Abstract
Previously, we demonstrated triacylglycerol (TAG) accumulation and the in vivo ability to catalyze esters from exogenous short chain alcohol sources in Gordonia sp. strain KTR9. In this study, we investigated the effects that putative lipase (KTR9_0186) and wax ester synthase/acyl-CoA:diacylglycerol acyltransferase (WS/DGAT; KTR9_3844) gene knockouts had on TAG accumulation. Gene disruption of KTR9_0186 resulted in a twofold increase in TAG content in nitrogen starved cells. Lipase mutants subjected to carbon starvation, following nitrogen starvation, retained 75 % more TAGs and retained pigmentation. Transcriptome expression data confirmed the deletion of KTR9_0186 and identified the up-regulation of key genes involved in fatty acid degradation, a likely compensatory mechanism for reduced TAG mobilization. In vitro assays with purified KTR9_3844 demonstrated WS/DGAT activity with short chain alcohols and C16 and C18 fatty acid Co-As. Collectively, these results indicate that Gordonia sp. KTR9 has a suitable tractable genetic background for TAG production as well as the enzymatic capacity to catalyze fatty acid esters from short chain alcohols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the Actinomycetes group of bacteria are known to naturally accumulate intracellular lipid storage compounds composed of TAGs [2]. Interest in bacterial TAGs has grown over the years as these compounds have a potential application as a renewable feed-stock source for biofuels and other bio commodities, similar to plant derived oils. Synthesis of TAGs can be induced under unbalanced growth conditions in which carbon is present in excess and nitrogen, and to a lesser extent phosphate, is limiting [2]. Members of the genera Gordonia, Rhodococcus, Mycobacterium, Streptomyces, and Nocardia, can accumulate significant internal reserves of TAGs, as high as 80 % of bacterial cell dry weight [14, 31]. This response is likely a physiological adaptation for survival in nutrient poor environments given that Actinomycetes are widely distributed in the natural environment.
The search for the biological trigger that causes TAG accumulation upon nitrogen starvation in Actinomycetes has largely focused on the bifunctional wax esterase synthase/acyl-CoA diacylglycerol acyltransferase (WS/DGAT) enzymes encoded by the atf genes. This enzyme was first described in Acinetobacter baylyi ADP1 and catalyzes the condensation of acyl-CoA with diacylglycerol to form TAGs [26, 29]. These enzymes have since been described for other gram negatives as well as actinomycetes, each displaying a promiscuous activity for a variety of alcohol and fatty acid substrates [1, 3, 6, 11, 25, 29]. Reductions in TAG accumulation of 25–30 % were reported in Rhodococcus opacus PD630 that had atf2 inactivated, further confirming the importance of these enzymes in TAG synthesis [17]. A transposon-based genetic screen in Rhodococcus opacus PD630 for TAG accumulation deficient mutants identified a NADPH-dependent glyceraldehyde 3-phosphate dehydrogenase activity, which was believed to generate excess reducing power under nitrogen limitation for increased TAG synthesis [20]. Global transcriptome-based insights into TAG accumulation have largely come from experimentation on the green algae Chlamydomonas reinhardtii [7, 8, 22]. Changes in transcript abundance in this organism following nitrogen deprivation reveal a general redirection of metabolism with carbon flowing directly to fatty acid biosynthesis. Interestingly, the majority of genes encoding fatty acid metabolism were only subjected to a modest twofold increase in expression. However, stronger transcript responses were observed for a diacylglycerol transferase gene, phosphatidic acid phosphatases, membrane bound desaturases, and lipases [22]. These regulated genes offer potential targets to manipulate the synthesis and degradation of TAGs in bacterial systems like the Actinomycetes.
