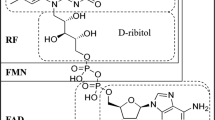
Abstract
Riboflavin (vitamin B2) is an essential nutrient for humans and animals that must be obtained from the diet. To ensure an optimal supply, riboflavin is used on a large scale as additive in the food and feed industries. Here, we describe a historical overview of the industrial process of riboflavin production starting from its discovery and the need to produce the vitamin in bulk at prices that would allow for their use in human and animal nutrition. Riboflavin was produced industrially by chemical synthesis for many decades. At present, the development of economical and eco-efficient fermentation processes, which are mainly based on Bacillus subtilis and Ashbya gossypii strains, has replaced the synthetic process at industrial scale. A detailed account is given of the development of the riboflavin overproducer strains as well as future prospects for its improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Discovery of riboflavin
Despite the fact that human beings have suffered vitamin-deficiency diseases, such as scurvy, beriberi, night blindness, xerophthalmia, pellagra and etcetera since the beginning of their existence, the recognition of vitamins as essential nutritional factors was not established until about the start of the twentieth century. Descriptions of diseases linked to diet were already reported in ancient documents like the Ebers Papyrus (ca. 1150 b.c.) and the writings of Hippocrates (ca. 420 b.c.). However, until the beginning of the twentieth century, the nutritional value of food was only exclusively considered in terms of its ability to provide energy and the basic building units of life [54].
In the early 1900s, the pioneer work of Christiaan Eijkman (Nijkerk, The Netherlands), Frederick Hopkins (Eastbourne, UK), Casimir Funk (Warsaw, Poland), Elmer McCollum (Kansas, US), and others, firmly established the existence of a new class of essential nutrients, and, in only five decades, paved the way to the isolation of more than a dozen vitamins as pure chemical substances (the last vitamin to be discovered was vitamin B12 in 1948).
In 1927, it was recognized that the so-called vitamin B complex contained two different components: the heat-labile antineuritic factor, vitamin B1 (thiamine), and vitamin B2 (riboflavin), a more heat-stable factor required by the rat for the maintenance of growth and the prevention of skin lesions. In 1933, the Heidelberg University team including Paul György, Richard Kuhn and Theodore Wagner-Jauregg was successful, for the first time, in the isolation and purification of vitamin B2 using the growth response of rats fed a purified diet as an assay. Kuhn suggested that the bright yellow fluorescent compound associated with the growth-promoting activity be given the name of flavin. This vitamin was first isolated from egg white (ovoflavin), from urine (uroflavin), from liver (hepatoflavin), and, later on, in large amounts from whey (lactoflavin) (1 g of crystallized lactoflavin from 5400 l of whey). Pure crystalline flavin compounds were found to contain ribose and be identical, and thus, the name riboflavin became standard when referring to these compounds.
Production by chemical synthesis
Two years later, Richard Kuhn at Heidelberg [20], and Paul Karrer at the University of Zurich [18], almost simultaneously synthesized the vitamin, although Karrer’s process was adapted for commercial production of riboflavin by Hoffman-La Roche.
In 1933, Randolph Major, Merck’s first director of R&D, decided to focus the company’s research efforts on vitamins and envisioned that synthetic vitamins could be produced in bulk and sold at prices that would allow for their use as food additives. Major also took the initiative and hired promising young scientists, including Max Tishler, who developed both a new synthesis of and industrial process for manufacturing riboflavin [52, 53].
For almost five decades, commercial riboflavin was almost exclusively produced by chemical synthesis processes, which essentially consisted of six to eight chemical steps starting from glucose [21]. In 1980, a combined chemoenzymatic process was developed that proceeds through a four-step reaction sequence also starting from glucose. In the first step, d-ribose is produced by fermentation from glucose using Bacillus tkt mutants in which transketolase, a major enzyme of the pentose phosphate pathway, is absent [42]. Afterwards, a reaction with xylidine is used to convert ribose into a riboside, which is then hydrogenated to produce ribamine and purified by crystallization. The subsequent step involves a reaction between ribamine and a phenyl diazonium salt derived from aniline, yielding phenylazoribitylamine. This compound is crystallized, dried and converted into vitamin B2 by cyclocondensation with barbituric acid [50]. An overall product yield of over 60 and 96 % purity can be achieved using this process. However, several chemical steps involve the use of toxic agents and produce many waste products that require stringent environmental control and special forms of effluent treatment.
