Abstract
The bioremediation of tetrachloroethene (perchloroethene; PCE) contaminated sites generally requires a supply of some fermentable organic substrates as an electron donor. On the other hand, organic substrates can induce the massive growth of microorganisms around the injection wells, which can foul the contaminated subsurface environment. In this study, PCE dechlorination to ethene was performed in a microbial electrochemical system (MES) using the electrode (a cathode polarized at −500 mV vs. standard hydrogen electrode) as the electron donor. Denaturing gel gradient electrophoresis and pyrosequencing revealed a variety of non-Dehalococcoides bacteria dominant in MES, such as Acinetobacter sp. (25.7 % for AS1 in suspension of M3), Rhodopseudomonas sp. (10.5 % for AE1 and 10.1 % for AE2 in anodic biofilm of M3), Pseudomonas aeruginosa (22.4 % for BS1 in suspension of M4), and Enterobacter sp. (21.7 % for BE1 in anodic biofilm of M4) which are capable of electron transfer, hydrogen production and dechlorination. The Dehalococcoides group, however, was not detected in this system. Therefore, these results suggest that a range of bacterial species outside the Dehalococcoides can play an important role in the microbial electrochemical dechlorination process, which may lead to innovative bioremediation technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tetrachloroethene (perchloroethene, PCE) has been detected widely in contaminated soil and groundwater due to the improper handling and disposal of solvents and degreasing agents in industrial processes [16, 19]. Several studies have been conducted to effectively remove PCE from contaminated soil and groundwater because of its toxic, carcinogenic and mutagenic nature [20]. A range of microorganisms and microbial consortia have been reported to be able to reductively dechlorinate PCE successively to trichloroethene (TCE), cis-dichloroethene (cis-DCE), vinyl chloride (VC), and finally, to ethene (ETH), a non-toxic product. Dechlorinating microbes can use a range of electron donors such as the H2 molecule and H2-releasing fermentable organic substrates [10, 15, 18].
The in situ bioremediation of PCE-contaminated sites by utilizing or stimulating the PCE-dechlorinating microbial communities generally requires a supply of some fermentable organic substrates as suitable electron donor sources [2]. On the other hand, organic electron donors can induce the massive growth of non-dechlorinating microorganisms around the injection wells, which can foul the contaminated subsurface environment [3, 19].
The microbial electrochemical system (MES) has been recognized as a novel technology to remove PCE, because it can provide electrons from solid electrodes directly to dechlorinating bacteria without stimulating the growth of other unwanted bacteria [1]. A dissimilative Fe(III)-reducing bacterium, Geobacter lovleyi, is bacterial strain capable of dechlorinating PCE to cis-DCE with a graphite electrode as the sole electron donor for reductive dechlorination [29]. The microbial electrochemical dechlorination of TCE to ETH by an enrichment culture using a graphite electrode as the electron donor has been observed in MES using methyl viologen (MV) as the electron mediator. In the microbial community, the presence of the well-known dechlorinating bacteria, Dehalococcoides spp., was confirmed by fluorescence in situ hybridization (FISH) analysis using a species-targeted probe [1]. More recently, the microbial electrochemical complete dechlorination of PCE to ETH was observed in MES (anode compartment) using acetate as the electron donor and PCE as the electron acceptor. Microbial community analysis results showed that the pronounced electrochemically active microorganisms, such as Geobacteraceae, Desulfuromonas, Desulfitobacteriacea, and Dehalococcoides groups, and the indigenous non-Dehalococcoides community (Spirochaetes, Firmicutes, Bacteroidetes and Protebacteria) contributed to electron transfer for PCE reduction in the anode chamber, which could prove to be a cost-effective bioremediation technology [22].
Although the microbial community in MES has been well studied, the relationship between the bacteria and how they contribute to the dechlorination of PCE is not completely understood. This study examined the PCE dechlorination in MES using an electrode, MV as the electron donor, and PCE as the electron acceptor, and analyzed the microbial community structure in MES.