The metabolic diversity of genera like the Gordonia [4] suggests this genus is especially well suited for TAG production, in that they possess a large number of genes dedicated to fatty acid and lipid metabolism [9] and are capable of utilizing alternative feedstocks as a cheap carbon source [16]. Previously, we demonstrated TAG accumulation and the in vivo ability to catalyze esters from exogenous short chain alcohol sources in Gordonia sp. KTR9 [14], a strain isolated from arid soil [27]. We identified a number of pathways and associated genes involved in TAG synthesis and degradation including 7 atf homologs, genes involved in the Kennedy pathway, and multiple fatty acid synthases, lipases, fatty acyl-CoA reductases, and diacylglycerol kinases. Here, based on insights from system-wide transcriptome studies in Chlamydomonas reinhardtii [7, 8, 22] and the KTR9 genome [9], we investigated the effects of putative lipase and wax ester synthase/acyl-CoA:diacylglycerol acyltransferase gene knockouts on triacylglycerol accumulation and transcriptome expression in KTR9.
Materials and methods
Bacterial strain and culturing conditions
All bacterial strains used in this study are listed in Table 1. Gordonia sp. KTR9 was grown on mineral salts medium (MSM) [14] supplemented with 1 g/l NH4Cl and 20 mM each of fructose, gluconate, and succinate at 30 °C, shaking at 180 rpm. All studies were done in 50 ml of MSM media in 250 ml flasks. For nitrogen limited studies, cultures were grown for 3 days, centrifuged at 3,220×g for 5 min, washed twice with 5 ml phosphate buffered saline (PBS), then re-suspended in fresh, nitrogen free MSM media. Following re-suspension, cultures were incubated for 2 days prior to harvesting. Complete depletion of NH4Cl from the media was verified by analysis with an Aquanal ammonium assay kit (Sigma-Aldrich Corp, St. Louis, MO, USA). Controls were treated the same but re-suspended in MSM media with 1 g/l NH4Cl. Carbon starved studies were performed the same as described above except that following nitrogen starvation, the cells were centrifuged at 3,220×g for 20 min, washed twice with 5 ml phosphate buffered saline (PBS), then re-suspended in fresh, carbon free MSM media with 1 g/l NH4Cl for 72 h. All experiments were done in triplicate.
Gene deletions
Based on DNA sequence information of KTR9, gene deletion constructs were synthesized (Eurofins Genomics, Huntsville, AL USA) with a total of 1.0-kb flanking sequences (500 bp on each side) in the pCR2.1 vector and pBSII SK(+) vector for KTR9_0186 and KTR9_3844, respectively. Gene deletion inserts were liberated from the vectors via EcoRI restriction enzyme digestion, gel purified with a Wizard SV Gel and PCR Cleanup System kit (Promega, Madison, WI), and sub-cloned into the EcoRI site of the mobilizable plasmid pK18mobsacB [24]. The resulting plasmids, pKTR9_0186 and pKTR9_3844, were used for gene deletion in Gordonia sp. KTR9 based on a conjugation strategy [30] using a sacB counter selectable marker, with the exceptions of using kanamycin and nalidixic acid at 50 g/ml. Transconjugants were replica plated on the same medium with and without 10 % sucrose. Sucrose-sensitive colonies were propagated overnight at 30 °C in 25 ml of LBP (1 % (wt/vol) Bacto peptone (Difco), 0.5 % (wt/vol) yeast extract (BBL Becton–Dickinson and Company), and 1 % (wt/vol) NaCl) medium and then spread on LBP plates supplemented with 10 % sucrose. Sucrose-resistant colonies were checked for kanamycin sensitivity and screened for the deleted gene by PCR amplification and DNA sequencing using primers listed in Table 1. PCR amplification with primers NKO_0186F and NKO_0186R was done to verify deletion of KTR9_0186. The PCR conditions were an annealing temperature of 59 °C with a 1 min extension time. The expected product size in the mutant was 806 bp. The KTR9_3844 deletion was verified with primers NKO_3844F and NKO_3844R and an annealing temperature of 60 °C with a 1:10 min extension time. The expected PCR product size in the mutant was 660 bp. Gene deletions were further confirm by sequencing using an ABI 3100 Genetic Analyzer (Life Technologies, Grand Island, NY USA) and Big Dye® Terminator v 3.1 Cycle Sequencing kit (Life Technologies #4337455) according to the manufacturers’ directions.
