Abstract
Mycobacterium neoaurum ST-095 and its mutant M. neoaurum JC-12, capable of transforming phytosterol to androst-1,4-diene-3,17-dione (ADD) and androst-4-ene-3,17-dione (AD), produce very different molar ratios of ADD/AD. The distinct differences were related to the enzyme activity of 3-ketosteroid-Δ1-dehydrogenase (KSDD), which catalyzes the C1,2 dehydrogenation of AD to ADD specifically. In this study, by analyzing the primary structure of KSDDI (from M. neoaurum ST-095) and KSDDII (from M. neoaurum JC-12), we found the only difference between KSDDI and KSDDII was the mutation of Val366 to Ser366. This mutation directly affected KSDD enzyme activity, and this result was confirmed by heterologous expression of these two enzymes in Bacillus subtilis. Assay of the purified recombinant enzymes showed that KSDDII has a higher C1,2 dehydrogenation activity than KSDDI. The functional difference between KSDDI and KSDDII in phytosterol biotransformation was revealed by gene disruption and complementation. Phytosterol transformation results demonstrated that ksdd I and ksdd II gene disrupted strains showed similar ADD/AD molar ratios, while the ADD/AD molar ratios of the ksdd I and ksdd II complemented strains were restored to their original levels. These results proved that the different ADD/AD molar ratios of these two M. neoaurum strains were due to the differences in KSDD. Finally, KSDD structure analysis revealed that the Val366Ser mutation could possibly play an important role in stabilizing the active center and enhancing the interaction of AD and KSDD. This study provides a reliable theoretical basis for understanding the structure and catalytic mechanism of the Mycobacteria KSDD enzyme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
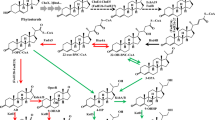
As the second largest category drugs after antibiotics, steroid drugs play an important role in treating and preventing various diseases. Since 1952 when Murray and Peterson [17] discovered the process of 11α-hydroxylation of progesterone by Rhizopus nigricans, the production of steroid drugs by microbial technology has attracted the attentions of many microbiologists. When compared with the traditional chemical synthesis process, microbial transformation of steroid compounds has the advantages of yielding products of high purity at low cost and under environmentally friendly conditions [5]. Over the past half century, numerous microbial transformations of steroid compounds, including steroid hydroxylation, Δ1-dehydrogenation and sterol side chain cleavage, have been reported [4]. Among these studies, microbial sterol side chain cleavage has received much attention in the pharmaceutical industry, and has become an important pathway to produce many steroid intermediates [11]. Since the discovery that microorganisms could degrade the side chain of sterols to produce 17-ketosterols [12], mainly androst-4-ene-3,17-dione (AD) and androst-1,4-diene-3,17-dione (ADD), phytosterol has become a major raw material in pharmaceutical industry for its low cost, abundant availability, and the efficiency of its transformation into steroid intermediates [13]. Among the products of microbial side chain cleavage obtained from phytosterol, AD and ADD are important and valuable steroid drug intermediates, widely used for the commercial production of mineralocorticoids, corticosteroids, oral contraceptives, and other pharmaceutical steroids [33].
Many microbial strains have been described as biocatalysts of sterol bioconversions and different approaches for steroid microbial conversion have been applied [4]. For example, it has been reported that the bioconversion of cholesterol to ADD could be enhanced by the addition of lecithin, with a final ADD yield of 59 % (w/w) [27]. Wang et al. reported that resting cell biotransformation of phytosterol to ADD in a cloud point system could improve ADD production with yields up to 12 g/L [26]. Shen et al. showed that use of hydroxypropyl-β-cyclodextrin (HP-β-CD) as a cosolvent could increase AD and ADD yield with phytosterol as the substrate [23]. In our recent study of phytosterol biotransformation by M. neoaurum, we proposed a three-stage fermentation strategy for enhancing ADD production with methyl-β-cyclodextrin (Me-β-CD) used as cosolvent [21].
In the process of phytosterol biotransformation, 3-ketosteroid-Δ1-dehydrogenase (KSDD) is the key enzyme that catalyzes the conversion of AD to ADD [33]. During microbial steroid transformations, both AD and ADD are formed. As the only structural difference between AD and ADD is the C1,2 double bond [33], so the structures of AD and ADD are highly similar. This high structural similarity greatly increases the cost of the downstream processes for the separation and purification of AD from ADD [28]. Therefore, it is crucial to obtain strains capable of efficient transformation of phytosterol into AD and ADD with as high ADD/AD or AD/ADD ratios as possible.
