloning of fsy1 (GenBank: KC147727.1) and ffzi1 (GenBank: KC147728.1) genes. A series of engineered S. cerevisiae strains (MP1 to MP8) were derived from liquor yeast (S. cerevisiae: CGMCC 2.773), a typical wild-type Chinese liquor brewing industry strain obtained from CGMCC (Beijing, China). The genome of the liquor yeast strain (S. cerevisiae: CGMCC 2.773) was used as a PCR template for cloning of gcr1 gene (NCBI Reference Sequence: NM_001183889.1).
Plasmid pUG6 was used as a PCR template to amplify the loxP-KanRMX-loxP fragment. Plasmid pUC19 was used as a vector to construct a series of plasmids: pMP2 to pMP8. Plasmid pPICZαA::Cre contains the expression cassette of Cre recombinase and the selective marker gene for zeocin resistance, which was used for the removal of the selective marker KanR in the construction of the engineered S. cerevisiae strains.
LB medium (5 g/L yeast extract, 10 g/L tryptone, 10 g/L NaCl) was used for the culture of E. coli DH5α cells and the construction of the plasmids (pMP2–pMP8). YPD medium (10 g/L yeast extract, 20 g/L tryptone, 20 g/L glucose) was used for the cultures of C. magnoliae and S. cerevisiae. Resistance media (LB medium with 100 µg/mL of ampicillin, or/and 50 µg/mL of kanamycin, or/and 20 µg/mL of zeocin; YPD medium with 400 µg/mL of G418, or/and 20 µg/mL of zeocin) were used for the selection of positive clones when necessary.
DNA manipulation techniques
The manipulation of recombinant DNA was performed using standard techniques of in vitro fusion PCR [4, 12] and multi-fragments in vitro recombination [2]. A DNA fragment (UP mel1-5′-loxP-Kan R MX-loxP-DW mel1-3′) was constructed for the deletion of the mel1 gene in the liquor yeast S. cerevisiae, to develop the engineered S. cerevisiae strain MP1 by using standard fusion PCR. The construction strategy is shown in Figure S1, and the primers used are listed in Table S1. Similarly, the recombinant expression cassette of the fsy1 gene from C. magnoliae (P tdh3 -fsy1 cm -T cyc1 ; Figure S2; Table S2), the recombinant expression cassette of the ffzi1 gene from C. magnoliae (P tdh3 -ffzi1 cm -T cyc1 ; Figure S3; Table S3), and the recombinant expression cassette of the gcr1 gene from S. cerevisiae (P tdh3 -gcr1 sc -T cyc1 ; Figure S4; Table S4) were constructed using standard fusion PCR.
The recombinant DNA fragments for the development of engineered S. cerevisiae strains MP2 to MP8 were PCR amplified from the constructed corresponding plasmid. The plasmids pMP2 to pMP8 were constructed using ClonExpress® MultiS One Step Cloning Kit, for which the construction strategy and primers used are shown in the supplementary materials (pMP2: Figure S5, Table S5; pMP3: Figure S6, Table S6; pMP4: Figure S7, Table S7; pMP5: Figure S8, Table S8; pMP6: Figure S9, Table S9; pMP7: Figure S10, Table S10; and pMP8: Figure S11, Table S11).
Development of engineered strains
The DNA fragments for the development of engineered strains MP1 to MP8 were transformed into the liquor yeast S. cerevisiae strain using the AcLi induced method (standard), and positive clones were selected using resistance medium (YPD medium with 400 µg/mL of G418). The genomes of candidate clones were extracted for PCR identification of the gene deletion and recombination after expanding cultured in YPD with 400 µg/mL of G418. For removal of the selective marker KanR, plasmid pPICZαA::Cre was transformed into the confirmed clones, and positive clones were selected using resistance medium (YPD medium with 20 µg/mL of zeocin). After the candidate clones were subcultured for 3–5 generations in YPD medium to remove the plasmid pPICZαA::Cre, PCR identification was implemented using extracted genome from subculture of the candidate clones as templates. The confirmed clones were denoted as MP1 to MP8.
Whole-cell preparation of wild-type and engineered strains
A single colony of the liquor yeast (wild-type) or engineered strain (MP1–MP8) was placed into 5 mL of liquid YPD medium and cultivated at 30 °C and 200 rpm for 24 h. Subsequently, the cultures were transferred into 50 mL of liquid YPD and cultivated at 30 °C and 200 rpm for 14–18 h. Cells were collected by centrifugation at 4 °C and 7000×g for 20 min. The collected cells were washed twice with sterile water precooled on ice and centrifuged at 4 °C and 7000×g for 20 min.
Functional verification of the engineered strains in whole-cell biocatalysis
Functional verification of the engineered strain with the mel1 gene deletion
Samples (extracellular) from the whole-cell catalytic process of the MP1 (Δmel1) strain with the raffinose substrate were analyzed by HPLC, and the chromatogram maps were compared with those of the wild-type strain (liquor yeast) at 0, 2, 4, and 6 h. Those whole-cell catalytic reactions were performed at that whole-cell concentration of each testing strain was OD600 of 1 in 50 mL of raffinose substrate (50 g/L) cultivated at 30 °C and 200 rpm for 6 h.