Materials and methods
Enrichment of PCE-dechlorinating bacterial cultures
To establish an efficient enrichment culture for PCE dechlorination, ten environmental samples consisting of two PCE-contaminated groundwater samples, two soil samples, three sewage sludge samples, and three sediment samples (SE) were tested. Each sample (1 g or 1 mL) was added to a 100-mL serum bottle containing 49-mL of an anaerobic basal medium [1, 4, 35]. Pyruvate (4 g/L) and PCE (75 μmol/L) were provided as the electron donor and electron acceptor, respectively. The pH of the medium was adjusted to 7.5. Resazurin (100 μl/L) was used as an anaerobic indicator. The bottles were then incubated for 30 days at 25 ± 1 °C, and the PCE concentration was measured every 3 days. Among the samples tested, the bacterial culture enriched from SE showed the highest PCE-dechlorinating activity, and was chosen for the following experiments. After complete dechlorination of the PCE added to the medium, the culture (1 mL) was sub-cultured into a new 100-mL serum bottle containing fresh medium (49 mL). This sub-cultivation was repeated until the culture showed stable PCE dechlorination activity. After 6 months of enrichment-cultivation, the sediment enrichment culture could finally dechlorinate 75 μmol/L of PCE within 2 days, and was then used for the microbial electrochemical PCE dechlorination experiments. The obtained enrichment culture was maintained in a 2-L fill-and-draw bioreactor using the method reported by Aulenta et al. [1]. PCE (75 μmol/L) was supplied to the reactor every week. The anaerobic conditions in the reactor were achieved by flushing with N2 gas.
Microbial electrochemical reactor operation
The microbial electrochemical reactors used consisted of two identical gas-tight glass bottles (380 mL) separated by a cation exchange membrane (Nafion 117; DuPont Co.; Wilmington, DE, USA). Graphite felt (GF-S6-06, Electrolytica Inc. NY, USA) was used as the anode and cathode electrode (30 × 50 × 5 mm) in each chamber, and the distances between the two electrodes was approximately 45 mm. The enrichment culture (SE-2, 110 mL) was added to the cathode chamber as a microbial inoculum.
The two MESs with the enrichment culture were operated according to MV (10 μmol/L) (M3 and M4). The MES cathodes were polarized at −500 mV versus the standard hydrogen electrode (SHE) using a direct current power supply (IT6322; ITECH Electronic Co. Ltd.; Korea). One MES without MV and inoculum (M2), and one reactor with a carbon source and without a potential were run as controls (M1) (Table 1). All dechlorinating experiments were carried out at 25 ± 1 °C for 6 days. The concentrations of PCE, TCE, cis-DCE, VC, and ETH were monitored daily in duplicate. These dechlorinating experiments were repeated in 3-batch cycles with minimal medium (pH 7.5) at a PCE concentration of 75 μmol/L.
Analytical techniques
PCE and its dechlorinated products, i.e., TCE, cis-DCE, VC, and ETH, were measured in 100-μL gaseous samples using a gas chromatograph (GC M600D; Young-Lin Instrument Co. Ltd.; Korea) equipped with a flame ionization detector. The concentrations were calculated using tabulated Henry’s law constants [14]. H2 was analyzed in a 500-µL gaseous sample using a Trace Analytical TA3000R reduction gas detector (Menlo Park, CA, USA).
The cyclic voltammograms were measured at 20 mV/s using a potentiostat (KST-P1; Kosentech Co.; Korea) to compare the electron transfer efficiency of each MES. After each batch experiment, the cathode chamber was then flushed with N2 gas to remove the accumulated volatile compounds and maintain anaerobic conditions.
