Abstract
Purpose
There is considerable interindividual variability in the perception of pain. Given that pain management is a major public health problem, gaining insight into the underlying physiology of these perceptual differences is important. We tested the hypothesis that when interindividual variability in initial muscle sympathetic nerve activity (MSNA) responses to a cold pressor test (CPT) is identified, the divergent responses will be linked to differences in pain perception in healthy young men and women.
Methods
In the supine position, blood pressure (BP) and MSNA were measured at baseline and during a 2-min CPT. Immediately following the CPT, pain was rated (range 0–10).
Results
Two groups were established: positive responders (Pos, n = 12) and negative responders (Neg, n = 12) based on the initial (first 30 s) MSNA response profiles (Pos: 12 ± 9, Neg: −3 ± 3 bursts/min, P < 0.0001). MSNA response profiles throughout the CPT were different between groups (P < 0.0001). Peak MSNA increases were different (Pos: 27 ± 11, Neg: 9 ± 5 bursts/min, P < 0.0001) and corresponded with initial MSNA responses (R2 = 0.6881, P < 0.0001). Blood pressure responses were also different throughout the CPT (P < 0.0001). Most importantly, the perception of pain induced by the CPT was different between the two groups (Pos: 8 ± 1, Neg: 4 ± 1, P < 0.0001).
Conclusions
The results indicate that in healthy young men and women, there are divergent initial sympathetic neural responses to a given painful stimulus that are linked to the magnitude of pain perception. These findings highlight the distinctive sympathetic patterns that may contribute to the considerable interindividual variability in the perception of pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is a major public health problem, with more than 100 million American adults experiencing chronic pain conditions, costing society at least US$560–635 billion annually to treat and manage [27]. The pathophysiology and subsequent experience of pain is highly complex and results in substantial variability in its perception [36, 58]. One of many factors that may modulate the responses to and perception of pain is the health of the cardiovascular system [45]. In fact, the systems modulating pain and blood pressure (BP) appear to be inherently linked [6, 23, 43, 44]. This is demonstrated in several recent observational studies suggesting that a relationship exists between pain responses and the risk of cardiovascular disease [7, 19, 25].
Acute pain leads to a rapid rise in BP, which in turn may reveal both short- and long-term cardiovascular risk [9, 34, 35, 50, 57]. Experimentally, inducing noxious stimuli such as strong pressure in the nail, mechanical pressure on the skin, hypertonic saline injection in the skin and muscle, and immersion of the hand in ice water (cold pressor test, CPT) leads to the activation of the sympathetic nervous system via a somatosensory reflex. The subsequent increase in efferent sympathetic outflow [muscle sympathetic nerve activity (MSNA)] evokes downstream cardiovascular responses and an increase in BP [8, 30, 40, 41, 57]. Effectively, experimentally induced pain allows for a functional model to demonstrate the strong relationship between sympathetically driven cardiovascular changes and the perception of pain [47].
Very much like the considerable interindividual variability in the perception of pain [20, 47], there is interindividual variability in efferent sympathetic outflow during established sympathoexcitatory stimuli. Indeed, recent studies have demonstrated that interindividual differences exist in the MSNA response during mental stress and hand grip [18, 26]. Particularly interesting is that MSNA response variability during mental stress was apparent even in the initial 30 s of the stress, wherein individuals exhibited either a rise or fall in sympathetic activity [26]. Early studies evaluating MSNA observed initial sympathoinhibition responses to noxious stimuli, including the CPT [14, 30, 47]. Adaptation to pain is composed of both inhibitory and facilitatory influences [6, 38]; however, it is unclear how the initial, individual MSNA responses relate to subjective perception of pain.
Thus, the primary objective of this study is to determine whether distinct MSNA response patterns are linked with differences in the perception of pain. Based on the previous studies [30, 33, 47], we hypothesized that there would be substantial interindividual variability in initial sympathetic neural responses to CPT-induced acute pain. We further hypothesized that the initial MSNA responses would correspond to the peak MSNA, BP responses, and perception of pain. Understanding this relationship may set the foundation for future research on chronic pain as well as pain-induced changes related to cardiovascular health.
Methods
Ethical approval
All participants were informed of the purpose, procedures, and risks of the study before providing written informed consent. The protocol and consent were approved by the institutional review boards at the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas (IRB nos. STU092013-036, STU082016-057, STU092017-068). This research study conformed to standards set by the Declaration of Helsinki, except for registration in a database.
Subjects
Twenty-four healthy subjects (14 women and 10 men; 32 ± 9 [mean ± SD] years old, body mass index (BMI): 26 ± 3 kg/m2) participated. Descriptive characteristics of the subjects are outlined in Table 1. All subjects were normotensive and had no evidence of cardiopulmonary, neurological or renal disease, based on history and physical examination at the time of the study. No subject smoked, used recreational drugs, or had other significant medical problems. No woman was pregnant during the experiment. Parental history of hypertension was obtained in each subject.