RNA extraction, labeling, and microarray experimentation
Total RNA was extracted from KTR9 cells using the Qiagen RNeasy kit (Qiagen, Valencia, CA). Prior to extraction, an enzymatic lysis/mechanical disruption step was added to the protocol, whereby the cell pellet was re-suspended in 100 µl TE buffer and 200 µl lysozyme (20 mg/µl), transferred to an MP Biomedicals Lysing Matrix B tube (MP Biomedicals, Santa Ana, CA, USA) and vortexed for 1 min. An optional on-column DNA digestion using the Qiagen RNase-Free DNase Kit was performed to remove any remaining DNA. The quantity and quality of RNA were assessed with the Agilent 2100 Bioanalyzer using the Agilent RNA 6000 Kit (Agilent, Santa Clara, CA, USA). The cDNA for microarray analysis was generated using the Invitrogen Superscript II Double-Stranded cDNA Synthesis Kit (Life Technologies, Grand Island, NY, USA) and the quality of the cDNA was confirmed using the Agilent 2100 Bioanalyzer and Agilent DNA 1000 Kit. The cDNA was Cy3-labeled with the NimbleGen One-Color DNA Labeling Kit (Roche NimbleGen, Madison, WI, USA). Two micrograms of the labeled cDNA was used for hybridization to NimbleGen Bacterial Gene Expression Arrays (Roche, Madison, WI, USA) using a Gordonia sp. KTR9 (12 × 135 K) array developed from the annotated KTR9 genome [9]. The arrays were hybridized for 18 h at 42 °C in a NimbleGen Hybridization system and subsequently washed using the NimbleGen Wash Buffer Kit. Washed arrays were scanned using the Agilent Microarray Scanner and images were analyzed using NimbleScan v2.5.26 software and ArrayStar v4.0.0 software (DNAStar, Madison, WI, USA). Expression data across multiple transcriptomes were log2 transformed and statistical significance was determined by Moderated t test (P value = 0.05, twofold induction). The microarray data set obtained from this effort is available from the NCBI gene expression and hybridization array data repository (www.ncbi.nlm.nih.gov/geo) under accession GSE60245.
Protein purification and activity assay
A 1,401 bp fragment representing a 50.7 kDa putative WS/DGAT protein encoded by KTR9_3844 was codon optimized for expression in Escherichia coli and synthesized (Celtek Biosciences) in the pGH vector (Invitrogen, Carlsbad, CA, USA) based on the predicted protein sequence information of KTR9. A 1,413 bp fragment was liberated from the pGH vector via EcoRI restriction enzyme digestion, gel purified with a Wizard SV Gel and PCR Cleanup System kit (Promega, Madison, WI, USA), and cloned into the EcoRI site of the pASK-IBA5plus protein expression vector (IBA Life Sciences, Germany). Recombinant clones were transformed into E. coli One-Shot® BL21 Star™. Clones were screened for the correctly oriented insert using primers KTR9_3844RF and KTR9_3844RR, which yielded a 1,237 bp insert. Step-TagII labeled KTR9_3844 protein was expressed and purified according to the manufacturer’s instructions using Strep-Tactin® columns. Briefly a 100 ml culture of the KTR9_3844 containing E. coli clone was induced with 10 μl of 2 mg/ml anhydrotetracycline in Dimethylformamide (DMF). Following induction, cultures were incubated at 30 °C, 200 rpm for 3 h. Cells were harvested by centrifugation at 4,500×g for 12 min at 4 °C. Cell lysate was prepared by three passages through a French Press (Glen Mills Model 11, Clifton, NJ, USA) at 20,000 psi. Cleared lysates were prepared by centrifuging the lysate at 14,000×g, 15 min at 4 °C. Cleared lysates were loaded on Strep-Tactin® columns and the proteins were purified following the manufacturer’s instructions.