Functional verification of the engineered strains with that heterologous integration of the fructose transporter gene(s)
Concentrations of fructose (extracellular) at 6 h in those whole-cell biocatalytic processes of that MP2 (Δmel1::fsy1 cm ), MP3 (Δmel1::ffzi1 cm ), and MP4 (Δmel1::fsy1 cm ::ffzi1 cm ) strains with fructose or raffinose substrates were analyzed by HPLC, and compared with the liquor yeast (wild-type) strain and/or MP1(Δmel1) strain. Those whole-cell catalytic reactions were performed at that whole-cell concentration of each testing strain was OD600 of 5 in 50 mL of fructose (or raffinose) substrate (50 g/L) cultivated at 30 °C and 200 rpm for 6 h.
Functional verification of engineered strains with overexpression of the gcr1 gene
Concentrations of fructose (extracellular) at 6 h in those whole-cell biocatalytic processes of the MP5 (Δmel1::gcr1 sc ), MP6 (Δmel1::fsy1 cm ::gcr1 sc ), MP7 (Δmel1::ffzi1 cm ::gcr1 sc ), and MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) strains with fructose or raffinose substrates were analyzed by HPLC, and compared with the MP1 (Δmel1), MP2 (Δmel1::fsy1 cm ), MP3 (Δmel1::ffzi1 cm ), and MP4 (Δmel1::fsy1 cm ::ffzi1 cm ) strains correspondingly. Those whole-cell catalytic reactions were performed at that whole-cell concentration of each testing strain was OD600 of 5 in 50 mL of fructose (or raffinose) substrate (50 g/L) cultivated at 30 °C and 200 rpm for 6 h.
Determination of the optimal engineered strain
To determine the optimal engineered strain, we compared the melibiose yield, productivity, and purity of the MP1–MP8 engineered strains at 6 h in those whole-cell biocatalytic processes. Those whole-cell catalytic reactions were performed at the whole-cell concentration of each testing strain was OD600 of 5 in 50 mL of raffinose substrate (50 g/L) cultivated at 30 °C and 200 rpm for 6 h. The substrate (raffinose), product (melibiose), and by-product (glucose) concentrations (extracellular) were determined by HPLC analysis. The melibiose yield (g/L) was determined by HPLC analysis directly; the melibiose productivity (%) was defined as that (determined melibiose yield/theoretical melibiose yield) × 100%; the melibiose purity (%) was defined as that [determined melibiose yield/(determined melibiose yield + determined fructose by- product yield) × 100%. The melibiose yield, productivity, and purity of the optimal engineered strain were compared with the wild-type strain similarly.
HPLC analysis
All samples from the above whole-cell biocatalysises were centrifuged at 20,000×g for 20 min to remove cell precipitation. Supernatant samples were incubated at 100 °C for 10 min, and centrifugation at 20,000×g for 20 min was performed on those samples to obtain the supernatant. These supernatant samples were filtered with 0.22 μm membranes for HPLC analysis to determine the substrate and product concentrations.
The HPLC system used was an Aglient 1200 series (Aglient Co., Ltd, CA, USA). Experiments were performed with a sugar-ParTM chromatographic column (6.5 × 300 mm) and Refractive Index Detector (RID), with deionized water as the mobile phase at a flow rate of 0.4 mL/min and column temperature of 80 °C.
Statistical analysis
Experiments were performed in three replicates. Data were expressed as arithmetic means or average ± standard deviation. Student’s t test or Mann–Whitney rank sum test was used to analyze the statistical significance of the observed differences. A p value of less than 0.05 was considered statistically significant. All the analyses were performed with Sigmaplot for Windows Version 12.0 (Systat Software, Inc).
Results
Engineered strain with the mel1 gene deletion could eliminate side reactions
For functional verification of the engineered strain with the mel1 gene deletion (Δmel1), samples from that whole-cell catalytic process of the MP1 (Δmel1) strain with raffinose substrate were analyzed by HPLC, and the chromatogram maps were compared with of the wild-type strain (liquor yeast) at 0 (Fig. 2a), 2 (Fig. 2b), 4 (Fig. 2c), and 6 h (Fig. 2d).
Functional verification of engineered strain with the mel1 gene deletion through comparison of that whole-cell catalytic process with raffinose substrate by the wild-type and Δmel1 strains using HPLC analysis. Samples from the whole-cell catalytic process of the MP1 (Δmel1) strain with raffinose substrate were analyzed by HPLC and were compared with the wild-type strain (liquor yeast) at 0 (a), 2 (b), 4 (c), and 6 h (d). Those whole-cell catalytic reactions were performed at that whole-cell concentration of each testing strain was OD600 of 1 in 50 mL of raffinose substrate (50 g/L) cultivated at 30 °C and 200 rpm for 6 h
At 0 h, the two chromatogram maps of the Δmel1 and wild-type strains were identical as they are the signal from the raffinose substrate. However, there were some miscellaneous peaks in addition to the characteristic peaks of raffinose in the maps, which indicated that the raffinose substrate contained some impurities such as sucrose, galactose, glucose, and fructose (Fig. 2a).