Measurements
Cardiovascular variables
Heart rate (HR) was determined from lead II of the electrocardiogram (ECG) (Hewlett-Packard). Arm BP was measured by electrosphygmomanometry (model 4240, Suntech); with a microphone placed over the brachial artery to detect Korotkoff sounds. Mean arterial pressure (MAP) was calculated as [(SBP − DBP)/3] + DBP, where SBP and DBP are systolic and diastolic BP, respectively. Respiration was monitored by a nasal cannula (Criticare Systems, model 602-11).
Muscle sympathetic nerve activity
Multi-unit MSNA signals were obtained utilizing the microneurographic technique [55]. Briefly, a recording electrode was placed in either the peroneal nerve (n = 19) at the popliteal fossa or the radial nerve at the spiral groove (n = 5), and a reference electrode was placed subcutaneously 2–3 cm from the recording electrode [12, 39, 59]. The nerve signals were amplified (gain 70,000–160,000), band-pass filtered (700–2000 Hz), full-wave rectified, and integrated with a resistance–capacitance circuit—time constant 0.1 s (662C-3, Department of Biomedical Engineering, University of Iowa). Criteria for adequate MSNA recording included the following: (1) pulse synchrony; (2) facilitation during the hypotensive phase of the Valsalva maneuver, and suppression during the hypertensive overshoot after release; (3) increases in response to breath holding; and (4) insensitivity to a gentle skin touch or a loud shout [55].
Protocol
The experiment was performed ≥ 2 h after a light meal and ≥ 12 h after the last caffeinated or alcoholic beverage was consumed, in a quiet, environmentally controlled laboratory with an ambient temperature of about 25 °C. Women were studied during the mid-luteal phase of the menstrual cycle (19–22 days after the onset of menstruation, when both estrogen and progesterone are high) or during the high-hormone phase of oral contraceptive use. A urine pregnancy test was performed prior to each testing to confirm that the women were not pregnant.
Participants were studied in the supine position. At least 10 min after an acceptable nerve recording site had been found, baseline MSNA and cardiovascular data were collected for 6 min during spontaneous breathing. After that, the subject performed the CPT, where the hand up to the wrist was immersed in an ice water bath (< 0.1°C) for 2 min, after which the subject’s hand was immediately dried and warmed. The subject was instructed to breathe normally, and avoid breath holding during the hand immersion. MSNA and cardiovascular data were recorded continuously during the CPT. Immediately following the CPT, the subject was asked to rate their perception of pain on a numeric rating scale of 0–10, with 0 being no pain and 10 being the worst pain imaginable. The scale accommodates the full range of human pain perceptions from non-painful, discomfort to intensely painful sensations [4, 49].
Data analysis
Data were sampled at 625 Hz with a commercial data acquisition system (Biopac Systems, Santa Barbara, CA, USA) and analyzed using LabVIEW software (National Instruments, Austin, TX, USA). Beat-by-beat HR was calculated from the R–R interval of the ECG. MSNA bursts were identified by a custom-made computer program [11], and confirmed by trained personnel. Burst frequency was defined as the number of bursts per minute, and burst incidence was used to normalize burst frequency per 100 heartbeats.
MSNA and cardiovascular data during supine baseline measurements were averaged. During the CPT, data were collected for 2 min and averaged for every 30 s, and MAP was collected every minute. In order to achieve our specific aims, we divided our participants into two groups based on the initial directionality of responses to the CPT. Subjects presenting with an increase in MSNA burst frequency during the initial 30 s of the CPT were classified and labeled as positive responders (Pos); and those that presented with no change or decrease in MSNA initially were classified and labeled as negative responders (Neg).
Statistical analysis
All data are expressed as mean ± standard deviation. A two-way repeated-measures ANOVA was utilized to compare the changes in HR, BP, and respiratory and MSNA responses between the two groups of responders. As determined a priori, if a significant interaction was identified, multiple post hoc comparisons of the changes (peak and/or initial) in responses were performed utilizing t tests with a Bonferroni adjustment. The perceived pain levels for the positive and negative responders were compared utilizing an unpaired t test, and parental history of hypertension reported by offspring was compared using a Chi-squared analysis. P values < 0.05 were considered statistically significant. GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA) was utilized to perform the statistical analysis and graphing.
Results
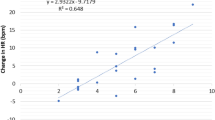
Baseline participant characteristics are presented in Table 1. Two groups were established based upon the initial (first 30 s) MSNA response to CPT (Pos: 12 ± 9, Neg: −3 ± 3 bursts/min, P < 0.0001). The two groups did not differ in any characteristic at rest. Representative laboratory tracings of data acquired during baseline, CPT, and recovery are presented in Fig. 1. Time course of changes in MAP, MSNA, HR, and respiratory responses to CPT are presented in Figs. 2a, 3, and 5, respectively. A significant interaction was revealed in the analysis of MAP, and MSNA expressed as burst incidence and burst frequency. Post hoc analysis demonstrated significant differences between the two groups in peak changes in MAP (Fig. 2b), as well as initial and peak changes in MSNA (Fig. 4). The relationship between the initial changes in MSNA and peak changes in MSNA are presented in Fig. 6. Significant differences in the reported perception of pain on a scale of 0–10 between the two groups are presented in Fig. 7. Finally, a significant difference existed between groups with respect to self-reported parental history of hypertension (Fig. 8).