Purified proteins were quantified via the Bradford assay, solubilized in 2× Laemmli Sample Buffer (Bio-Rad #161-0737) and analyzed on a Bio-Rad 4–20 % Criterion Stain-Free™ Tris–HCl gel (Bio-Rad #345-0412) to confirm the size and purity of the target protein. WS/DGAT activity for various short and long-chain alcohols and C16 and C18 fatty acyl-CoA substrates was determined using a 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) based colorimetric protein assay as described previously [6, 21]. Briefly, proteins were diluted to a final concentration of 0.5 µg/µl in 1 ml of 50 mM potassium phosphate (pH 7) and 300 mM NaCl buffer. Ten microliters of 18 mg DTNB/ml dimethyl sulfoxide (DMSO) was added along with 50 µl of the appropriate alcohol (methanol, ethanol, dodecanol, or hexadecanol). Fatty alcohols were dissolved in DMSO. Fifteen microliters of either 1 mM pamitoyl-CoA or stearoyl-CoA was added to initiate the reaction and the absorbance at 412 nm was measured for 5 min. Initial reaction rates were calculated in Excel (Microsoft, Redmond, WA, USA) using the slope from the best fit line obtained from the spectrophotometric assays during the first minute of the reaction and calculating nmol of NTB2− dianion formed per second using the extinction coefficient of the NTB22− dianion at 412 nm of 14,150 M−1 cm−1 [6].
Lipid extraction and analysis
Total lipids were extracted from 40 to 50 mg of dry cells as described in Eberly et al. [14]. Briefly, total lipids were recovered from cells by extraction in 1.5 ml methanol (MeOH) and 5 ml methyl tert-butyl ether (MTBE) following incubation for 1 h at room temperature with constant mixing. One-half of the recovered total lipids were subjected to fractionation on silica gel columns to isolate the neutral or non-polar lipids from the glyco- and polar lipids. Neutral lipids were eluted in 4.5 ml of CHCl3 containing 1 % acetic acid to enhance the recovery of free fatty acids. Recovered total and neutral lipids were assayed via GC–MS following strong acid methylation in methanol:chloroform:concentrated hydrochloric acid (10:1:1, v:v:v) with heat (80 °C) for 1 h as described previously [14]. Total TAG content was determined by colorimetric assay using an ABcam Triglyceride Quantification Kit (ABcam, Cambridge, MA, USA).
Results
Deletion of KTR9_0186 and its effect on TAG accumulation and transcriptome expression
Deletion mutants were constructed using a counter selection recombination strategy and genotypes were confirmed by DNA sequencing and PCR (Fig. 1) as described in the “Materials and methods”. The amount of TAGs produced by deletion strain KTR9_0186, a putative lipase was twice that produced by the wild type when grown under similar conditions. (Fig. 2a). The amount of TAGs accumulated by these mutants under balanced growth conditions was greater than those measured in the wild type when propagated under unbalanced growth conditions to stimulate TAG synthesis. The ability of this mutant strain to mobilize TAGs, relative to the wild type, was assessed by first stimulating TAG synthesis under carbon rich, nitrogen limiting conditions followed by carbon starvation in the presence of nitrogen. Following 3 days of carbon starvation, deletion strain KTR9_0186 retained twofold greater neutral lipid fatty acid content compared to wild type suggesting a functional role for KTR9_0186 in TAG mobilization (Fig. 2b). Cultures of the carbon starved mutant also retained their characteristic pigmentation, which was in stark contrast to the wild-type cultures that lost all pigmentation (by visual inspection, data not shown).