Production by microbial fermentation
Between the 1960s and 1990s, chemical industrial production attempted to minimize the adverse impact generated by this process by treating effluent and removing pollutants from an already damaged environment. Designing industrial processes and technologies that prevented pollution did not become a priority until just recently. Nevertheless, attempts to obtain riboflavin by fermentation instead of using the less nature-friendly chemical processes were already initiated during the middle of the last century.
The first commercial fermentations for riboflavin production were based on the anaerobic bacterium Clostridium acetobutylicum [36] and the two natural riboflavin-overproducer molds Eremothecium ashbyii, in 1940, and Ashbya gossypii, in 1946 [36, 55]. In 1965, several companies established fermentation processes for riboflavin at industrial scale, but the production plants were closed down a few years later, because they were not competitive with the chemical process [22].
In 1954, a young Arnold Demain joined Merck’s Microbiology Department, and a decade later, Merck’s Vice President Karl Pfister asked him to form a new department devoted to the improvement of product biosynthesis in microbial strains. In addition to the investigation of the biosynthetic process of cephalosporin, streptomycin, monosodium glutamate, and other products, Demain and his group in the new Department of Fermentation Microbiology also initiated the development of a microbiological riboflavin process. He chose the fungus Ashbya gossypii because it was already known to make 5 g/L [5]. By the time Demain left Merck in 1969, they had greatly boosted the production of riboflavin by A. gossypii fermentation [16]. However, the process was not competitive enough to be used for industrial production of vitamin B2 at that time. From 1974 to 1984, riboflavin fermentation with A. gossypii was resumed at Merck, and an intense strain development program by classical mutagenesis was developed, which generated overproducer strains able to yield up to 15 g/L riboflavin [2].
In the late 1980s, some information appeared in the literature about organisms that could possibly synthesize riboflavin with appropriate yields, and a feasibility study was undertaken to compare the biological with the chemical process on the basis of yield, space yield (productivity) and titer. Another leader company in the vitamin B2 sector, Roche, initiated the production of riboflavin in 1942 using chemical processes. In 1988, Roche started the development of a new, entirely biological process, based on the Gram-positive, non-toxigenic bacterium Bacillus subtilis. After several years of genetic engineering research in collaboration with external partners (BioTechnica International/OmniGene Bioproducts), a B. subtilis strain was constructed, which was able to efficiently convert glucose into riboflavin. Subsequently, the genetically engineered strain was further developed by classical mutagenesis, aiming to reduce the use of raw materials and increase productivity. After a pilot-scale phase carried out in Japan in 1996, a large production plant was constructed at Grenzach-Wyhlen, Germany, in 2000, which finally replaced Roche’s chemical production utilizing the B. subtilis bioprocess. This plant had an initial capacity of 2000 tons per year, with the capability of increasing to 3000 tons/year. In September 2003, the Dutch DSM multinational, a nutritional and specialty food ingredients manufacturer, acquired Roche’s Vitamin Division including the vitamin B2 production business.
After several years of running commercial production of riboflavin using a chemical synthesis process (4000 tons/year), the multinational chemical company BASF implemented, in 1990, the production of vitamin B2 at industrial scale using the A. gossypii fermentation process initially developed by Merck. In collaboration with our Metabolic Engineering group at the University of Salamanca, researchers at the company succeeded in increasing the productivity of the microorganism by 20 % [1]. For almost 6 years, both the chemical and the biotechnological industrial processes were simultaneously exploited, proving that the single-step fermentation route was economically advantageous. Consequently, the synthetic process was abandoned and the chemical plant closed down in 1996.
Metabolic engineering of riboflavin production
The development of strains with high riboflavin productivity has been achieved by applying methods of classical mutagenesis and, more recently, by modern strategies of metabolic engineering. Random, chemical and radiation mutagenesis is a rapid and efficient method used at the beginning stages of strain development for metabolite production. This process is highly efficient when antimetabolites specific for the target biosynthetic pathway(s) are available.
Bacillus subtilis
Initial improvement of B. subtilis riboflavin-producing strains was accomplished by selecting mutants resistant to purine (8-azaguanine, methionine sulfoxide, decoyinine) or riboflavin analogs (roseoflavin) [35, 50]. However, classical mutagenesis soon reached its limitation and no additional improvements were achieved after several rounds of mutagenesis and selection, and therefore, other strategies like gene-targeted metabolic engineering were employed. To obtain industrially competitive B. subtilis strains, integration of multiple copies of the RIB operon in the genome as well as the substitution of the native promoter with strong, constitutive promoters was performed [35]. Further strain development has been addressed mainly to increase the supply of precursors for the riboflavin biosynthetic pathway by increasing the carbon flux through the PP pathway [7, 46, 56] and enhancing the expression of the purine biosynthetic genes (pur operon) [47, 48].