Wild-type M. neoaurum ST-095 (formerly Mycobacterium sp.), typically produces an ADD/AD molar ratio of 1:2 when used for phytosterol biotransformation [34]. In our previous study, mutant strain M. neoaurum ZADF-4 was isolated [9], which gave AD/ADD molar ratios of 8:1 when used for steroid biotransformations, but activity was reduced [9]. By analyzing the DNA sequence of the mutant ksdd gene, a nine nucleotide deletion and two point mutations were revealed, which resulted in amino acid changes [9]. To develop steroid biotransformation systems capable of producing high AD/ADD or ADD/AD ratios and while retaining high levels of biotransformation activity, it is necessary to understand the behavior of the key enzyme KSDD [29]. As a flavin adenine dinucleotide (FAD)-dependent enzyme, KSDD not only catalyzes the conversion of AD to ADD, but also plays an important role in microbial sterol catabolism as well as other steroid drug transformations [15, 24]. However, the catalysis mechanism of Mycobacterium KSDD enzymes is not well resolved, and structural information on KSDD is still limited. Previous studies focused on the investigation of the molecular, catalytic and spectral characteristics of KSDD from different strains, and based on chemical modification, mutagenesis, and kinetics experiments, several key amino acid residues were identified [6, 14]. In a recent study, Rohman et al. revealed the first structure and catalytic mechanism of the KSDD enzyme from Rhodococcus erythropolis SQ1 [19]. They confirmed that the enzyme contains two domains: an FAD-binding domain and a catalytic domain, and they also found that the active site contains four key residues: Tyr119, Tyr318, Tyr487, and Gly491. Xie et al. further reported that Ser138 was a key amino acid residue of KSDD and changes at that position led to large differences in the ADD/AD ratio [29].
By UV-NTG mutation, M. neoaurum JC-12 (formerly Mycobacterium sp.-11), a strain capable of producing an ADD/AD molar ratio of 10:1, was derived from strain M. neoaurum ST-095 [34]. Furthermore, total amounts of ADD and AD produced by these two strains were the same. Having shown that KSDD enzyme activity directly affects ADD/AD molar ratios, we investigated the KSDDs from M. neoaurum ST-095 (named KSDDI) and M. neoaurum JC-12 (named KSDDII) to attempt to explain the different characteristics of these two strains. After searching the Protein Data Bank (PDB) database for structural similarity, we found that KSDDI and KSDDII have the highest similarity to KSDD from R. erythropolis SQ1 [29].
In this study, by sequence alignment, we found that the only difference between KSDDI and KSDDII was the mutation of Val366 to Ser366. The enzymatic characteristics of KSDDI and KSDDII were studied by heterologous expression in Bacillus subtilis 168, and the results revealed that the mutation of Val366 to Ser366 enhanced KSDD enzyme activity. We further investigated the function of KSDDI and KSDDII in AD and ADD production by gene disruption and complementation, which confirmed that the different characteristics of these two M. neoaurum strains with respect to ADD/AD molar ratios attained were caused by this mutation. The mechanism responsible for the different characteristics of KSDDI and KSDDII was further unraveled at the molecular level by modeling the complex structure of KSDD-AD. The results suggested that the change of Val366 to Ser366 in KSDD could facilitate the interaction of KSDD with AD and thereby enhance KSDD enzyme activity. This study not only serves as a basis for further studies on the structural analysis and catalytic mechanism of this dehydrogenase, but also provides a reliable theoretical basis for site-directed mutagenesis to further improve ADD/AD molar ratios in future research.
Materials and methods
Strains, plasmids and primers
The strains, plasmids and primers used in this work are listed in Table 1.
Reagents, media and cultivation
The AD and ADD standards were purchased from Sigma-Aldrich (Shanghai, China). Substrate phytosterol (95 % purity) was obtained from Wuhan Kaidi Fine Chemical Industrial Co., Ltd. (Wuhan, China). Tryptone and yeast extract were purchased from Oxoid Ltd. (Basingstoke Hampshire, England). Methyl-β-cyclodextrin (Me-β-CD) was obtained from Zhiyuan Biotechnology Co., Ltd (Shandong, China). Methanol and ethyl acetate obtained from Jiangsu Hanbon Science & Technology CO., Ltd. (Jiangsu, China) were chromatographic grade. All other chemicals and reagents were analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), unless otherwise stated.
Escherichia coli and B. subtilis were cultivated in Luria Broth (LB) medium and were used as the cloning and expression hosts, respectively. The cultivation of M. neoaurum ST-095 and M. neoaurum JC-12 was performed according to our previous study [21].
Expression and purification of KSDD in B. subtilis 168
Both the ksdd I and ksdd II genes were cloned with the forward primer P1 and the reverse primer P2, from the genome DNA of M. neoaurum ST-095 and M. neoaurum JC-12, respectively. The plasmids pMA5-ksdd I and pMA5-ksdd II were constructed by inserting the ksdd I and ksdd II genes into the BamH I and Nde I sites of pMA5. The transformation of B. subtilis cells was in accordance with the procedure described by Anagnostopoulos et al. [1], and the selection of the recombinant strains BS168I and BS168II followed the process described in our previous study [20].