When whole-cell catalytic reactions were performed for 2 h, the map of the wild-type strain showed that some by-products had been generated; especially galactose, which indicated that the raffinose substrate or melibiose product had been hydrolyzed by the α-galactosidase (Fig. 2b). However, the map of the Δmel1 strain showed that no galactose was generated, which indicated that the unwanted reactions catalyzed by the α-galactosidase were eliminated. This confirms that the Δmel1 strain had achieved the desired effect.
When the reactions proceeded for 4 (Fig. 2c) and 6 h (Fig. 2d), it was more obvious that the desired function of the Δmel1 strain had been achieved by eliminating reactions induced by the α-galactosidase. In the catalytic system of the wild-type strain, the by-products of glucose, galactose, and fructose were still detected. Importantly, the galactose by-product significantly increased from 4 to 6 h and was accompanied by other by-products with also increased significantly (the peaks of other by-products are marked as 1, 2, and 3 in the maps of the wild-type strain in Fig. 2c, d). However, in the catalyzed system of the MP1 (Δmel1) strain, no galactose by-product was generated, which was accompanied by the complete conversion of the raffinose substrate. The only significant peaks were of the target product melibiose and the fructose by-product, which could be observed at 4 or 6 h, although there were some other by-products detected (marked with 2 and 3 in the maps of the MP1 (Δmel1) strain in Fig. 2c, d).
The sugar concentrations (extracellular) for the MP1 (Δmel1) and wild-type strain at (only) 6 h whose were determined by HPLC were presented in Table 2, a description on data.
In summary, by comparison of the whole-cell catalytic processes of the MP1 (Δmel1) strain and the wild-type strain with raffinose substrate, the side reactions mediated by α-galactosidase were eliminated, although there was a reduction in the whole-cell catalyzed speed of raffinose after mel1 gene deletion. The degradation of the target product melibiose and formation of the by-product galactose were eliminated in the MP1 (Δmel1) strain. This indicates that the function of the engineered strain MP1 (Δmel1) reached the expected goal.
Engineered strains with that heterologous integration of fructose transporter gene(s) could increase the fructose transporter
In the fructose substrate test (Fig. 3a), when compared with the liquor yeast (wild-type) strain and MP1 (Δmel1) strain, there were significant reductions in fructose concentrations (extracellular) in the whole-cell biocatalytic process of the MP2 (Δmel1::fsy1 cm ), MP3 (Δmel1::ffzi1 cm ), and MP4 (Δmel1::fsy1 cm ::ffzi1 cm ) strains at 6 h. Strikingly, there was a significant difference with the MP4 (Δmel1::fsy1 cm ::ffzi1 cm ) strain, which has simultaneous integration of fsy1 and ffzi1 genes, when comparing the fructose concentrations (extracellular) with the control strain. This indicates that the engineered strains with that heterologous integration of fructose transporter genes from C. magnoliae showed significant improvement in fructose transport during the whole-cell biocatalysis.
Functional verification of engineered strains with that heterologous integration of fructose transporter genes. The fructose concentration (extracellular) of engineered strains were compared with those of the wild-type and/or Δmel1 strains, at 6 h of in the whole-cell catalytic process with fructose substrate (a), or with raffinose substrate (b). Those whole-cell catalytic reactions were performed at that whole-cell concentration of each testing strain was OD600 of 5 in 50 mL of fructose (or raffinose) substrate (50 g/L) cultivated at 30 °C and 200 rpm for 6 h. Fructose concentrations (extracellular) were determined by HPLC analysis. Mean values of the results of triplicate experiments are shown with error bars indicating the relative standard deviation. *p < 0.05 (compared to the control strain)
In the raffinose substrate test (Fig. 3b), fructose concentrations (extracellular) in the whole-cell biocatalytic process of the MP2 (Δmel1::fsy1 cm ), MP3 (Δmel1::ffzi1 cm ), and MP4 (Δmel1::fsy1 cm ::ffzi1 cm ) strains at 6 h were all significant reductions compared with the MP1 (Δmel1) strain. Incidentally, engineered strains with that heterologous integration of fructose transporter genes from C. magnoliae also had significant improvement in fructose transport during the whole-cell biocatalysis with raffinose substrate. Integration of the fsy1 gene in an engineered strain was more efficient in improving fructose transport than integration of the ffzi1 gene, and the simultaneous integration of the fsy1 and ffzi1 genes exhibited some accumulated or synergistic effects on fructose transport.
With fructose and raffinose substrates, the engineered strains consistently had enhanced fructose transport capacity. Hence, the function of engineered strains with heterologous integration of fructose transporter genes from C. magnoliae was confirmed during the whole-cell biocatalytic processes.