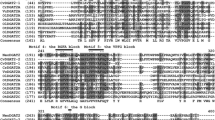
PCR confirmation of KTR9_0186 and KTR9_3844 gene deletions in Gordonia sp. KTR9. a PCR with NKO_0186F/R primers. Product size is 806 bp with 0186 deleted. Lanes 1–3 are clones that have the correct 806 bp knockout region. Lane 4 is a Thermo Scientific GeneRuler 1 kb Plus DNA ladder. b PCR with NKO 3844F/R primers. Product size is 660 bp with the KTR9_3844 gene deleted. Lanes 1–2 Thermo Scientific GeneRuler 1 kb Plus and 100 bp DNA ladder, lanes 3-6 knockout clones
To further investigate the mechanism(s) of TAG accumulation in the KTR9_0186 deletion strain, microarray experiments were performed on wild-type and mutant strains grown under the conditions described above. Transcript-expression analysis of carbon starved KTR9 0186 mutant cells identified 475 targets with statistically significant differences in gene expression compared to the wild-type controls. A total of 358 of the gene targets had twofold or greater increases in expression, whereas the remaining 117 gene targets had twofold or greater decreases in expression. A subset of transcripts involved in lipid metabolism induced/repressed as a result of deletion of KTR9-0186 is presented in Table 2. Expression data confirmed the deletion of KTR9_0186 with a zerofold change in expression. In addition, a number of genes involved in fatty acid metabolism, beta-oxidation in particular (KTR9_0374, 0584, 0585, 0721, 2316, 3121, 3122, 3480, 3505, 3836, 4289, and 5406), were found to be differentially expressed in the KTR9_0186 mutant. Alternative putative lipase/esterase activities (KTR9_0645, 0995, 151, 3686) were also up-regulated in the KTR9_186 mutant, possibly as a compensatory mechanism for the loss of lipase activity.
Expression and characterization of KTR9_3844
The WS/DGAT enzyme encoded by KTR9_3844 was cloned, heterologously expressed and purified as described in the “Materials and methods”. A prominent expected band of around 52 kDa was observed when the sample was electrophoresed through an SDS-PAGE gel (Fig. 3). Spectrophotometric assays were used to monitor enzyme activity as described in the “Materials and methods”. Michaelis–Menten kinetic parameters were calculated using a Lineweaver–Burk double reciprocal plot to calculate V m and K m. Substrates for these assays included methanol, ethanol, hexadecanol and dodecanol as acyl acceptors and palmitoyl acyl-CoA and stearoyl acyl-CoA as acyl donors. Of the substrates tested, methanol and ethanol with palmitoyl-CoA produced the highest activity with a calculated V max of 25.9 ± 9.6 and 21.5 ± 8.6 nmol product/min/mg protein respectively and K m values of 17.3 ± 5.6 and 6.5 ± 3.7 %, respectively (Table 3). Longer chain hexadecanol and dodecanol acyl acceptors exhibited much less activity (Fig. 4) due in part to their low solubility. Deletion strain KTR9_3844 did not result in a statistically significant change in total TAG content compared to wild type under the growth conditions tested (data not shown).
Discussion
Previously, we demonstrated TAG accumulation and the in vivo ability to catalyze esters from exogenous short chain alcohol sources in Gordonia sp. KTR9 [14]. As part of this study, we revisited a subset of our previous transcriptome data in which KTR9 was grown under nitrogen rich vs nitrogen starved conditions to look at changes in expression of genes and pathways that might implicated a “biological trigger” for TAG accumulation [18]. Interestingly, in that study the majority of genes encoding fatty acid metabolism were only subjected to a modest twofold or less change in expression similar to what others have reported [7, 22]. Studies in Chlamydomonas strengthen these observations indicating that TAG accumulation appears to be largely independent of de novo protein synthesis, suggesting that carbon availability is a key factor in TAG biosynthesis [15]. While prior transcriptome studies combined with the KTR9 annotation provided little insights into mechanisms of TAG accumulation [18], they did confirm that the genes targeted in this study for deletion were expressed.