Microbial community analyses
To characterize the microbial community responsible for catalyzing the reductive dechlorination in the MES cathode chambers, the microbial samples were collected in duplicate from the suspension after each batch cycle (first batch: 1st, second batch: 2nd, and third batch: 3rd) and from the biofilms formed on the electrode at the 3rd cycle. The microbial community DNA was extracted using a PowerSoil DNA isolation kit (Mo Bio Lab.; Carlsbad, CA, USA) according to the manufacturer’s instructions. The DNA products were processed for genomic analysis. The overall changes in the microbial community structure were investigated by polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE), whereas pyrosequencing was used for a more detailed characterization of the changes in the bacterial species. Principal component analysis (PCA) was performed based on the band position and intensity in the DGGE profile using Fingerprinting II Informatix software version 3.0 (Bio-Rad; Hercules, CA, USA) and SPSS software version 14.0 (SPSS Inc.; Chicago, IL, USA). Detailed procedures are described in supplementary information.
Results and discussion
Microbial electrochemical PCE dechlorination
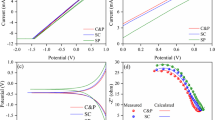
M4 using the electrode and MV as the electron donor showed the best PCE dechlorination activity. The dechlorination activity of M3 using only the electrode as an electron donor was slightly lower than that of M4. M1 using a carbon source as the electron donor showed the lowest PCE dechlorination (Fig. 1). In the case of M1 with pyruvate as the electron donor, at 6 days, all PCE was reduced to TCE and cis-DCE, and small amounts of VC and ETH were observed (Fig. 1a). On the other hand, PCE dechlorination did not occur in M2 (Fig. 1b). In contrast, M3 and M4 with the electrode as the electron donor showed rapid PCE dechlorination to ETH (Fig. 1c, d), and 91 % of PCE was reduced to TCE (14 % of PCE) and cis-DCE (77 % of PCE) after 1 day, and then further dechlorinated to VC (64 % of PCE for M3 and 80 % of PCE for M4) and ETH (15 % of PCE for M3 and 18 % of PCE for M4) after 7 days. This similar dechlorination pattern in both M3 and M4 indicates that the presence of MV was not a decisive factor in the dechlorination of PCE. It seems that certain bacteria in SE may have achieved PCE dechlorination by the electrode directly as the electron donor or using a self-excreted electron mediator. H2 may have been produced via the microbially mediated reduction of protons and consumed immediately by the PCE-dechlorinating bacteria as the electron donor, because a small amount of liquid phase H2 was observed. These results correspond well to previous report that TCE can be reduced to non-chlorinated products by TCE dechlorinating bacteria in the presence of MV with the cathode polarized at −500 mV vs. SHE [2]. It is also consistent with that dechlorinating bacteria can reduce TCE to ETH via cis-DCE at the polarized cathode without MV using H2 or electrode as the electron donor [1].
The microbial electrochemical dechlorination without MV will be more favorable for practical applications. A recent study also reported that PCE can be dechlorinated to ETH without any electron mediator in the MES anode chamber [22]. On the other hand, the mechanism and the microbial community involved in the PCE dechlorination would have been different from those in the present study, because the MES used acetate as the electron donor and PCE as the electron acceptor. The acetate-utilizing exoelectrogens may not have been the PCE-dechlorinating bacteria, and acetate may have enhanced the growth of unwanted bacteria such as acetoclastic methanogens.
Cyclic voltammetry analysis of the biocathodes
Cyclic voltammetry (CV) revealed apparently different patterns of anodic and cathodic peaks according to MV (Fig. 2). When MV was not introduced in M3 (Fig. 2a), the record of the two anodic peaks (around −350 and +350 mV) and one cathodic peak (around −550 mV) revealed the presence of unknown redox-active molecules (Fig. 2a). These molecules may have been excreted by some bacteria on the biocathode in response to continuous polarization at −500 mV vs. SHE, and become involved in the electron transfer process for microbial electrochemical PCE. The M4 with MV showed one anodic peak (around −400 mV) and one cathodic peak (around −520 mV), which is indicative of a pair of oxidized/reduced MV (Fig. 2b). This suggests that the major role of MV is to support electron transfer during dechlorination.