Engineered strains with overexpression of the transcription factor (GCR1) could increase the fructose transport and consumption
For functional verification of engineered strains with overexpression of a transcription factor gene (gcr1), the concentrations of fructose (extracellular) at 6 h during the whole-cell biocatalytic processes of the MP5 (Δmel1::gcr1 sc ), MP6 (Δmel1::fsy1 cm ::gcr1 sc ), MP7 (Δmel1::ffzi1 cm ::gcr1 sc ), and MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) strains with fructose or raffinose substrate were compared with those of the corresponding MP1 (Δmel1), MP2 (Δmel1::fsy1 cm ), MP3 (Δmel1::ffzi1 cm ), and MP4 (Δmel1::fsy1 cm ::ffzi1 cm ) strains.
With fructose substrate, at 6 h of the whole-cell biocatalytic process, the fructose concentrations of MP6 (Δmel1::fsy1 cm ::gcr1 sc ), MP7 (Δmel1::ffzi1 cm ::gcr1 sc ), and MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) all showed significant differences compared with the respective control strains (Fig. 4a). However, overexpression of a transcription factor gene (gcr1) in the MP1 (Δmel1) strain did not shown a difference in the fructose concentrations (extracellular) in the whole-cell biocatalyzed process compared with the MP1 (Δmel1) strain (Fig. 4a). Taking these results together, we have found that overexpression of a transcription factor (GCR1) could contribute to improved fructose transport efficiency in engineered strains with the heterologous integration of fructose transporter gene(s), especially in which strains with the heterologous integration of the fsy1 gene and the simultaneous integration of the fsy1 and ffzi1 genes. Hence, the functions of engineered strains with overexpression of a transcription factor (GCR1) were confirmed in this fructose substrate testing.
Functional verification of engineered strains with overexpression of a transcription factor (GCR1). Concentrations of fructose (extracellular) at 6 h in the whole-cell biocatalytic process of the MP5 (Δmel1::gcr1 sc ), MP6 (Δmel1::fsy1 cm ::gcr1 sc ), MP7 (Δmel1::ffzi1 cm ::gcr1 sc ), and MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) strains in fructose or raffinose substrates were compared with that of the of respective MP1 (Δmel1), MP2 (Δmel1::fsy1 cm ), MP3 (Δmel1::ffzi1 cm ), and MP4 (Δmel1::fsy1 cm ::ffzi1 cm ) strains. Those whole-cell catalytic reactions were performed at that whole-cell concentration of each testing strain was OD600 of 5 in 50 mL of fructose (or raffinose) substrate (50 g/L) cultivated at 30 °C and 200 rpm for 6 h. Fructose concentrations (extracellular) were determined by HPLC analysis. a 6 h with fructose substrate; b 6 h with raffinose substrate. Mean values of the results of triplicate experiments are shown with error bars indicating the relative standard deviation. *p < 0.05 (compared to the controlled strain)
To verify the fructose transport capacity of the engineered strains with overexpression of the transcription factor (GCR1) in the whole-cell biocatalyzed production of melibiose from raffinose, we implemented a reaction with raffinose substrate (Fig. 4b).
Compared with the control strain, there were some reductions in fructose concentrations in the whole-cell biocatalytic process of the MP6 (Δmel1::fsy1 cm ::gcr1 sc ), MP7 (Δmel1::ffzi1 cm ::gcr1 sc ), and MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) strains at 6 h (Fig. 4b). Notably, in the whole-cell biocatalytic process with raffinose substrate, fructose concentrations of MP6 (Δmel1::fsy1 cm ::gcr1 sc ), MP7(Δmel1::ffzi1 cm ::gcr1 sc ), and MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) showed significant differences compared with that of the respective control strains (Fig. 4b). Hence, the function of the engineered strains with overexpression of a transcription factor (GCR1) was confirmed in this raffinose substrate testing.
Summarizing the results from the above verification tests, we had concluded that overexpression of the transcription factor (GCR1) in the engineered strains with that heterologous integration of fructose transporter gene(s) could enhance the fructose transport capacity in this whole-cell biocatalyzed process using fructose or raffinose substrate.
Determination of the optimal engineering strain for the whole-cell biocatalytic production of melibiose from raffinose
We had compared yield, productivity, and purity of the target product (melibiose) in the whole-cell biocatalytic production of melibiose from raffinose using strains MP1–MP8 to determine the optimal engineered strain. Those whole-cell catalytic reactions were performed at that whole-cell concentration of each testing strain was OD600 of 5 in 50 mL of raffinose substrate (50 g/L) cultivated at 30 °C and 200 rpm for 6 h. The substrate (raffinose), product (melibiose), and by-product (glucose) concentrations (extracellular) were determined by HPLC analysis for calculation of yield, productivity, and purity of the target product (melibiose).
At 6 h in the whole-cell biocatalytic process with strains MP1–MP8 and the wild-type strain (liquor yeast), the raffinose substrates were all transformed completely. At this time, the wild-type strain (liquor yeast) had a melibiose yield of 25.25 ± 1.46 g/L (mixed with sugar by-product). However, all the engineered strains had higher melibiose yields compared with the wild-type strain (liquor yeast), achieving 32–33 g/L.