Expression and in vitro characterization of KTR9_3844 confirmed that this enzyme can catalyze formation of fatty acid methyl esters (FAME) and fatty acid ethyl esters (FAEE) in the presence of methanol and ethanol with both palmitoyl acyl-CoA and stearoyl-CoA as an acyl donor. This finding is consistent with our previous work demonstrating the in vivo ability to catalyze esters from exogenous short chain alcohol sources in Gordonia sp. KTR9 [14]. Deletion strain KTR9_3844, a putative WS/DGAT enzyme, did not lead to a decrease in total TAGs as reported previously for other atf deletions [17] however, sequence analysis indicated that there are multiple atf genes present and therefore KTR9_3844 may only play a limited role in TAG synthesis. Indeed, further analysis showed that this enzyme had greatest activity with short chain alcohols, indicating it may play a greater role in transesterification of TAGs rather than TAG synthesis. Previous studies have shown that the WS/DGAT enzyme has a broad substrate range that includes short and long-chain alcohols and acyl-CoA esters ranging from C12 to C20 resulting in the synthesis of both wax esters (WE) and TAG’s [26]. However, some species show a marked preference for specific alcohols and acyl-CoA chain lengths. For example, the WS/DGAT from Acinetobacter sp. ADP1 showed the highest specific activity with medium chain (C14-C18) alcohols and palmitoyl-CoA acyl-CoA [26]. In contrast, a more recent study of 5 WS/DGAT’s by Barney [6] showed significantly higher rates of activity with dodecanol (C12) than hexadecanol (C16) with palmitoyl-CoA as the acyl donor. As shown in Fig. 4, the WS/DGAT from KTR9 preferred even shorter alcohols and had significantly higher activity with short chain alcohols such as methanol and ethanol. This is significant in the context of biofuels production as short chain alcohols such as methanol and ethanol are the primary alcohols used for biodiesel synthesis.
Deletion of a putative lipase gene, KTR9_0186, led to a twofold increase in TAG accumulation. Previously, a fivefold increase in TAG accumulation was reported in a lipase knockout in Acinetobacter baylyi ADP1 [23]. While the role of this lipase in TAG accumulation was unclear, the authors suggested that the enzyme might be involved in the mobilization of TAGs when the cells are exposed to carbon starvation. Studies in both Mycobacterium tuberculosis and Chlamydomonas reinhardtii provide additional evidence that lipases do have a role in the mobilization of TAGs during carbon starvation [12, 19]. Similarly, when we subjected KTR9_186 mutants to carbon starvation following TAG accumulation, we observed a 75 % higher TAG content indicating reduced TAG mobilization compared to the wild-type strain (Fig. 2b). Gene expression data confirmed the deletion of KTR9_0186 and identified the up-regulation of key genes involved in fatty acid degradation. These findings in combination with the TAG accumulation data suggest that the KTR9_0186 lipase mutant is less efficient at retrieving carbon from TAGs under carbon starvation growth conditions. It is possible that the cells compensate by recruiting the activity of other lipase/esterases as well as increase basal expression levels of genes involved in the subsequent beta-oxidation of fatty acids. Interestingly, a Phage shock protein, PspA, was also found to be up-regulated in the lipase mutant (KTR9_2116). This protein was recently identified as a functional component of prokaryotic lipid granules and a deletion of this gene resulted in changes to the structure of the associated lipid granules [13]. It is intriguing to speculate that the lipase knockout resulted in tertiary structural changes to the TAG granules suggesting a possible mechanism for TAG mobilization.
During carbon starvation we observed significant differences in the intensity of pigmentation of the mutant strain compared to wild-type, when cultured in liquid media, with the mutant strain displaying a more orange rather than pink pigmentation. Previously, transposon insertional mutants displaying altered pigmentation have been observed in Gordonia polyisoprenivorans [5]. In particular, a dark orange phenotype was associated with an anti-anti-sigma regulatory factor mutant. A KTR9 homlog (KTR9_0062) to this gene was up-regulated in the KTR9_0186 lipase mutant. Links between TAG accumulation and pro-oxidant molecules like carotenoids have recently been investigated in Rhodococcus species [28]. It is intriguing to speculate that reduced mobilization of TAGs limits NADPH generating lipolysis reactions under carbon starvation conditions, exposing the cells to increased oxidative stress which the cells respond to by modulating pro-oxidant levels.
Declining petroleum reserves and the growing demand for energy have led to an increased interest in alternative, renewable fuels. Towards the goal of producing renewable biofuels in microorganisms, a host of synthetic biology and metabolic engineering approaches are being pursued for biosynthesis of biodiesel in well characterized production hosts, like E. coli [10]. As an alternative to using E. coli, members of the Actinomycetes group of bacteria are known to naturally accumulate TAGs and therefore may be a more suitable bacterial host for de novo biosynthesis of fatty acid esters. Collectively, these results indicate that Gordonia sp. KTR9 has a suitable tractable genetic background for TAG production as well as the enzymatic capacity to catalyze fatty acid esters from short chain alcohols.