Consistent with the CV results, a previous study reported the presence of a self-produced redox mediator that was involved in extracellular electron transfer from the electrode to the dechlorinating bacteria [1]. Furthermore, c-type cytochromes, which are extracellular proteins, exhibit a wide range of potentials in both direct the anodic electron transfer mechanisms and in the cathodic electron transfer of the MES [26].
Community structure variations by DGGE
Over three consecutive PCE dechlorination batch cycles in the MES (M1, M3 and M4), the microbial community structure of M1 was different from M3 and M4, and the microbial community structures of the suspension in M3 and M4 were changed over the batch cycles (Fig. 3a). Interestingly, MV led to clear differences between the bacterial communities. In addition, the communities in the suspension and the biofilm on the cathode were also different from each other in both MESs. PCA showed the microbial communities of M1, M3 and M4 were clearly diverged (Fig. 3b). The values for the microbial community attached to the electrode of MES without MV were very close to those of the microbial communities suspended in the medium. On the other hand, the values derived from the microbial community attached to the electrode of MES with MV were different from those of the microbial communities suspended in the medium.
Denaturing gel gradient electrophoresis (DGGE) profiles (a) of the 16S rRNA gene fragments amplified from microbial communities catalyzing perchloroethene dechlorination in M1, M3 and M4 and principal components analysis (b) based on the DGGE profiles; M: marker; suspension: sampled at the end of the 1st, 2nd, and 3rd batches, respectively; and biofilm: sampled at the end of the 3rd batch
As shown in Table S1 (supplementary information), non-Dehalococcoides, such as Gammaproteobacteria, Bacteroidetes, Deltaproteobacteria and Firmicutes were detected in M3 and M4. In M3 (Fig. 3a), the high intensity bands in the 1st batch suspension decreased throughout successive batch cultivations and only limited members remained in the 3rd batch suspension. The remaining bacteria in the suspension such as bands #1, #3, and #8 might play important roles in PCE dechlorination through the use of self-producing electron mediators, or H2 produced by other bacteria near the electrode. Acinetobacter sp. (band #1) [23, 27, 34], uncultured bacteria (bands #3 and #8) [12, 13], Clostridium species (bands #4 and #7) [8], and Thiobacillus sp. (band #5) [34] related to dechlorination and electron transfer or hydrogen production were detected. These bacterial species prefer to inhabit areas near the electrode to have the advantage of taking an electron to dechlorinate PCE or produce H2, which might be utilized as an electron donor by the dechlorinating bacteria.
The DGGE band profile of the microbial community in the M4 appeared to be relatively simple (Fig. 3a). Most of the high intensity bands (such as bands #10 to #14) in the suspended growth bacterial community were maintained throughout consecutive batch cycles. Some (such as bands #11 to #13) were also observed in the biofilm community, but the band intensities were weakened considerably. Instead of these bands, other high intensity bands such as bands #9 and #15 appeared in the biofilm community. Uncultured bacteria (bands #10 and #14) [5, 6], Citrobacter sp. (band #12) [30, 33], Pantoea agglomerans (band #13) [15, 28], Pseudomonas sp. (band #9) [24], and Clostridium bifermentans (band #15) [8] related to electron transfer and dechlorination were found. This suggests that the microbial species capable of utilizing MV as an electron mediator were selected and kept in the suspension and biofilm. These bacterial species might play a significant role in microbial electrochemical dechlorination with MV.
Overall community structure analyzed by pyrosequencing
Pyrosequencing analysis also revealed the apparent divergence of the bacterial communities catalyzing the microbial electrochemical PCE dechlorination in the suspension and biofilm of the MESs according to the MV. Microbial diversity indices, such as the Chao index, Shannon index, and Evenness, which were calculated from the pyrosequencing data, quantitatively showed that the microbial communities were affected by inhabitation (the suspension and biofilm), and MV (Table 2).