Ashbya gossypii
Prior to the application of metabolic engineering strategies in A. gossypii, a detailed molecular characterization of the riboflavin biosynthetic genes (RIB genes) [10, 37, 40], as well as the development of a molecular toolbox specific for this organism, was required. This set of molecular methods included an efficient electrotransformation method [8], recycling selectable markers [23], an assortment of constitutive and regulatory promoters of different strength [38], an insertional mutagenesis technique [41], and the sequencing of the genome [6].
A successful collaborative research program between BASF-based research groups (H. Seulberger, O. Zelder, B. Kroeger, H. Althoefer, M. Pompejus, C. Bollschweiler, M. Karos, R. Thummer, S. Haefner and B. Hoff), research teams from the Jülich Institute for Biotechnology, Germany, (H. Sahm, R. Krämer, and KP. Stahmann), and our own Metabolic Engineering group at the University of Salamanca, Spain, was established to develop industrially competitive A. gossypii strains. Rational metabolic design in A. gossypii has been focused on different processes, which a priori seemed relevant for riboflavin production. Since GTP is one of the committed precursors for riboflavin biosynthesis, the de novo purine biosynthetic pathway attracted considerable attention. Purine biosynthesis is a tightly regulated pathway at the transcriptional and metabolic levels. Two enzymes, PRPP amidotransferase (encoded by AgADE4 in A. gossypii) and PRPP synthetase (encoded by four different genes: AgPRS1, AgPRS2,4, AgPRS3 and AgPRS5), are subjected to feedback inhibition by their end products and are major control steps of the purine pathway (Fig. 1). Accordingly, strains overexpressing inhibition-resistant forms of PRPP amidotransferase and PRPP synthetase were constructed and showed tenfold and twofold increases, respectively, in riboflavin production [14, 15]. Insertional mutagenesis allowed the isolation of several mutants with improved vitamin B2 production yields [41]. One of these mutations, shown to be in the transcription factor (AgBAS1), has been reported to transcriptionally control the purine biosynthetic pathway in S. cerevisiae. By mimicking the insertional mutant, the construction of a strain expressing a AgBAS1 truncated factor lacking the regulatory domain led to a deregulated, constitutive transcription of the genes involved in the purine biosynthetic pathway, and a tenfold enhanced riboflavin overproduction (Fig. 1) [31].
Metabolic engineering strategies for riboflavin overproduction in Ashbya gossypii. Schematic diagram of the interconnection of the pentose phosphate (blue box) purine biosynthesis (green box) and riboflavin biosynthesis pathways (yellow box). Green arrows indicate gene overexpression; red lines indicate gene knockout or underexpression; red broken lines indicate abolished end-product inhibition mechanism; green broken line indicates transcription activation of the purine biosynthesis genes by the truncated transcription factor BAS1t. AMP adenosine monophosphate, DHBP 3,4-dihydroxy-2-butanone-4-phosphate, Gly glycine, GTP guanosine triphosphate, IMP inosine monophosphate, PRA 5-phosphoribosylamine, PRPP phosphoribosylpyrophosphate, R5P ribose 5-phosphate, Ru5P ribulose 5-phosphate, Ser serine, Thr threonine
Glycine, which also participates in the biosynthesis of purines, stimulates riboflavin production in A. gossypii, and several examples illustrate how increasing the levels of intracellular glycine can enhance riboflavin production. To improve the supply of the purine precursor glycine, the gene encoding threonine aldolase (AgGLY1) was overexpressed resulting in a remarkable enhancement in riboflavin production [33]. Similarly, the disruption of the gene encoding one of two isoenzymes of serine hydroxymethyltransferase (AgSHM2) also increased the production of riboflavin [44]. Heterologous expression of the alanine:glyoxylate aminotransferase encoding gene (AGX1) from Saccharomyces cerevisiae was also used to enlarge the pool of glycine precursor [19].