After cultivation in 50 mL LB medium for 24 h, recombinant cells were harvested by centrifugation (10,000 rpm, 10 min and 4 °C) and washed twice with 50 mM Tris–HCl buffer (pH 7.0). Then the harvested cells were resuspended with 5 mL Tris–HCl buffer. After ultrasonic disruption, cell debris was removed by centrifugation at 10,000 rpm and 4 °C for 30 min. The supernatant was then used for enzyme activity assays, SDS-PAGE analysis and enzyme purification. Enzyme purification was carried out using HisTrap™ HP columns according to the manufacturer’s instructions [30].
Enzyme activity assay of KSDD
The KSDD enzyme activity was determined spectrophotometrically at 30 °C←using phenazine methosulphate (PMS) and 2,6-dichlorophenolindophenol (DCPIP). Reaction mixtures (1 mL) contained 50 mM Tris–HCl buffer (pH 7.0), 1.5 mM PMS, 40 µM DCPIP, appropriate volumes of the supernatant or cell extract, and 200 mM AD in methanol (2 %) [33]. KSDD activity is expressed as U/mg of protein, and 1 U is defined as the amount of enzyme giving a reduction of 1 µmol/min DCPIP (ξ 600 = 11.3 × 103/cm/M) [28].
Kinetic parameters of KSDDI and KSDDII were performed in 50 mM Tris–HCl buffer (pH 7.0) at 30 °C by changing the concentration of the substrate (AD). Kinetic parameters (K m, V max and k cat) were determined by fitting a plot of rate versus substrate concentration to the Michaelis–Menten equation using nonlinear regression in the software GraphPad Prism 5.0 (GraphPad Software, Inc. La Jolla, CA, USA).
AD and phytosterol transformation analysis
The conversion of AD by the purified enzymes was done in 50-mL shake flasks and the transformation system was similar to the enzyme assay system. The reaction mixture (10 mL) consisted of 50 mM Tris–HCl buffer (pH 7.0), 1.5 mM PMS, 40 µM DCPIP, appropriate amounts of the purified enzymes and 10 mg AD in methanol (2 %). The conversion was carried out at 30 °C and 160 rpm for 12 h. Phytosterol transformation was carried out in 250-mL shake flasks and was performed as described in our previous study [21].
The ksdd gene disruption and complementation
Gene deletion in M. neoaurum was performed as described by Gordhan and Parish with minor modifications [7]. To delete the ksdd I and ksdd II genes, a 425 bp upstream sequence and a 350 bp downstream sequence were amplified by PCR with primers P3/P4 and P5/P6. Then the two purified fragments were mixed to be used as template to amplify a single large fragment using primers P3/P6. The amplification products (containing the upstream and downstream regions of ksdd I and ksdd II , 775 bp) were digested with KpnI and HindIII and cloned into the plasmid p2 NIL to construct recombinant plasmid p2 N-Δksdd I and p2 N-Δksdd II which also included a selectable marker gene cassette, which was removed from pGOAL19 by digestion using PacI and then inserted into the PacI site of p2 NIL. Finally, after alkali treatment and UV irradiation, the plasmids p2N-Δksdd I and p2N-Δksdd II were transformed into M. neoaurum ST-095 and M. neoaurum JC-12 by electrotransformation, respectively [7]. Prospective deletion mutants of ksdd I and ksdd II were confirmed by PCR and gene sequencing, and named Mut ksddI and Mut ksddII , respectively.
Plasmid pMV306 was employed as an integrating expression vector to prepare plasmids for complementation of the deleted mutants of ksdd I and ksdd II . To construct the complementation plasmids, we first constructed plasmids p261-ksdd I and p261-ksdd II . Using primers P1/P6, ksdd I and ksdd II genes were obtained through PCR techniques. Then ksdd I and ksdd II were inserted into the BamH I/Hind III sites of plasmid pMV261 to create plasmids p261-ksdd I and p261-ksdd II , respectively. Then expression cassettes containing ksdd I and ksdd II under the control of the heat-shock promoter hsp60 were removed from plasmids p261-ksdd I and p261-ksdd II , respectively, by double digestion with XbaI/ClaI, and subsequently inserted into the corresponding sites of pMV306 to obtain the complementation plasmids p306-ksdd I and p306-ksdd II . The plasmids p306-ksdd I and p306-ksdd II were further transferred into Mut ksddII and Mut ksddI by electrotransformation to generate the KSDD complemented strains Comp ksddI and Comp ksddII , respectively.
Analytical methods
The methods used for structural analysis of KSDDI and KSDDII were in accordance with our recent report [10]. The structural models of KSDDI and KSDDII were acquired by homology modeling using SWISS-MODEL Workspace (http://swissmodel.expasy.org/). Analysis of the three-dimensional structures of the KSDDI and KSDDII enzymes were conducted using Discovery Studio 2.5 software.