Comparing the yield, productivity, and purity of the target product (melibiose) at 6 h with strains MP1–MP8 in the whole-cell biocatalytic production of melibiose from raffinose, it was obvious that there were no significant differences in the yield and productivity. However, the purity of the melibiose from the MP4 (Δmel1::fsy1 cm ::ffzi1 cm ), MP6 (Δmel1::fsy1 cm ::gcr1 sc ), MP7 (Δmel1::ffzi1 cm ::gcr1 sc ), and MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) strains was significantly different compared with the MP1(Δmel1) strain as a control (Fig. 5). Hence, the difference in performance of the engineered strains (MP1–MP8) was in the increase in the purity of the target product (melibiose) from the whole-cell biocatalytic production of melibiose from raffinose, which was meeting our expectation. Notably, the MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) strain had the most significant improvement in the purity of the target product (melibiose) among the engineered strains. Compared with the MP1 (Δmel1) strain which purity of the melibiose product was 75.19 ± 4.50%, the MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) strain had about 10% improvement in the purity of the melibiose product which was reach to 82.97 ± 3.60%. Hence, we could determine that the MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) strain was the optimal engineered strain for the whole-cell biocatalytic production of melibiose from raffinose.
Compared purity of the target product (melibiose) from the whole-cell biocatalytic production of melibiose from raffinose using the MP1–MP8 engineered strains at 6 h. Those whole-cell catalytic reactions were performed at that whole-cell concentration of each testing strain was OD600 of 5 in 50 mL of raffinose substrate (50 g/L) cultivated at 30 °C and 200 rpm for 6 h. The substrate (raffinose), product (melibiose), and by-product (glucose) concentrations (extracellular) were determined by HPLC analysis for calculation of yield, productivity, and purity of the target product (melibiose). The melibiose yield (g/L) was determined by HPLC analysis directly; the melibiose productivity (%) was defined as that (determined melibiose yield/theoretical melibiose yield) × 100%; the melibiose purity (%) was defined as that [determined melibiose yield/(determined melibiose yield + determined fructose by-product yield) × 100%. Mean values of the results of triplicate experiments are shown with error bars indicating the relative standard deviation. *p < 0.05 (compared to the control strain MP1(Δmel1)
In addition, compared with the wild-type strain (melibiose yield: 25.25 ± 1.46 g/L, melibiose productivity: 75.76 ± 4.38%, and melibiose purity: 60.74 ± 3.52%), the optimal engineered strain MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) had significant improvements on the yield, productivity, and purity of the target product (melibiose) in this whole-cell biocatalytic production of melibiose from raffinose (Fig. 6), which produced a melibiose yield: 32.86 ± 1.43 g/L, melibiose productivity: 98.98 ± 4.30%, and melibiose purity: 82.97 ± 3.61%, and had improved about 30% on the yield, about 31% on the productivity, and about 36% on the purity of melibiose product compared with the wild-type strain.
The melibiose yield, productivity, and purity of the optimal engineered strain (MP8) were compared with the wild-type strain similarly. a Compared yield of melibiose product; b compared productivity of melibiose product; c Compared purity of melibiose product. Mean values of the results of triplicate experiments are shown with error bars indicating the relative standard deviation. *p < 0.05 (compared to the wild-type strain)
Conclusion
An engineered strain was constructed to eliminate the by-products of the whole-cell biocatalytic production of melibiose from raffinose based on strategy involving the intracellular–extracellular interaction mechanism. Deletion of the mel1 gene could eliminate the side reactions mediated by α-galactosidase and led to elimination of the degradation of melibiose in this whole-cell biocatalysis. Recombination of the fsy1 or/and ffzi1 gene(s), and over-expression of the gcr1 gene could enhance the bio-purification rate of the fructose (by-product) in this whole-cell biocatalysis. The optimal engineered strain (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) had improved significantly on yield, productivity, and purity of the melibiose product compared with the wild-type strain in this whole-cell biocatalysis.
Discussion
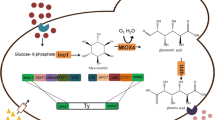
Whole-cell biocatalysis is a kind of biological technology that uses living cells as biological catalysts to catalyze specific reactions for obtaining target product [3]. During the process of whole-cell biocatalysis, there may be some side reactions, such as the ineffective catalytic consumption of specific substrates and target products. We could analyze the principle underlying specific whole-cell biocatalysis, identify key genes that cause side reactions, and knock them out to eliminate side reactions during the reaction process. For example, the sucA gene encoding α-ketoglutaric acid (α-KG) dehydrogenase was deleted to block α-KG degradation, further increasing the α-KG titer in a Bacillus subtilis whole-cell biocatalysis [15]. In this study, the mel1 gene encoding α-galactosidase was deleted to eliminate α-galactosidase expression (Fig. 7, ⑥ → k), which blocked the ineffective degradation of raffinose (substrate) and melibiose (target product). This engineered strain with the mel1 gene deletion could increase the melibiose yield from 25.25 to 32.86 g/L compared with the wild-type strain in the whole-cell biocatalysis using raffinose as substrate.