Since oils are the preferred carbon source for industrial riboflavin fermentation in A. gossypii, an efficient glyoxylate cycle is required for acetyl-CoA to be converted into the carbohydrate precursors needed for riboflavin biosynthesis [50]. Improvement of riboflavin production was achieved by the isolation of mutants resistant to itaconate, an inhibitor of the key isocitrate lyase enzyme that exerts the main control of the glyoxylate shunt [43]. Introduction of an additional copy of the ICL1 gene, encoding isocitrate lyase, enhanced riboflavin production in a medium containing soybean oil [3, 30]. Overexpression of the second enzyme of the glyoxylate pathway, malate synthase, was also performed in an attempt to improve the efficiency of oil consumption and riboflavin production [51].
In addition, attention has also been paid to the riboflavin transport processes. In A. gossypii, an unidentified high-activity efflux carrier capable of maintaining a concentration gradient of at least two orders of magnitude over several hours exports riboflavin out of the cell. Riboflavin is also stored in the vacuolar compartment, leading to product retention and thereby reducing the excretion yields and requiring the disruption of cells to obtain the full amount of the product [9]. Knockout of the gene encoding vacuolar ATPase (AgVMA1), which energizes active riboflavin transport from the cytoplasm to the vacuole, resulted in complete excretion of the synthesized riboflavin into the medium (Fig. 1) [8].
Recent approaches guided by computational metabolic modeling have led to the overexpression of RIB genes [24]. Although RIB1 and RIB3 were the major limiting steps in riboflavin production, the strain overexpressing all the RIB genes showed the highest production yield (Fig. 2) [25].
It has been reported that A. gossypii industrial riboflavin strains accumulate more than 15 g/L of riboflavin [2]. However, these data do not consider the improvements achieved by recent metabolic engineering approaches, and current industrial producer strains could surely accumulate much higher titers.
Future prospects and perspectives
Industrial production of riboflavin has come a long way but is still subject to further improvement [45]. In the recent years, traditional metabolic engineering has suffered a rapid expansion thanks to the development of novel techniques, which has settled the concept of systems metabolic engineering. This term refers to the applications to metabolic engineering of systems biology, synthetic biology, metabolic modeling and advanced genome engineering techniques [29].
Systems biology permits a wide analysis of the metabolic network through the analysis of genomes, transcriptome, proteome, metabolome and fluxome. These omics techniques allow us to define novel target for engineering by the identification of bottlenecks and regulatory systems. So far, transcriptomic and proteomic analyses has been carried out in both the B. subtilis and A. gossypii cell factories [11, 12] but only with the aim of improving riboflavin in the case of transcriptomic analysis in B. subtilis [47] and proteomic analysis in A. gossypii [34].
Metabolomic analysis has recently been applied to improve inosine production [26], whose synthetic pathway is shared for riboflavin production. Lately, fluxomics-metabolic flux analysis (MFA) has been carried out to elucidate the different fluxes between a parental strain and an overproducer mutant of A. gossypii, which highlighted the importance of pentose phosphate and purine pathways [13]. In B. subtilis, MFA studies revealed the distribution of fluxes along different phases during fed-batch cultivation of an industrial riboflavin producer strain [39].
The recent developments in synthetic biology have also had a high impact in metabolic engineering. Novel genetic circuits can be developed to finely control the desired pathway expression. In addition, synthetic biosensors can be applied for evolutionary engineering approaches or to reduce intermediate and undesired metabolites. A mutant library of B. subtilis has been successfully screened in nanoliter reactors using engineered E. coli cells that transform riboflavin into FMN, which is sensed by an RNA riboswitch triggering the expression of GFP [32]. In addition, assembly methods will facilitate the construction of large genetic cassettes [4]. In A. gossypii, Golden Gate assembly has been successfully developed by our group [23, 27].
Genome-scale metabolic models have been proven to be useful tools for metabolic engineering [49], not only to analyze in silico metabolic fluxes but also to study omics data in the context of the metabolic network, which may lead to the identification of novel target genes. In A. gossypii, our group has recently reconstructed and validated the model iRL766 and it has been successfully combined with transcriptomic data [17] to obtain a better understanding of those genes involved in riboflavin production [24].