The preparation and analysis of biotransformation products were performed as follows: 1 mL of sample was withdrawn from culture broth and extracted with 4 ml of ethyl acetate. After centrifugation, 2 ml of the supernatant was analyzed on a Shimadzu HPLC instrument equipped with a C18 column (Diamonsil ®C18, 5 µm particles, 250 mm × 4.6 mm) and a UV/visible detector. ADD and AD were detected at 240 nm and the mobile phase consisted of methanol and water (70/30, v/v). The flow rate was 1 mL/min and the column temperature was 30 °C [21, 22].
Results and discussion
Sequence analysis of KSDDI and KSDDII
Using the ksdd gene sequence from M. neoaurum NwIBL-01 (Gene ID: 251736854) as the basis, we designed the primers P1/P2 (Table 1) to obtain the complete ksdd gene sequence from M. neoaurum ST-095 and M. neoaurum JC-12 by PCR techniques. Both the ksdd I and ksdd II gene fragments were complete open reading frames and contained 1701 bp nucleotides. Rohman et al. have reported that the four key amino acid residues in the active center of KSDD from R. erythropolis SQ1 are Tyr119, Tyr318, Tyr487, and Gly491, and so we determined the corresponding amino acid residues of KSDD from M. neoaurum by sequence alignment (Fig. 1). As shown in Fig. 1, by the amino acid sequence alignment analysis, we predicted the key amino acid residues in the active center of KSDD from M. neoaurum were Tyr125, Tyr365, Tyr541, and Gly545. By further analysis of the nucleotide sequences of ksdd I and ksdd II , we found the ksdd I and ksdd II genes showed 99.9 % similarity, and there were only two differences located at nucleotides 1096 and 1097 (Fig. 2a), which caused the Val366 of KSDDI to change into Ser366 of KSDDII (Fig. 2b). Mutations in the amino acid sequence of KSDD have been shown previously to lead to changes in KSDD structure, which resulted in different enzyme activities [29]. Therefore, we speculated that the residue change from Val366 to Ser366 might have enhanced KSDD enzyme activity, and thereby increased the ADD/AD molar ratio. Similar results have also been reported in studies on Mycobacterium sp. VKM Ac-1816D and Mycobacterium sp. VKM Ac-1815D, where a single point mutation apparently led to differences in accumulation of the main product [2].
Sequences alignment of KSDD from different strains. The key residues in the active center were marked with black frames and the mutation site (Ser366) was indicated by hollow inverted triangle. GenBank accession numbers for KSDD were in brackets: DNAMAN1, KSDDI; DNAMAN2, KSDDII; DNAMAN3, KSDD from M. neoaurum NwIBL-01 (ACT10280.1); DNAMAN4, KSDD from R. erythropolis SQ1 (ABW74859.1); DNAMAN5, KSDD from R. rhodochrous (BAA22789.1); DNAMAN6, KSDD from M. fortuitum (ALI25697.1); DNAMAN7, KSDD from Pimelobacter simplex (AIY19527.1); DNAMAN8, KSDD from Pseudoalteromonas haloplanktis TAC125 (CAI87189.1); DNAMAN9, KSDD from Comamonas testosteroni (BAP91417.1); DNAMAN10, KSDD from Pseudomonas resinovorans NBRC 106553 (BAN46966.1)
Enzymatic characteristics of KSDDI and KSDDII
To confirm that the differences in KSDD activity were caused by amino acid differences between KSDDI and KSDDII, the ksdd I and ksdd II genes, together with 6 His-tag coding regions appended at their 3′-termini, were expressed in B. subtilis 168. The KSDD enzyme activity of the recombinant strains was detected and the results are shown in Table 2. BS168I and BS168II showed KSDD activities of 0.65 and 1.72 U/mg, respectively. No enzyme activity was detected in BS168E. These results gave preliminary indication that the enzyme activity of KSDDII was higher than that of KSDDI.
Recombinant KSDDI and KSDDII were then purified using HisTrap™ HP columns to yield products with the expected molecular weight of about 61 kDa, as shown by SDS-PAGE analysis in Fig. 3. Enzyme activity and Michaelis–Menten kinetics of the purified KSDD were measured at 30 °C and pH 7.0 with AD as substrate. As shown in Table 3, the specific activity of KSDDII (4.03 U/mg) was much higher than that of KSDDI (1.46 U/mg) indicating that the mutation of Val366 to Ser366 greatly improved the KSDD enzyme activity. The Michaelis constant (K m value) of KSDDII was lower than that of KSDDI suggesting that the AD affinity of KSDD was increased by the Val366 to Ser366 mutation. The k cat/K m value of KSDDII (0.53 µM−1 min−1) was higher than that of KSDDI (0.07 µM−1 min−1), which further showed that the catalytic efficiency of KSDDII was improved considerably. Finally, the conversion of AD to ADD by the purified enzymes, KSDDI and KSDDII, was investigated. As shown in Fig. 4, AD transformation was catalyzed more efficiently by KSDDII than by KSDDI indicating the KSDDII enzyme activity was higher than KSDDI. Since concentrations of KSDDI and KSDDII enzymes added into the conversion system were the same, we conclude that the major factor that leads to the difference in KSDD enzyme activity is the amino acid differences in the two forms of KSDD enzyme.