Construction of engineered strains for that whole-cell biocatalytic production of melibiose from raffinose. I Extracellular system. Raffinose substrate is catalyzed by invertase secreted from S. cerevisiae cell to produce melibiose and fructose. II Cell wall and cell membrane. There are raffinose, melibiose, and fructose transporters, as well as secretory pathways for invertase and α-galactosidase. III Extracellular system. There are that gene expression and regulation of two key enzymes (invertase and α-galactosidase), gene expression and regulation of three sugar transporter proteins (raffinose, melibiose, and fructose transporter proteins), and the fructose metabolism. Fructose metabolism results in acetyl-CoA via a series of metabolic conversion, which run into the TCA cycle to produce ATP as the energy supply maintaining the whole-cell biocatalysis. Cross symbol indicate that no α-galactosidase expression via the deletion of its coding gene, and no raffinose, and melibiose transporter expression via deletion of their coding genes. Green arrow indicate that enhancement of that whole-cell biocatalytic production of melibiose from raffinose, which are including invertase expression and its secretion, hydrolysis reaction of raffinose substrate, fructose transporter expression, fructose transport (from extracellular into intracellular), and intracellular metabolism of fructose to produce ATP
Our hypothesis was that if the whole-cell biocatalysis was primarily extracellular, the by-product would be transported into the cytosol via an enhancement to reach a similar bio-purification. In this study, the raffinose substrate is hydrolyzed by invertase secreted from S. cerevisiae cell to produce melibiose and fructose (Fig. 7, I), which happens extracellularly. Fructose, as a major by-product, should be transmembrane-transported into the cytosol (Fig. 7, O → P) in the whole-cell biocatalysis process to increase the extracellular melibiose purity. However, the fructose transport capacity of the wild-type strain is not strong enough for the whole-cell biocatalysis. Hence, we have carried out insertion of the fsy1 or/and ffzi1 genes from C. magnoliae, which are coding genes for fructose transporters [20], to construct the engineered strains of MP2 (Δmel1::fsy1 cm ), MP3 (Δmel1::ffzi1 cm ), and MP4 (Δmel1::fsy1 cm ::ffzi1 cm ) based on deletion of the mel1 gene.
We designed experiments with fructose as the sole substrate and simulated reaction conditions for whole-cell biocatalysis to verify the function of the fructose transports in the engineered strains during whole-cell biocatalysis, which was different with verifying the fructose transport capacity in the growth process of the strain [20]. At the same time, we detected the transport capacity for the by-product fructose for the engineered strains during the process of whole-cell biocatalysis with raffinose substrate. Our results showed that integration of heterologous fructose transporter genes had enhanced the fructose transport capacity for the engineered strains with fructose or raffinose substrates. Hence, this confirmed our hypothesis that the by-product could be transported into the cytosol via an enhancement to reach a similar bio-purification during whole-cell biocatalysis.
Overexpression of suitable transcription factors could enhance transcription and expression of target genes for strengthening functions of the target gene. In this study, we hypothesized that overexpression of the gcr1 gene in the S. cerevisiae strain could improve fructose consumption via enhanced transcription level of glycolytic genes [5]. We constructed engineered strains of MP6 (Δmel1::fsy1 cm ::gcr1 sc ), MP7 (Δmel1::ffzi1 cm ::gcr1 sc ), and MP8 (Δmel1::fsy1 cm ::ffzi1 cm ::gcr1 sc ) with overexpression of the gcr1 gene (transcription factor) based on deletion of the mel1gene and integration of the fructose transporters gene(s). In both fructose and raffinose substrates, we observe that the fructose concentrations (extracellular) were reduced significantly during the whole-cell biocatalysis using engineered strains compared with the control strains. In view of the whole-cell biocatalysis process, this provides a strategy in which overexpression of suitable transcription factors can improve the by-products bio-purification.
Compared with the wild-type strain, the engineered strains constructed in this study have significantly increased yield, productivity, and purity of the target product (melibiose) for whole-cell biocatalytic production of melibiose from raffinose. However, further studies are still needed, such as how to increase the rate of substrate catalysis, increase the yield of the target product (melibiose), enhance the bio-purification rate for the fructose by-product, and maintain the state of whole-cell biocatalysis for a longer duration. Hence, insights into construction strategies for engineered strains based on this study are necessary, which should provide ideas and theoretical support for further study (Fig. 7).
In increasing the rate of substrate (raffinose) catalysis, invertase is the key enzyme in the whole-cell biocatalysis (Fig. 7a); hence, based on engineering enzymes to fit the manufacturing process [1], directed mutagenesis can be performed in the coding gene for invertase and its cofactor genes for secretion to improve its expression and secretion (Fig. 7, ② → e→f, ③ → g) and to strengthen its stability and catalytic activity (Fig. 7a).