The above-mentioned systems metabolic engineering techniques are in an early stage of development in the two major riboflavin producers, and thus, these organisms’ capacity to produce the vitamin is expected to be boosted in parallel to the improvement and standardization of such techniques. In addition to the technical advancement of the field, the selection of the target for the engineering process may also improve the current process of riboflavin production. So far, most of the efforts for strain engineering have been carried out around the synthetic pathways of the vitamin, but it seems interesting to explore other cell behaviors such as (1) the expansion of the substrate range to produce the vitamin from cheaper carbon sources such as lignocellulosic material or starch [28], (2) the increase of the robustness of the strain to reduce substrate sterilization or even to permit open-air fermentations, and (3) the increase of the temperature resistance of the strains to diminish the cost in bioreactor refrigeration and save time for the cooling of the feeding streams.
References
Althöefer H, Revuelta JL, Seulberger H, Zelder O (1999) Genetic method for producing riboflavin. Patent WO1999061623 A2
Bigelis R (1989) Industrial products of biotechnology: application of gene technology. In: Rehm HJ, Reed G (eds) Biotechnology, vol 7b. VCH, Weinheim, p 243
Boeddecker T, Kaesler B, Sahm H, Schmidt G, Seulberger H, Stahmann KP (1997) Riboflavin production process by means of microorganisms with modified isocitrate lyase activity. Patent WO1997003208 A1
Casini A, Storch M, Baldwin GS, Ellis T (2015) Bricks and blueprints: methods and standards for DNA assembly. Nat Rev Cell Mol Biol 16(9):568–576
Demain AL (1972) Riboflavin oversynthesis. Annu Rev Microbiol 26:369–388
Dietrich FS, Voegeli S, Brachat S, Lerch A, Gates K, Steiner S, Mohr C, Pöhlmann R, Luedi P, Choi S, Wing RA, Flavier A, Gaffney TD, Philippsen P (2004) The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science 304(5668):304–307
Duan YX, Chen T, Chen X, Zhao XM (2010) Overexpression of glucose-6-phosphate dehydrogenase enhances riboflavin production in Bacillus subtilis. Appl Microbiol Biotechnol 85(6):1907–1914
Förster C, Santos MA, Ruffert S, Krämer R, Revuelta JL (1999) Physiological consequence of disruption of the VMA1 gene in the riboflavin overproducer Ashbya gossypii. J Biol Chem 274(14):9442–9448
Förster C, Revuelta JL, Krämer R (2001) Carrier-mediated transport of riboflavin in Ashbya gossypii. Appl Microbiol Biotechnol 55(1):85–89
Garcia-Ramirez JJ, Santos MA, Revuelta JL (1995) The Saccharomyces cerevisiae RIB4 gene codes for 6,7-dimethyl-8-ribityllumazine synthase involved in riboflavin biosynthesis. Molecular characterization of the gene and purification of the encoded protein. J Biol Chem 270:23801–23807
Gattiker A, Rischatsch R, Demougin P, Voegeli S, Dietrich FS, Philippsen P, Primig M (2007) Ashbya Genome Database 3.0: a cross-species genome and transcriptome browser for yeast biologists. BMC Genom 8:9
Hahne H, Mäder U, Otto A, Bonn F, Steil L, Bremer E, Hecker M, Becher D (2010) A comprehensive proteomics and transcriptomics analysis of Bacillus subtilis salt stress adaptation. J Bacteriol 192(3):870–882
Jeong BY, Wittmann C, Kato T, Park EY (2015) Comparative metabolic flux analysis of an Ashbya gossypii wild type strain and a high riboflavin-producing mutant strain. J Biosci Bioeng 119(1):101–106
Jimenez A, Santos MA, Pompejus M, Revuelta JL (2005) Metabolic engineering of the purine pathway for riboflavin production in Ashbya gossypii. Appl Environ Microbiol 71(10):5743–5751
Jimenez A, Santos MA, Revuelta JL (2008) Phosphoribosyl pyrophosphate synthetase activity affects growth and riboflavin production in Ashbya gossypii. BMC Biotechnol 8:67