Phytosterol degradation into AD and ADD by mycobacteria is complicated and the metabolic pathway remains unresolved. However, KSDD has been identified as a key enzyme in the metabolic pathway for many years [28]. Since Plesiat et al. successfully heterologous expressed the KSDD from Pseudomonas testosterone in E. coli in 1991 [18], the ksdd genes from different species, such as Arthrobacter, Rhodococcus and Mycobacterium, were heterologously expressed in different hosts including E. coli, B. subtilis, Streptomyces lividans, etc. [3, 8, 16, 33]. These previous studies of KSDD mainly focused on heterologous expression and molecular characterization. Recently, Rohman et al. provided the first crystal structure and identified the key active site residues of the KSDD enzyme from R. erythropolis [19]. Xie et al. also found that Ser138 was a critical amino acid in the KSDD enzyme from M. neoaurum [29]. Despite all these studies, the structure and catalytic mechanism of the KSDD enzyme from M. neoaurum were still unclear. In the present study, we heterologously expressed the KSDDI and KSDDII from M. neoaurum in B. subtilis 168 to reveal their enzymatic characteristics. Our results indicate that the mutation of Val366 to Ser366 appears to improve KSDD affinity toward AD and enhance KSDD enzyme activity. This study suggests possible directions for future site-directed mutagenesis studies of KSDD to improve its enzyme activity.
The function of KSDDI and KSDDII in phytosterol biotransformation
To eliminate the possible impact of other factors on the different ADD/AD molar ratios in M. neoaurum ST-095 and M. neoaurum JC-12, the ksdd I and ksdd II genes were disrupted and then complemented to clarify their function in phytosterol conversion. The cell growth curves showed that there were no differences in biomass and residual glucose between the ksdd gene disrupted strains and the parent strains (Fig. 5), which indicated that ksdd gene disruption has no observable effect on the growth of M. neoaurum under these conditions. The results of phytosterol transformation by the parent strains, ksdd gene disrupted strains, and ksdd gene complemented strains are shown in Fig. 6. As seen in Fig. 6, with the disruption of ksdd in M. neoaurum ST-095 and M. neoaurum JC-12, ADD production decreased markedly, while AD accumulation increased. The ADD/AD molar ratios of the mutant strains Mut ksddI and Mut ksddII were almost the same (1:29), but production levels were very low. Then the mutant strains Mut ksddI and Mut ksddII were complemented with ksdd II and ksdd I to construct the complemented strains Comp ksddI and Comp ksddII , respectively. As a result, the ADD/AD molar ratio of the complemented strain Comp ksddI returned to a level very similar to that of M. neoaurum JC-12, while the ADD/AD molar ratio of the complemented strain Comp ksddII was similar to that of M. neoaurum ST-095 (Fig. 6). Xie et al. [29] reported that disruption of ksdd in M. neoaurum MNR M3 led to the strain completely losing KSDD activity and that no ADD was detected. In this study, ksdd gene disruption caused ADD production to decrease dramatically. However, there was still a small amount of ADD detected during the phytosterol conversion, and this result was in accordance with that reported by Wei et al. [28]. This phenomenon may be explained by the possible presence of other enzymes that possess C1,2 dehydrogenation ability [32]. All of these results suggest that the most reasonable explanation for the difference in the ADD/AD molar ratios and KSDD enzyme activities between M. neoaurum ST-095 and M. neoaurum JC-12 is that the amino acid residue difference in 366 position considerably influences KSDD activity.
The cell growth curves of the parent strains and their ksdd gene disrupted mutants. a M. neoaurum ST-095 and Mut ksddI ; b M. neoaurum JC-12 and Mut ksddII . All assays were performed in triplicate with three independent measurements. Standard deviations of the biological replicates were represented by error bars
AD and ADD molar yields of the engineered M. neoaurum strains. The transformation was carried out for 7 days with 10 g/L phytosterol as substrate. The phytosterol was not completely converted to AD and ADD, and the AD and ADD molar yield was not up to 100 %. The balance is essentially unconverted phytosterol. All assays were performed in triplicate with three independent measurements. Standard deviations of the biological replicates were represented by error bars
Structure analysis of KSDDI and KSDDII
As the amino acid residues play important roles in enzyme structure and catalytic reactions, single amino acid mutations can significantly influence enzyme activity [29]. The structure of KSDDI and KSDDII was modeled using the crystal structure of KSDD (3-ketosteroid-Δ1-dehydrogenase from R. erythropolis SQ1, PDB entry 4c3x; 43.1 % sequence identity) as template. The resulting models were used to explain the differences in enzyme catalytic efficiency between KSDDI and KSDDII in terms of structure using the software of Discovery Studio 2.5 according to our recent report [10]. The analysis of the three-dimensional structure of the wild-type KSDDI revealed the extremely hydrophobic environment of the active center (Fig. 7a). Molecular docking of complexes KSDD-AD with the active site of KSDD obtained from M. neoaurum JC-12 (with 43.1 % sequence identity of KSDD from R. erythropolis SQ1, PDB entry 4c3x) was performed by the AUTODOCK 4.2 program suite according to the previous study [31]. The docking input files were generated using the AutoDockTools program. A grid box size of 30 × 30 × 30 pointing in x, y and z directions was built. A grid spacing of 0.375 Å was used and fifty runs were generated for the best conformation. The O atoms in AD served as hydrogen bond acceptors forming three strong hydrogen bonding interactions with Tyr125, Trp326 and Try468. The distances of these hydrogen bonds were 3.089, 2.809 and 2.852 Å, respectively. Compared with the Val366 residue of KSDDI, the Ser366 residue of KSDDII has a hydroxyl group that could possibly interact with the active catalytic center by hydrogen bonding (Fig. 7c). Furthermore, due to steric hindrance, the two methyl groups of Val366 may interfere with the interaction of the substrate and the active center (Fig. 7b). In this way, the Val366 Ser mutation could possibly improve KSDD affinity toward AD and further enhance KSDD enzyme activity. Thus, the mutation of Val366 to Ser may directly affect the dehydrogenation activity of KSDD.