In order to increase the yield of the target product (melibiose), the loss of raffinose substrates and the melibiose product, which could be transported into the cytosol by the raffinose transporter (Fig. 7, b → d) and melibiose transporter (Fig. 7, h → i), should be considered. Based on membrane transporter engineering in industrial biotechnology and whole-cell biocatalysis [17], raffinose transporter genes should be deleted to yield no raffinose transporter expression (Fig. 7, ① → c). Similarly, melibiose transporter genes should be deleted to yield no melibiose transporter expression (Fig. 7, ④→ j).
In this study, we realized the heterologous integration of fructose transporter genes (Fig. 7, ⑦ → n) and overexpression of the transcription factor (gcr1 gene) in engineered strains to enhance transmembrane-transport (Fig. 7, o → p) and consumption of the fructose by-product for increasing the extracellular melibiose purity.
In addition, after the extracellular fructose by-product is transported into the cytosol (Fig. 7, o → p), it will change into acetyl-CoA via a series of metabolic conversion, and run into the TCA cycle to produce ATP and supply energy maintaining the whole-cell biocatalysis (Fig. 7, q → r→s). Then, fructose metabolism should contribute to enhance the bio-purification rate of the fructose by-product and extend the duration of state maintenance for this whole-cell biocatalysis. Hence, transformation of the metabolic pathways of fructose, such as improving key enzymes expression involved in fructose metabolism to strengthen metabolic pathway flow, is worth considering in further studies [28].
References
Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485(7397):185–194. doi:10.1038/nature11117
Cao P, Wang L, Zhou G, Wang Y, Chen Y (2014) Rapid assembly of multiple DNA fragments through direct transformation of PCR products into E. coli and Lactobacillus. Plasmid 76:40–46. doi:10.1016/j.plasmid.2014.09.002
de Carvalho CC (2011) Enzymatic and whole cell catalysis: finding new strategies for old processes. Biotechnol Adv 29(1):75–83. doi:10.1016/j.biotechadv.2010.09.001
de Jong BW, Shi S, Valle-Rodríguez JO, Siewers V, Nielsen J (2015) Metabolic pathway engineering for fatty acid ethyl ester production in Saccharomyces cerevisiae using stable chromosomal integration. J Ind Microbiol Biotechnol 42(3):477–486. doi:10.1007/s10295-014-1540-2
Deminoff SJ, Santangelo GM (2001) Rap1p requires Gcr1p and Gcr2p homodimers to activate ribosomal protein and glycolytic genes, respectively. Genetics 158(1):133–143
De Sousa HR, Spencer-Martins I, Gonçalves P (2004) Differential regulation by glucose and fructose of a gene encoding a specific fructose/H+ symporter in Saccharomyces sensu stricto yeasts. Yeast 21(6):519–530. doi:10.1002/yea.1118
Diezemann A, Boles E (2003) Functional characterization of the Frt1 sugar transporter and of fructose uptake in Kluyveromyces lactis. Curr Genet 43(4):281–288. doi:10.1007/s00294-003-0392-5
Ford CW (1972) Oligosaccharides from Phaseolus atropurpureus. Aust J Chem 25(4):889–892. doi:10.1071/CH9720889
Galeote V, Novo M, Salema-Oom M, Brion C, Valério E, Gonçalves P, Dequin S (2010) FSY1, a horizontally transferred gene in the Saccharomyces cerevisiae EC1118 wine yeast strain, encodes a high-affinity fructose/H+ symporter. Microbiology 156(12):3754–3761. doi:10.1099/mic.0.041673-0
Gonçalves P, de Sousa HR, Spencer-Martins I (2000) FSY1, a novel gene encoding a specific fructose/H+ symporter in the type strain of Saccharomyces carlsbergensis. J Bacteriol 182(19):5628–5630. doi:10.1128/JB.182.19.5628-5630.2000
Guimarães VM, de Rezende ST, Moreira MA, de Barros EG, Felix CR (2001) Characterization of α-galactosidases from germinating soybean seed and their use for hydrolysis of oligosaccharides. Phytochemistry 58(1):67–73. doi:10.1016/S0031-9422(01)00165-0
Hegemann JH, Heick SB (2011) Delete and repeat: a comprehensive tool kit for sequential gene knockout in the budding yeast Saccharomyces cerevisiae. In: Strain engineering: methods and protocols, pp 189–206. doi:10.1007/978-1-61779-197-0_12
Heljo VP, Filipe V, Romeijn S, Jiskoot W, Juppo AM (2013) Stability of rituximab in freeze-dried formulations containing trehalose or melibiose under different relative humidity atmospheres. J Pharm Sci 102(2):401–414. doi:10.1002/jps.23392
Hendrix DL, Peelen KK (1987) Artifacts in the analysis of plant tissues for soluble carbohydrates. Crop Sci 27(4):710–715. doi:10.2135/cropsci1987.0011183X002700040022x