Kaplan L, Demain AL (1970) Nutritional studies on riboflavin overproduction by Ashbya gossypii. In: Ahearn DG (ed) Recent trends in yeast research, vol 1. Georgia State University, Atlanta, pp 137–159
Karos M, Vilariño C, Bollschweiler C, Revuelta JL (2004) A genome-wide transcription analysis of a fungal riboflavin overproducer. J Biotechnol 113(1–3):69–76
Karrer P, Schopp K, Benz F (1935) Synthesen von flavinen IV. Helv Chim Acta 18:426–429
Kato T, Park EY (2006) Expression of alanine:glyoxylate aminotransferase gene from Saccharomyces cerevisiae in Ashbya gossypii. Appl Microbiol Biotechnol 71(1):46–52
Kuhn R, Reinemund K, Weygand F (1934) Synthesis of lumilactoflavins. Ber 67:1460–1463
Kurth R, Paust W, Haenlein W (1996) Vitamins, Chapter 7. In: Ullmann’s encyclopedia of industrial chemistry. VCH, Weinheim, A27: 521–530
Lago BD, Kaplan L (1981) Vitamin fermentations: B2 and B12. Adv Biotechnol 3:241–246
Ledesma-Amaro R, Santos MA, Jimenez A, Revuelta JL (2014) Strain design of Ashbya gossypii for single-cell oil production. Appl Environ Microbiol 80:1237–1244
Ledesma-Amaro R, Kerkhoven EJ, Revuelta JL, Nielsen J (2014) Genome scale metabolic modeling of the riboflavin overproducer Ashbya gossypii. Biotechnol Bioeng 111(6):1191–1199
Ledesma-Amaro R, Serrano-Amatriain C, Jimenez A, Revuelta JL (2015) Metabolic engineering of riboflavin production in Ashbya gossypii through pathway optimization. Microb Cell Fact 14(1):163
Ledesma-Amaro R, Buey RM, Revuelta JL (2015) Increased production of inosine and guanosine by means of metabolic engineering of the purine pathway in Ashbya gossypii. Microb Cell Fact 14:58
Ledesma-Amaro R, Lozano-Martinez P, Jimenez A, Revuelta JL (2015) Engineering Ashbya gossypii for efficient biolipid production. Bioengineered 6(2):119–123
Ledesma-Amaro R, Nicaud JM (2016) Metabolic engineering for expanding the substrate range of Yarrowia lipolytica. Trends Biotechnol. doi:10.1016/j.tibtech.2016.04.010
Lee JW, Kim TY, Jang YS, Choi S, Lee SY (2011) Systems metabolic engineering for chemicals and materials. Trends Biotechnol 29(8):370–378
Maeting I, Schmidt G, Sahm H, Revuelta JL, Stierhof YD, Stahmann KP (1999) Isocitrate lyase of Ashbya gossypii––transcriptional regulation and peroxisomal localization. FEBS Lett 444:15–21
Mateos L, Jimenez A, Revuelta JL, Santos MA (2006) Purine biosynthesis, riboflavin production, and trophic-phase span are controlled by a Myb-related transcription factor in the fungus Ashbya gossypii. Appl Environ Microbiol 72(7):5052–5060
Meyer A, Pellaux R, Potot S, Becker K, Hohmann HP, Panke S, Held M (2015) Optimization of a whole-cell biocatalyst by employing genetically encoded product sensors inside nanolitre reactors. Nat Chem 7(8):673–678
Monschau N, Sahm H, Stahmann KP (1998) Threonine aldolase overexpression plus threonine supplementation enhanced riboflavin production in Ashbya gossypii. Appl Environ Microbiol 64(11):4283–4290
Park EY, Ito Y, Nariyama M, Sugimoto T, Lies D, Kato T (2011) The improvement of riboflavin production in Ashbya gossypii via disparity mutagenesis and DNA microarray analysis. Appl Microbiol Biotechnol 91(5):1315–1326
Perkins JB, Sloma A, Hermann T, Theriault K, Zachgo E, Erdenberger T, Hannett N, Chatterjee NP, Williams V, Rufo GA, Hatch R, Pero J (1999) Genetic engineering of Bacillus subtilis for the commercial production of riboflavin. J Ind Microbiol Biotechnol 22(1):8–18
Perlman D (1979) Microbial process for riboflavin production. In: Pepplev HJ, Pevlman D (eds) Microbial technology, vol I. Academic Press, New York, pp 52–527
Revuelta JL, Buitrago MJ, Santos MA (I995) Riboflavin synthesis in fungi. Patent US 5,821,090
Revuelta JL, Santos MA, Pompejus M, Seulberger H (I999) Promoter from Ashbya gossypii. Patent WO1999033993 A1
Ruhl M, Zamboni N, Sauer U (2010) Dynamic flux responses in riboflavin overproducing Bacillus subtilis to increasing glucose limitation in fed-batch culture. Biotechnol Bioeng 105(4):795–804