Purification of KSDD has been complicated by the presence of hydrophobic transmembrane domains which embed the enzyme in the membrane [8,15], and as a result progress in determining KSDD structure and its catalytic mechanism has been slow [29]. Oosterwijk et al. proposed a method for analyzing membrane protein structure and successfully revealed the crystal structure of Δ4-(5α)-KSDD from R. jostii RHA1 [25]. Rohman et al. resolved the crystal structure of KSDD from R. erythropolis SQ1 and clarified its catalytic mechanism with the active site containing four key residues: Tyr119, Tyr318, Tyr487 and Gly491 [19]. Xie et al. revealed that Ser138 played an important role in maintaining the active center in the hydrophobic environment of KSDD [29]. Despite all these studies, the structure of KSDD from M. neoaurum remains unresolved. The analysis methods used for revealing the crystal structure of KSDD from Rhodococcus sp. could provide a feasible way for KSDD structural analysis in mycobacteria. In this study, the structural differences in KSDD caused by the mutation of Val366 to Ser366 resulted in obvious changes in the ADD/AD molar ratio between M. neoaurum ST-095 and M. neoaurum JC-12. This result suggested that, in addition to the identified active site residues, other amino acids such as Val366 are also important determinants of the C1,2 dehydrogenation activity of the KSDD enzyme. This work provides a feasible way for analyzing the key amino acids of KSDD from M. neoaurum by site-directed mutagenesis. As the key residues of KSDD can greatly affect final ratios of products formed in industrial production strains [29], it is crucial to further identify the key residues to reveal the catalytic mechanism of the KSDD enzyme.
Conclusion
In this study, we clarified the different effects on ADD/AD molar ratio of strain M. neoaurum ST-095 and its mutant M. neoaurum JC-12 when used for phytosterol transformation. By PCR techniques and DNA sequence alignment, we found that the only difference between KSDDI and KSDDII was the change of Val366 to Ser366. Enzyme assay of KSDDI and KSDDII showed that KSDDII activity was higher than KSDDI, which indicated that the mutation could possibly enhance KSDD enzyme activity and improve the ADD/AD molar ratio. The analysis of the KSDDI and KSDDII functions in phytosterol bioconversion further verified that the differences in the ADD/AD molar ratio seen during phytosterol transformation by strains M. neoaurum ST-095 and M. neoaurum JC-12 were possibly due to the KSDD mutation. The KSDD structure analysis showed how the mutation in the amino acid sequence of KSDD might lead to KSDD structural modification and thereby result in the different KSDD enzyme activity, which in turn would alter the ADD/AD molar ratio.