Hossain GS, Li J, Shin HD, Liu L, Wang M, Du G, Chen J (2014) Improved production of α-ketoglutaric acid (α-KG) by a Bacillus subtilis whole-cell biocatalyst via engineering of l-amino acid deaminase and deletion of the α-KG utilization pathway. J Biotechnol 187:71–77. doi:10.1016/j.jbiotec.2014.07.431
Hudson CS, Harding TS (1915) The preparation of melibiose. J Am Chem Soc 37(12):2734–2736. doi:10.1021/ja02177a020
Kell DB, Swainston N, Pir P, Oliver SG (2015) Membrane transporter engineering in industrial biotechnology and whole cell biocatalysis. Trends Biotechnol 33(4):237–246. doi:10.1016/j.tibtech.2015.02.001
Kim D, Song JY, Hahn JS (2015) Improvement of glucose uptake rate and production of target chemicals by overexpressing hexose transporters and transcriptional activator Gcr1 in Saccharomyces cerevisiae. Appl Environ Microbiol 81(24):8392–8401. doi:10.1128/AEM.02056-15
Leandro MJ, Sychrová H, Prista C, Loureiro-Dias MC (2011) The osmotolerant fructophilic yeast Zygosaccharomyces rouxii employs two plasma-membrane fructose uptake systems belonging to a new family of yeast sugar transporters. Microbiology 157(2):601–608. doi:10.1099/mic.0.044446-0
Lee DH, Kim SJ, Seo JH (2014) Molecular cloning and characterization of two novel fructose-specific transporters from the osmotolerant and fructophilic yeast Candida magnoliae JH110. Appl Microbiol Biotechnol 98(8):3569–3578. doi:10.1007/s00253-013-5225-y
Lee GC, Lin CH, Tao YC, Yang JM, Hsu KC, Huang YJ (2015) The potential of lactulose and melibiose, two novel trehalase-indigestible and autophagy-inducing disaccharides, for polyQ-mediated neurodegenerative disease treatment. Neurotoxicology 48:120–130. doi:10.1016/j.neuro.2015.03.009
Naumov GI, Naumova ES, Korshunova IV, Jakobsen M (2002) Yeast comparative genetics: a new mel15 α-galactosidase gene of Saccharomyces cerevisiae. Russ J Genet 38(10):1127–1132
Pina C, Gonçalves P, Prista C, Loureiro-Dias MC (2004) Ffz1, a new transporter specific for fructose from Zygosaccharomyces bailii. Microbiol 150(7):2429–2433. doi:10.1099/mic.0.26979-0
Silman RW, Black LT, McGhee JE, Bangley EB (1980) Hydrolysis of raffinose in a hollow-fiber reactor using an unrefined mixture of α-galactosidase and invertase. Biotechnol Bioeng 22(3):533–541. doi:10.1002/bit.260220306
Song C, Zhao L, Ono S, Shimasaki C, Inoue M (2001) Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from cottonseed oil and valeric acid in batch culture of Ralstonia sp. strain JC-64. Appl Microbiol Biotechnol 94(2):169–178. doi:10.1385/ABAB:94:2:169
Tanaka S, Shinoki A, Hara H (2014) Melibiose, a non-digestible saccharide, promotes absorption of quercetin glycosides in rat small intestine with a novel mechanism. FASEB 28(1 Supplement):1044–1049
Tomita K, Nagura T, Okuhara Y, Nakajima-Adachi H, Shigematsu N, Aritsuka T (2007) Dietary melibiose regulates Th cell response and enhances the induction of oral tolerance. Biosci Biotechnol Biochem 71(11):2774–2780. doi:10.1271/bbb.70372
Weber N, Gorwa-Grauslund M, Carlquist M (2014) Exploiting cell metabolism for biocatalytic whole-cell transamination by recombinant Saccharomyces cerevisiae. Appl Microbiol Biotechnol 98(10):4615–4624. doi:10.1007/s00253-014-5576-z
Xiao M, Tanaka K, Qian XM, Yamamoto K, Kumagai H (2000) High-yield production and characterization of α-galactosidase from Bifidobacterium breve grown on raffinose. Biotechnol Lett 22(9):747–751. doi:10.1023/A:1005626228056
Zhou Y, Zhu Y, Dai L, Men Y, Wu J, Zhang J, Sun Y (2016) Efficiency analysis and mechanism Insight of that whole-cell biocatalytic production of melibiose from raffinose with Saccharomyces cerevisiae. Appl Biochem Biotechnol 2016:1–17. doi:10.1007/s12010-016-2220-7
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (No. 2013AA102102) and the National Natural Science Foundation of China (General Program, 31571793).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations, which would inappropriately influence our work; there is no professional or other personal interest of any nature in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, this manuscript.
Ethical approval
This article does not include any studies involving human participants or animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, Y., Zhu, Y., Men, Y. et al. Construction of engineered Saccharomyces cerevisiae strain to improve that whole-cell biocatalytic production of melibiose from raffinose. J Ind Microbiol Biotechnol 44, 489–501 (2017). https://doi.org/10.1007/s10295-017-1901-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1901-8