Phylum-level analysis of the pyrosequencing result showed apparent differences between the microbial community structures in the inoculum culture and both MESs (Fig. 4). While M1 was dominated by Gammaproteobacteria (75 % of the total OTUs) and Firmicutes (13 %), the portions of Gammaproteobacteria decreased in both MESs and the other phylotypes belonging to Alpha-, Beta- and Deltaproteobacteria increased.
In M3, Gammaproteobacteria was still the most dominant bacteria (48 %) in the suspension, whereas the portion was greatly decreased (3 %) in the biofilm community and the distributions of Alphaproteobacteria (24 %), Betaproteobacteria (20 %), and Firmicutes (20 %) were increased. In contrast to M3, Gammaproteobacteria was dominant in both the suspension (52 %) and biofilm (68 %) of M4. There was a significant difference in the distribution of Firmicutes in the suspension (40 %) and in the biofilm (2 %). The distribution of Deltaproteobacteria was 1–5 % of the total OTUs in all investigated conditions.
This contrasts with a previous study, which reported that Deltaproteobacteria accounted for 69.5 % of the total bacteria in a MES designed for TCE degradation [1]. Interestingly, less than 1 % of all microbial communities was Chloroflexi including Dehalococcoides genus. Thus far, the results of phylum-targeted FISH analysis reported previously showed that non-Dehalococcoides bacterial populations affiliated with Betaproteobacteria (8.7 % of total bacteria), Deltaproteobacteria (69.5 %), and Gammaproteobacteria (3.5 %) might contribute to TCE dechlorination to ETH [1]. The indigenous non-Dehalococcoides such as Spirochaete, Firmicutes, Bacteroidetes, Gammaproteobacteria, and Deltaproteobacteria helped reduce PCE in MES [22]. Therefore, non-Dehalococcoides might play an important role in PCE dechlorination in the present system.
Diversity of dominant phylotypes
The five most abundant phylotypes of each microbial community were selected and compared (Table S2 in supplementary information). In M1, all four top phylotypes (EC1 to EC4) were affiliated with Enterobacter spp. (43 % of total OTUs). Some Enterobacter species were reported as facultative bacteria dechlorinating PCE to cis-DCE, [15] and played important roles in the primary step for the reductive dechlorination of chlorinated organic insecticides [27].
In M3, the suspension in M3 showed different major phylotypes from the biofilm of M3. The bacteria capable of dechlorination, hydrogen production and electron transfer such as Acinetobacter sp. (AS1) [23, 34], Rhodopseudomonas palustris (AE1 and AE2) [7, 10, 11, 32], were dominant. Aeromonas spp. (AS2 and AS4) [9] and uncultured bacteria (AS3 and AS5) [6, 25] related to electron transfer and Azonexus sp. (AE3) [31] capable of dechlorination were observed. In M4, Pseudomonas sp. (BS1 and BE2) [24], Clostridium sp. (BS2, BS4 and BS5) [8, 17], and Enterobacter spp. (BE1 and BS3) [15] related to dechlorination, hydrogen and electron transfer were dominant. Pseudomonas aeruginosa, is a well-known exoelectrogenic bacteria that utilizes redox mediators, in the suspension and in biofilms. Their ability to use MV as an electron mediator might have the advantage of being selected almost exclusively, and kept in the suspension and biofilms. In particular, Enterobacter spp., detected in M1 was still observed in the suspension and in the biofilm of M4. Enterobacter species are the only facultative bacteria capable of dechlorinating PCE to cis-DCE.
Thus far, Dehalococcoides is the only known genus that can dechlorinate chlorinated ETHs to VC and ETH anaerobically [21]. Most of the known dechlorinators grow slowly (e.g. Dehalococcoides) and they do not tend to be the dominant populations even in an enrichment culture [21]. This study demonstrated that a variety of bacterial species of the non-Dehalococcoides genus could be involved in dechlorination and electron transfer for microbial electrochemical PCE dechlorination. As mentioned above, non-Dehalococcoides bacterial populations can play a key role in microbial electrochemical PCE dechlorination [1, 22].