Abbreviations
- AD:
-
Androst-4-ene-3,17-dione
- ADD:
-
Androst-1,4-diene-3,17-dione
- KSDD:
-
3-Ketosteroid-Δ1-dehydrogenase
- FAD:
-
Flavin adenine dinucleotide
- Me-β-CD:
-
Methyl-β-cyclodextrin
- PMS:
-
Phenazine methosulphate
- DCPIP:
-
2,6-Dichlorophenolindophenol
References
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation in Bacillus subtilis. J Bacteriol 81:741
Bragin EY, Shtratnikova VY, Dovbnya D, Schelkunov M, Pekov YA, Malakho S, Egorova O, Ivashina T, Sokolov S, Ashapkin V (2013) Comparative analysis of genes encoding key steroid core oxidation enzymes in fast-growing Mycobacterium spp. strains. J Steroid Biochem Mol Biol 138:41–53
Choi K-P, Molnar I, Yamashita M, Murooka Y (1995) Purification and characterization of the 3-ketosteroid-Δ1-dehydrogenase of Arthrobacter simplex produced in Streptomyces lividans. J Biochem 117:1043–1049
Donova MV, Egorova OV (2012) Microbial steroid transformations: current state and prospects. Appl Microbiol Biotechnol 94:1423–1447. doi:10.1007/s00253-012-4078-0
Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS (2003) Microbial conversion of steroid compounds: recent developments. Enzyme Microb Technol 32:688–705. doi:10.1016/S0141-0229(03)00029-2
Fujii C, Morii S, Kadode M, Sawamoto S, Iwami M, Itagaki E (1999) Essential tyrosine residues in 3-Ketosteroid-Δ’-Dehydrogenase from Rhodococcus rhodochrous. J Biochem 126:662–667
Gordhan BG, Parish T (2001) Gene replacement using pretreated DNA. In: Parish T, Stoker NG (eds) Mycobacterium tuberculosis protocols, vol 54. Methods in Molecular Medicine. Humana Press Inc., New Jersey, pp 77–92. doi:10.1385/1-59259-147-7:077
Li Y, Lu F, Sun T, Du L (2007) Expression of ksdD gene encoding 3-ketosteroid-Δ1-dehydrogenase from Arthrobacter simplex in Bacillus subtilis. Lett Appl Microbiol 44:563–568
Liu C, Zhang X, Z-m Rao, Shao M-l WuD, Z-h Xu, Li H (2015) Mutation breeding of high 4-androstene-3, 17-dione-producing Mycobacterium neoaurum ZADF-4 by atmospheric and room temperature plasma treatment. J Zhejiang Univ Sci B 16:286–295
Long S, Zhang X, Rao Z, Chen K, Xu M, Yang T, Yang S (2016) Amino acid residues adjacent to the catalytic cavity of tetramer l-asparaginase II contribute significantly to its catalytic efficiency and thermostability. Enzyme Microb Technol 82:15–22. doi:10.1016/j.enzmictec.2015.08.009
Malaviya A, Gomes J (2008) Androstenedione production by biotransformation of phytosterols. Bioresour Technol 99:6725–6737. doi:10.1016/j.biortech.2008.01.039
Marsheck WJ, Kraychy S, Muir RD (1972) Microbial degradation of sterols. Appl Microbiol 23:72–77
Martin CK (1977) Microbial cleavage of sterol side chains. Adv Appl Microbiol 22:29–58
Matsushita H, Itagaki E (1992) Essential histidine residue in 3-ketosteroid-Δ1-dehydrogenase. J Biochem 111:594–599
Molnar I, Choi KP, Yamashita M, Murooka Y (1995) Molecular cloning, expression in Streptomyces livdans, and analysis of a gene cluster from Arthrobacter simplex encoding 3-ketosteroid-Δ1-dehydrogenase, 3-ketosteroid-Δ5-isomerase and a hypothetical regulatory protein. Mol Microbiol 15:895–905
Morii S, Fujii C, Miyoshi T, Iwami M, Itagaki E (1998) 3-Ketosteroid-Δ1-dehydrogenase of Rhodococcus rhodochrous: sequencing of the genomic DNA and hyperexpression, purification, and characterization of the recombinant enzyme. J Biochem 124:1026–1032
Murray HC, Peterson DH (1952) Oxygenation of steroids by Mucorales fungi. Google Patents
Plesiat P, Grandguillot M, Harayama S, Vragar S, Michel-Briand Y (1991) Cloning, sequencing, and expression of the Pseudomonas testosteroni gene encoding 3-oxosteroid delta 1-dehydrogenase. J Bacteriol 173:7219–7227
Rohman A, van Oosterwijk N, Thunnissen A-MW, Dijkstra BW (2013) Crystal structure and site-directed mutagenesis of 3-ketosteroid Δ1-dehydrogenase from Rhodococcus erythropolis SQ1 explain its catalytic mechanism. J Biol Chem 288:35559–35568
Shao M, Rao Z, Zhang X, Xu M, Yang T, Li H, Xu Z, Yang S (2015) Bioconversion of cholesterol to 4-cholesten-3-one by recombinant Bacillus subtilis expressing choM gene encoding cholesterol oxidase from Mycobacterium neoaurum JC-12. J Chem Technol Biotechnol 90:1811–1820. doi:10.1002/jctb.4491
Shao M, Zhang X, Rao Z, Xu M, Yang T, Li H, Xu Z (2015) Enhanced production of Androst-1, 4-Diene-3, 17-Dione by Mycobacterium neoaurum JC-12 using three-stage fermentation strategy. PLoS One 10:e0137658