References
Alvarez AF, Alvarez HM, Kalscheuer R, Waltermann M, Steinbuchel A (2008) Cloning and characterization of a gene involved in triacylglycerol biosynthesis and identification of additional homologous genes in the oleaginous bacterium Rhodococcus opacus PD630. Microbiology 154:2327–2335
Alvarez HM, Steinbuchel A (2002) Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol 60:367–376
Arabolaza A, Rodriguez E, Altabe S, Alvarez H, Gramajo H (2008) Multiple pathways for triacylglycerol biosynthesis in Streptomyces coelicolor. Appl Environ Microbiol 74:2573–2582
Arenskotter M, Baumeister D, Kalscheuer R, Steinbuchel A (2003) Identification and application of plasmids suitable for transfer of foreign DNA to members of the genus Gordonia. Appl Environ Microbiol 69:4971–4974
Banh Q, Arenskotter M, Steinbuchel A (2005) Establishment of Tn5096-based transposon mutagenesis in Gordonia polyisoprenivorans. Appl Environ Microbiol 71:5077–5084
Barney BM, Wahlen BD, Garner E, Wei J, Seefeldt LC (2012) Differences in substrate specificities of five bacterial wax ester synthases. Appl Environ Microbiol 78:5734–5745
Blaby IK, Glaesener AG, Mettler T, Fitz-Gibbon ST, Gallaher SD, Liu B, Boyle NR, Kropat J, Stitt M, Johnson S, Benning C, Pellegrini M, Casero D, Merchant SS (2013) Systems-level analysis of nitrogen starvation-induced modifications of carbon metabolism in a Chlamydomonas reinhardtii starchless mutant. Plant Cell 25:4305–4323
Boyle NR, Page MD, Liu B, Blaby IK, Casero D, Kropat J, Cokus SJ, Hong-Hermesdorf A, Shaw J, Karpowicz SJ, Gallaher SD, Johnson S, Benning C, Pellegrini M, Grossman A, Merchant SS (2012) Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J Biol Chem 287:15811–15825
Chen HP, Zhu SH, Casabon I, Hallam SJ, Crocker FH, Mohn WW, Indest KJ, Eltis LD (2012) Genomic and transcriptomic studies of an RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine)-degrading actinobacterium. Appl Environ Microbiol 78:7798–7800
Clomburg JM, Gonzalez R (2010) Biofuel production in Escherichia coli: the role of metabolic engineering and synthetic biology. Appl Microbiol Biotechnol 86(2):419–434
Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE (2004) Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol 186:5017–5030
Deb C, Daniel J, Sirakova TD, Abomoelak B, Dubey VS, Kolattukudy PE (2006) A novel lipase belonging to the hormone-sensitive lipase family induced under starvation to utilize stored triacylglycerol in Mycobacterium tuberculosis. J Biol Chem 281:3866–3875
Ding Y, Yang L, Zhang S, Wang Y, Du Y, Pu J, Peng G, Chen Y, Zhang H, Yu J, Hang H, Wu P, Yang F, Yang H, Steinbuchel A, Liu P (2012) Identification of the major functional proteins of prokaryotic lipid droplets. J Lipid Res 53:399–411
Eberly JO, Ringelberg DB, Indest KJ (2013) Physiological characterization of lipid accumulation and in vivo ester formation in Gordonia sp. KTR9. J Ind Microbiol Biotechnol 40:201–208
Fan J, Yan C, Andre C, Shanklin J, Schwender J, Xu C (2012) Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant Cell Physiol 53:1380–1390
Gouda MK (2008) Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J Microbiol Biotechnol 24:1703–1711
Hernandez MA, Arabolaza A, Rodriguez E, Gramajo H, Alvarez HM (2013) The atf2 gene is involved in triacylglycerol biosynthesis and accumulation in the oleaginous Rhodococcus opacus PD630. Appl Microbiol Biotechnol 97:2119–2130
Indest KJ, Hancock DE, Jung CM, Eberly JO, Mohn WW, Eltis LD, Crocker FH (2013) Role of nitrogen limitation in transformation of RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) by Gordonia sp. strain KTR9. Appl Environ Microbiol 79:1746–1750