Possible dechlorination pathways in MES
The presence of MV in the cathode of MES altered the microbial community structure. It indicates that the mechanism of microbial electrochemical PCE dechlorination was altered when an external redox mediator was added. Although more detailed studies will be needed to elucidate the specific role of each bacterial species in the community, it was assumed that four possible dechlorination mechanisms occurred because a variety of bacteria related to electron transfer, hydrogen production and dechlorination were detected in this system (Fig. 5). First, the dechlorinating bacteria or non-Dehalococcoides bacteria attached to the electrode may receive electrons directly from it. Second, hydrogenotrophic dechlorinating bacteria or hydrogenotrophic non-Dehalococcoides bacteria might utilize the H2 (produced through microbial electrochemical proton reduction) as an electron donor for the dechlorination of PCE. Third, dechlorinating bacteria or non-Dehalococcoides bacteria might receive electrons indirectly from the electrode via self-excreting electron mediators. Finally, when MV, an external electron shuttle, is added to the reactors, dechlorinating bacteria or non-Dehalococcoides bacteria might receive electrons from the electrode through MV.
Proposed dechlorinating mechanisms employed by bacteria using the cathode of a microbial electrochemical system as the electron donor. Direct electron transfer (far left) from the cathode to bacteria, the production of H2 for subsequent microbial dechlorination (middle left), indirect electron transfer through a self-producing mediator (middle right), and indirect electron transfer through methyl viologen as an artificial mediator from the electrode to dechlorinating bacteria (far right)
Conclusions
The dechlorination of PCE to ETH was performed successfully in MESs. An analysis of the microbial community structures showed that various bacterial species outside of the Dehalococcoides genus have the potential to play an important role in microbial electrochemical PCE dechlorination. There still remains the possibility that non-Dehalococcoides bacteria capable of using an electrode as an electron donor might microbial electrochemical dechlorinate PCE to ethane. These results are expected to provide new insights into research for the microbial electrochemical reductive PCE dechlorination by non-Dehalococcoides group. This microbial electrochemical process can be a cost-effective and sustainable bioremediation technology. On the other hand, further study will be needed to determine if Dehalococcoides are present in MES, and ensure novel microbial electrochemical technology by non-Dehalococcoides.
References
Aulenta F, Canosa A, Reale P, Rossetti S, Panero S, Majone M (2009) Microbial reductive dechlorination of trichloroethene to ethene with electrodes serving as electron donors without the external addition of redox mediators. Biotechnol Bioeng 103:85–91. doi:10.1002/bit.22234
Aulenta F, Catervi A, Majone M, Panero S, Reale P, Rossetti S (2007) Electron transfer from a solid-state electrode assisted by methyl viologen sustains efficient microbial reductive dechlorination of TCE. Environ Sci Technol 41:2554–2559. doi:10.1021/es0624321
Aulenta F, Pera A, Rossetti S, Papini MP, Majone M (2007) Relevance of side reactions in anaerobic reductive dechlorination microcosms amended with different electron donors. Water Res 41:27–38. doi:10.1016/j.watres.2006.09.019