Shao M, Zhang X, Rao Z, Xu M, Yang T, Li H, Xu Z, Yang S-T (2015) Efficient testosterone production by engineered Pichia pastoris co-expressing human 17β-hydroxysteroid dehydrogenase type 3 and Saccharomyces cerevisiae glucose 6-phosphate dehydrogenase with NADPH regeneration. Green Chem. doi:10.1039/C5GC02353J
Shen YB, Wang M, Li HN, Wang YB, Luo JM (2012) Influence of hydroxypropyl-β-cyclodextrin on phytosterol biotransformation by different strains of Mycobacterium neoaurum. J Ind Microbiol Biotechnol 39:1253–1259
Van der Geize R, Hessels G, Van Gerwen R, Vrijbloed J, Van der Meijden P, Dijkhuizen L (2000) Targeted disruption of the kstD gene encoding a 3-Ketosteroid Δ1-Dehydrogenase isoenzyme of rhodococcus erythropolis strain SQ1. Appl Environ Microbiol 66:2029–2036
van Oosterwijk N, Knol J, Dijkhuizen L, van der Geize R, Dijkstra BW (2012) Structure and catalytic mechanism of 3-ketosteroid-Δ4-(5α)-dehydrogenase from Rhodococcus jostii RHA1 genome. J Biol Chem 287:30975–30983
Wang Z, Zhao F, Chen D, Li D (2006) Biotransformation of phytosterol to produce androsta-diene-dione by resting cells of Mycobacterium in cloud point system. Process Biochem 41:557–561
Wang ZF, Huang YL, Rathman JF, Yang ST (2002) Lecithin-enhanced biotransformation of cholesterol to androsta-1, 4-diene-3, 17-dione and androsta-4-ene-3, 17-dione. J Chem Technol Biotechnol 77:1349–1357
Wei W, Wang FQ, Fan SY, Wei DZ (2010) Inactivation and augmentation of the primary 3-ketosteroid-Δ1-dehydrogenase in Mycobacterium neoaurum NwIB-01: biotransformation of soybean phytosterols to 4-androstene-3,17-dione or 1,4-androstadiene-3,17-dione. Appl Environ Microbiol 76:4578–4582. doi:10.1128/aem.00448-10
Xie RL, Shen YB, Qin N, Wang YB, Su LQ, Wang M (2015) Genetic differences in ksdD influence on the ADD/AD ratio of Mycobacterium neoaurum. J Ind Microbiol Biotechnol:1–7
Xu XX, Jin FL, Yu XQ, Ji SX, Wang J, Cheng HX, Wang C, Zhang WQ (2007) Expression and purification of a recombinant antibacterial peptide, cecropin, from Escherichia coli. Protein Expr Purif 53:293–301
Xu Y-P, Qin J, Sun S-M, Liu T-T, Zhang X-L, Qian S-S, Zhu H-L (2014) Synthesis, crystal structures, molecular docking and urease inhibitory activity of nickel (II) complexes with 3-pyridinyl-4-amino-5-mercapto-1, 2, 4-triazole. Inorganica Chim Acta 423:469–476
Yao K, Xu L-Q, Wang F-Q, Wei D-Z (2014) Characterization and engineering of 3-ketosteroid-△1-dehydrogenase and 3-ketosteroid-9α-hydroxylase in Mycobacterium neoaurum ATCC 25795 to produce 9α-hydroxy-4-androstene-3,17-dione through the catabolism of sterols. Metab Eng 24:181–191. doi:10.1016/j.ymben.2014.05.005
Zhang WQ, Shao ML, Rao ZM, Xu MJ, Zhang X, Yang TW, Li H, Xu ZH (2013) Bioconversion of 4-androstene-3,17-dione to androst-1,4-diene-3,17-dione by recombinant Bacillus subtilis expressing ksdd gene encoding 3-ketosteroid-Δ1-dehydrogenase from Mycobacterium neoaurum JC-12. J Steroid Biochem Mol Biol 135:36–42. doi:10.1016/j.jsbmb.2012.12.016
Zhong C, Rao Z, Xia H, Xu Z, Fang H, Zhuge J (2009) Mutation breeding of Mycobacterium sp. for transformation of phytosterol into androst-1,4-diene-3,17-dione. Chem Bioeng 7:43–46 (in Chinese)
Acknowledgments
We sincerely appreciate Professor W. R. Jacobs, Jr. (Howard Hughes Medical Institute, USA) for providing plasmids pMV261 and pMV306, and Professor T. Parish (Department of Infectious & Tropical Diseases, United Kingdom) for providing plasmids of p2NIL and pGOAL19. This work was supported by the National Basic Research Program of China (973 Program) (2012CB725202), the High-Tech Research and Development Programs of China (2011AA02A211, SS2015AA021004), the National Natural Science Foundation of China (31570085), Jiangsu Province Science Fund for Distinguished Young Scholars (BK20150002), the Fundamental Research Funds for the Central Universities (JUSRP51306A), the 111 Project (111-2-06) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shao, M., Zhang, X., Rao, Z. et al. A mutant form of 3-ketosteroid-Δ1-dehydrogenase gives altered androst-1,4-diene-3, 17-dione/androst-4-ene-3,17-dione molar ratios in steroid biotransformations by Mycobacterium neoaurum ST-095. J Ind Microbiol Biotechnol 43, 691–701 (2016). https://doi.org/10.1007/s10295-016-1743-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1743-9