Li X, Benning C, Kuo MH (2012) Rapid triacylglycerol turnover in Chlamydomonas reinhardtii requires a lipase with broad substrate specificity. Eukaryot Cell 11:1451–1462
MacEachran DP, Sinskey AJ (2013) The Rhodococcus opacus TadD protein mediates triacylglycerol metabolism by regulating intracellular NAD(P)H pools. Microb Cell Fact 12:104–108
McFie PJ, Stone SJ (2011) A fluorescent assay to quantitatively measure in vitro acyl CoA:diacylglycerol acyltransferase activity. J Lipid Res 52:1760–1764
Miller R, Wu G, Deshpande RR, Vieler A, Gartner K, Li X, Moellering ER, Zauner S, Cornish AJ, Liu B, Bullard B, Sears BB, Kuo MH, Hegg EL, Shachar-Hill Y, Shiu SH, Benning C (2010) Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol 154:1737–1752
Santala S, Efimova E, Kivinen V, Larjo A, Aho T, Karp M, Santala V (2011) Improved triacylglycerol production in Acinetobacter baylyi ADP1 by metabolic engineering. Microb Cell Fact 10:36–45
Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Shi S, Valle-Rodriguez JO, Khoomrung S, Siewers V, Nielsen J (2012) Functional expression and characterization of five wax ester synthases in Saccharomyces cerevisiae and their utility for biodiesel production. Biotechnol Biofuels 5:7–14
Stoveken T, Kalscheuer R, Malkus U, Reichelt R, Steinbuchel A (2005) The wax ester synthase/acyl coenzyme A:diacylglycerol acyltransferase from Acinetobacter sp. strain ADP1: characterization of a novel type of acyltransferase. J Bacteriol 187:1369–1376
Thompson KT, Crocker FH, Fredrickson HL (2005) Mineralization of the cyclic nitramine explosive hexahydro-1,3,5-trinitro-1,3,5-triazine by Gordonia and Williamsia spp. Appl Environ Microbiol 71:8265–8272
Urbano SB, Di Capua C, Cortez N, Farias ME, Alvarez HM (2014) Triacylglycerol accumulation and oxidative stress in Rhodococcus species: differential effects of pro-oxidants on lipid metabolism. Extremophiles 18:375–384
Uthoff S, Stoveken T, Weber N, Vosmann K, Klein E, Kalscheuer R, Steinbuchel A (2005) Thio wax ester biosynthesis utilizing the unspecific bifunctional wax ester synthase/acyl coenzyme A:diacylglycerol acyltransferase of Acinetobacter sp. strain ADP1. Appl Environ Microbiol 71:790–796
van der GR, Hessels GI, van Gerwen R, van der MP, Dijkhuizen L (2001) Unmarked gene deletion mutagenesis of kstD, encoding 3-ketosteroid Delta1-dehydrogenase, in Rhodococcus erythropolis SQ1 using sacB as counter-selectable marker FEMS Microbiol Lett 205:197–202
Waltermann M, Steinbuchel A (2005) Neutral lipid bodies in prokaryotes: recent insights into structure, formation, and relationship to eukaryotic lipid depots. J Bacteriol 187:3607–3619
Acknowledgments
This research was funded through the US Army Engineer Research and Development Center's Basic Research Program (Project 10–50, K. J. Indest). Views, opinions and/or findings contained herein are those of the authors and should not be construed as an official Department of the Army position or decision unless so designated by other official documentation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Indest, K.J., Eberly, J.O., Ringelberg, D.B. et al. The effects of putative lipase and wax ester synthase/acyl-CoA:diacylglycerol acyltransferase gene knockouts on triacylglycerol accumulation in Gordonia sp. KTR9. J Ind Microbiol Biotechnol 42, 219–227 (2015). https://doi.org/10.1007/s10295-014-1552-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1552-y