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Baptista II, Peeva LG, Zhou NY, Leak DJ, Mantalaris A, Livingston AG (2006) Stability and performance of Xanthobacter autotrophicus GJ10 during 1,2-dichloroethane biodegradation. Appl Environ Microbiol 72:4411–4418. doi:10.1128/AEM.02576-05
Borole AP, Hamilton CY, Vishnivetskaya TA, Leak D, Andras C, Morrell-Falvey J, Keller M, Davison B (2009) Integrating engineering design improvements with exoelectrogen enrichment process to increase power output from microbial fuel cells. J Power Sources 191:520–527. doi:10.1016/j.jpowsour.2009.02.006
Bose A, Gardel E, Vidoudez C, Parra E, Girguis P (2014) Electron uptake by iron-oxidizing phototrophic bacteria. Nat Commun. doi:10.1038/ncomms4391 (article number: 3391)
Chang YC, Hatsu M, Jung K, Yoo YS, Takamizawa K (2000) Isolation and characterization of a tetrachloroethylene dechlorinating bacterium, Clostridium bifermentans DPH-1. J Biosci Bioeng 89:489–491. doi:10.1016/S1389-1723(00)89102-1
Chung K, Okabe S (2009) Characterization of electrochemical activity of a strain ISO2-3 phylogenetically related to Aeromonas sp. isolated from a glucose-fed microbial fuel cell. Biotechnol Bioeng 104:901–910. doi:10.1002/bit.22453
DiStefano TD, Gossett JM, Zinder SH (1991) Reductive dechlorination of high concentrations of tetrachloroethene to ethene by an anaerobic enrichment culture in the absence of methanogenesis. Appl Environ Microbiol 57:2287–2292
Egland PG, Gibson J, Harwood CS (2001) Reductive, coenzyme A-mediated pathway for 3-chlorobenzoate degradation in the phototrophic bacterium Rhodopseudomonas palustris. Appl Environ Microbiol 67:1396–1399. doi:10.1128/AEM.67.3.1396-1399.2001
Flynn SJ, Löffler FE, Tiedje JM (2000) Microbial community changes associated with a shift from reductive dechlorination of PCE to reductive dechlorination of cis-DCE and VC. Environ Sci Technol 34:1056–1061. doi:10.1021//es9908164
Gu AZ, Hedlund BP, Staley JT, Strand SE, Stensel HD (2004) Analysis and comparison of the microbial community structures of two enrichment cultures capable of reductively dechlorinating TCE and cis-DCE. Environ Microbiol 6:45–54. doi:10.1046/j.1462-2920.2003.00525.x
Heimann AC, Jakobsen R (2006) Experimental evidence for a lack of thermodynamic control on hydrogen concentrations during anaerobic degradation of chlorinated ethenes. Environ Sci Technol 40:3501–3507. doi:10.1021/es052320u
Holliger C, Wohlfarth G, Diekert G (1998) Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol Rev 22:383–398. doi:10.1111/j.1574-6976.1998.tb00377.x
Huang B, Lei C, Wei C, Zeng G (2014) Chlorinated volatile organic compounds (Cl-VOCs) in environment—sources, potential human health impacts, and current remediation technologies. Environ Int 71:118–138. doi:10.1016/j.envint.2014.06.013
Ise K, Suto K, Inoue C (2011) Microbial diversity and changes in the distribution of dehalogenase genes during dechlorination with different concentrations of cis-DCE. Environ Sci Technol 45:5339–5345. doi:10.1021/es104199y
Jayaraj J, Rockne KJ, Makkar RS (2004) Reductive dechlorination of tetrachloroethene by a mixed bacterial culture growing on ethyl lactate. J Environ Sci Health Part A 39:1399–1414. doi:10.1081/ESE-120037841
Kim S, Park T, Lee W (2015) Enhanced reductive dechlorination of tetrachloroethene by nano-sized mackinawite with cyanocobalamin in a highly alkaline condition. J Environ Manage 151:378–385. doi:10.1016/j.jenvman.2015.01.004
Kyung D, Sihn Y, Kim S, Bae S, Amin MT, Alazba AA, Lee W (2016) Synergistic effect of nano-sized mackinawite with cyano-cobalamin in cement slurries for reductive dechlorination of tetrachloroethylene. J Hazard Mater 311:1–10. doi:10.1016/j.jhazmat.2016.02.074