Santos MA, Jimenez A, Revuelta JL (2000) Molecular characterization of FMN1, the structural gene for the monofunctional flavokinase of Saccharomyces cerevisiae. J Biol Chem 275:28618–28624
Santos MA, Mateos L, Stahmann KP, Revuelta JL (2005) Insertional mutagenesis in the vitamin B2 producer fungus Ashbya gossypii. In: Barredo JL (ed) Methods in biotechnology, vol 18., Microbial processes and productsHumana Press Inc, Totowa, pp 283–290
Sasajima K, Yoneda M (1971) Carbohydrate metabolism-mutants of a Bacillus species, Part II: d-ribose accumulation by pentose phosphate pathway mutants. Agric Biol Chem 35:509–517
Schmidt G, Stahmann KP, Sahm H (1996) Isolation and characterization of isocitrate lyase from the riboflavin-producing fungus Ashbya gossypii. Microbiology 142:411–417
Schlüpen C, Santos MA, Weber U, de Graaf A, Revuelta JL, Stahmann KP (2003) Disruption of the SHM2 gene, encoding one of two serine hydroxymethyltransferase isoenzymes, reduces the flux from glycine to serine in Ashbya gossypii. Biochem J 369:263–273
Schwechheimer SK, Park EY, Revuelta JL, Becker J, Wittmann C (2016) Biotechnology of riboflavin. Appl Microbiol Biotechnol 100(5):2107–2119
Shi SB, Chen T, Zhang ZG, Chen X, Zhao XM (2009) Transcriptome analysis guided metabolic engineering of Bacillus subtilis for riboflavin production. Metab Eng 11(4–5):243–252
Shi SB, Shen Z, Chen X, Chen T, Zhao XM (2009) Increased production of riboflavin by metabolic engineering of the purine pathway in Bacillus subtilis. Biochem Eng J 46(1):28–33
Shi T, Wang YC, Wang ZW, Wang GL, Liu DY, Fu J, Chen T, Zhao XM (2014) Deregulation of purine pathway in Bacillus subtilis and its use in riboflavin biosynthesis. Microb Cell Fact 13:101
Simeonidis E, Price ND (2015) Genome-scale modeling for metabolic engineering. J Ind Microbiol Biotechnol 42(3):327–338
Stahmann KP, Revuelta JL, Seulberger H (2000) Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical ribofl̄avin production. Appl Microbiol Biotechnol 53:509–516
Sugimoto T, Kanamasa S, Kato T, Park EY (2009) Importance of malate synthase in the glyoxylate cycle of Ashbya gossypii for the efficient production of riboflavin. Appl Microbiol Biotechnol 83(3):529–539
Tischler M, Wellman JW, Ladenburg K (1945) The preparation of riboflavin; the synthesis of alloxazines and isoalloxazines. J Am Chem Soc 67:2165–2168
Tischler M, Pfisterrd K, Babson RD, Ladenburg K, Fleming AJ (1947) The reaction between o-aminoazo compounds and barbituric acid. A new synthesis of riboflavin. J Am Chem Soc 69:1487–1492
Wagner AF, Folkers K (1962) Vitamins and coenzymes. Wiley (Interscience), New York
Wickerham LJ, Flickinger MH, Johnsten RM (1946) The production of riboflavin by Ashbya gossypii. Arch Biochem 9:95–98
Zhu Y, Chen X, Chen T, Shi S, Zhao X (2006) Over-expression of glucose dehydrogenase improves cell growth and riboflavin production in Bacillus subtilis. Biotechnol Lett 28(20):1667–1672
Acknowledgments
This work was supported in part by BASF SE and grant BIO2014-23901 from the Spanish Ministerio de Economía y Competitividad. Rubén M Buey was supported by a “Ramón y Cajal” contract from the Spanish Ministerio de Economía y Competitividad. P. L.-M. was recipient of an FPI fellowship from the Spanish Ministerio de Economía y Competitividad. R. L.-A. was recipient of FPI predoctoral fellowship from the Spanish Ministerio de Educación, Cultura y Deporte. D. F.-D. was recipient of a predoctoral fellowship from the Universidad de Salamanca, Spain. We thank M. D. Sánchez and S. Domínguez for excellent technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Revuelta, J.L., Ledesma-Amaro, R., Lozano-Martinez, P. et al. Bioproduction of riboflavin: a bright yellow history. J Ind Microbiol Biotechnol 44, 659–665 (2017). https://doi.org/10.1007/s10295-016-1842-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1842-7