Lu X, Wilson JT, Kampbell DH (2006) Relationship between Dehalococcoides DNA in ground water and rates of reductive dechlorination at field scale. Water Res 40:3131–3140. doi:10.1016/j.watres.2006.05.030
Patil SS, Adetutu EM, Rochow J, Mitchell JG, Ball AS (2014) Sustainable remediation: electrochemically assisted microbial dechlorination of tetrachloroethene-contaminated groundwater. Microb Biotechnol 7:54–63. doi:10.1111/1751-7915.12089
Prakash D, Kumar R, Jain RK, Tiwary BN (2011) Novel pathway for the degradation of 2-chloro-4-nitrobenzoic acid by Acinetobacter sp. strain RKJ12. Appl Environ Microbiol 77:6606–6613. doi:10.1128/AEM.00685-11
Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70:5373–5382. doi:10.1128/AEM.70.9.5373-5382.2004
Richardson RE, Bhupathiraju VK, Song DL, Goulet TA, Alvarez-Cohen L (2002) Phylogenetic characterization of microbial communities that reductively dechlorinate TCE based upon a combination of molecular techniques. Environ Sci Technol 36:2652–2662. doi:10.1021/es0157797
Rosenbaum M, Aulenta F, Villano M, Angenent LT (2011) Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour Technol 102:324–333. doi:10.1016/j.biortech.2010.07.008
Satsuma K, Masuda M (2012) Reductive dechlorination of methoxychlor by bacterial species of environmental origin: evidence for primary biodegradation of methoxychlor in submerged environments. J Agric Food Chem 60:2018–2023. doi:10.1021/jf2048614
Shan H, Kurtz HD Jr, Mykytczuk N, Trevors JT, Freedman DL (2010) Anaerobic biotransformation of high concentrations of chloroform by an enrichment culture and two bacterial isolates. Appl Environ Microbiol 76:6463–6469. doi:10.1128/AEM.01191-10
Strycharz SM, Woodard TL, Johnson JP, Nevin KP, Sanford RA, Loffler FE, Lovley DR (2008) Graphite electrode as a sole electron donor for reductive dechlorination of tetrachlorethene by Geobacter lovleyi. Appl Environ Microbiol 74:5943–5947. doi:10.1128/AEM.00961-08
Thompson LJ, Gray VM, Kalala B, Lindsay D, Reynolds K, von Holy A (2008) Biohydrogen production by Enterobacter cloacae and Citrobacter freundii in carrier induced granules. Biotechnol Lett 30:271–274. doi:10.1007/s10529-007-9527-y
Thrash JC, Pollock J, Torok T, Coates JD (2010) Description of the novel perchlorate-reducing bacteria Dechlorobacter hydrogenophilus gen. nov., sp. nov. and Propionivibrio militaris, sp. nov. Appl Microbiol Biotechnol 86:335–343. doi:10.1007/s00253-009-2336-6
Thygesen A, Marzorati M, Boon N, Thomsen AB, Verstraete W (2011) Upgrading of straw hydrolysate for production of hydrogen and phenols in a microbial electrolysis cell (MEC). Appl Microbiol Biotechnol 89:855–865. doi:10.1007/s00253-010-3068-3
Xu S, Liu H (2011) New exoelectrogen Citrobacter sp. SX-1 isolated from a microbial fuel cell. J Appl Microbiol 111:1108–1115. doi:10.1111/j.1365-2672.2011.05129.x
Yu J, Park Y, Kim B, Lee T (2015) Power densities and microbial communities of brewery wastewater-fed microbial fuel cells according to the initial substrates. Bioprocess Biosyst Eng 38:85–92. doi:10.1007/s00449-014-1246-x
Zeikus JG (1977) The biology of methanogenic bacteria. Bacteriol Rev 41:514–541
Acknowledgments
This study was financially supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1C1A1A01054204 and NRF-2015R1A2A1A15054528).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, J., Park, Y., Nguyen, V.K. et al. PCE dechlorination by non-Dehalococcoides in a microbial electrochemical system. J Ind Microbiol Biotechnol 43, 1095–1103 (2016). https://doi.org/10.1007/s10295-016-1791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1791-1