Group and individual summary data depicting initial (first 30 s) and peak MSNA changes from baseline in the positive and negative responder groups during CPT. a, b Initial and peak changes in MSNA presented as burst incidence (BI). c, d Initial and peak changes in MSNA presented as burst frequency (BF)
Discussion
The primary objectives of this study were to investigate and classify the initial sympathetic neural response profiles to a CPT and to subsequently compare pain perceptions between the two distinct groups. Four important findings were revealed. First, interindividual variability in initial MSNA responses to a cold pressor test was apparent in this study. The patterns of these initial MSNA responses permitted the classification and comparison of positive and negative responders. Secondly, the stratification of these two groups revealed differences in MSNA and BP response profiles throughout the cold pressor stimulus. In this regard, the initial phase (first 30 s) of the sympathetic response to a CPT (first 30 s) corresponds to peak changes in MSNA during the CPT, with positive responders showing greater peak sympathetic responsiveness than negative responders. Third, differences were discovered in the perception of pain between these two groups. Specifically, individuals with positive responses during the initial phase of CPT had greater pain scores than those with negative responses. Finally, individuals in the initial negative response group to CPT also reported a higher prevalence of parental hypertension. Taken together, these findings provide important insight into the efferent neural underpinnings of the relationship between the cardiovascular and pain regulatory systems when exposed to a noxious stimulus.
The relationship between blood pressure, MSNA, and pain perception in the current study is consistent with previous investigations. This pattern of cardiovascular and sympathetic neural responses to painful stimuli first emerged in a study by Schobel et al. in 1996 [47]. In normotensive male subjects, their data showed strong correlations between pain ratings and increases in blood pressure and MSNA in response to pain evoked by mechanostimulation, i.e. skin pinching. Our investigation extends these findings, providing additional evidence that the magnitude of change in blood pressure and MSNA correspond with ratings of pain in response to an alternative painful stimulus (i.e. CPT). Furthermore, in our study, this relationship between blood pressure, MSNA, and pain perception in response to a CPT was demonstrated in a sample that included both normotensive men and women. Individual differences in sympathetic nerve activity and cardiovascular responses to a painful stimulus were also described by Fazalbhoy et al. in 2012 [20]. Variable levels of saline were intramuscularly infused to induce consistent subject ratings of tonic muscle pain; again, distinctly divergent patterns in MSNA, blood pressure and heart rate were observed. In the current investigation, similar divergent MSNA and BP responses were identified under conditions of a consistent noxious stimulus, as opposed to a consistent subjective experience. It is possible that the baroreflex was involved in the initial sympathoinhibition in the negative responders.
The highly subjective nature of the pain experience may also be influenced by the intricate interplay between the peripheral and central nervous system. The observed initial divergence in the MSNA responses to CPT in our two groups may be explained by alterations of the peripheral nociceptors (e.g., changes in receptor density and/or sensitivity), modulations within the central nervous system (CNS), or both. While the absolute magnitude of CPT stimulus was consistent across participants, differences in the propagation of the stimulus by sensory afferents may alter the sympathetic, cardiovascular, and/or perceptual responses. Furthermore, CNS modulation encompasses transmissions of spinal cord inhibitory inter-neurons, cortical processing of nociceptive inputs, and facilitatory/inhibitory descending pain control mechanisms [32, 61, 62]. Thus, another factor underpinning the difference in pain perception intensity between the groups may be the brainstem’s descending modulatory network—both its pro- and antinociception [54]. Previous research has demonstrated that CPT-induced pain activates the autonomic nervous system, potentially resulting in hypoalgesia, a response driven by hypothalamic–pituitary–adrenal axis stress reactivity [24, 31, 46, 48] and/or baroreflex-mediated pain modulation [17]. Clinically relevant, CPT-induced hypoalgesia is absent in patients with chronic pain [42]. Ultimately, parsing out the exact cause of the different response patterns is beyond the scope of this investigation; rather, these findings reflect the final efferent arc of the summation of all facilitatory and inhibitory mechanisms of pain processing. Future research should take a more mechanistic approach towards understanding the distinct MSNA response patterns described in the current investigation. A potential avenue to further examine physiological mechanisms underlying the CPT response in healthy individuals is to selectively alter pain perception via pharmacologic manipulation. Importantly, extending this research into pathophysiological processes, i.e. maladaptive pain, may give important insight into the management of chronic pain.
The implications of the observed differences in parental history of hypertension between the two groups are unclear. Nearly four decades of research has demonstrated a consistent relationship between hypertension and reduced pain sensitivity [6, 22, 45]. In fact, similar to the results of the current study, a handful of investigations have demonstrated reduced cardiovascular responsiveness to acute pain in normotensive individuals with a family history of hypertension [1, 2, 5, 13, 15, 21, 51]. In stark contrast, however, an abundance of literature describes an exaggerated or hyperreactive pressor responses to CPT in hypertensive and pre-hypertensive individuals [3, 10, 28, 37, 53, 60, 63]. Furthermore, there are inherent limitations in assessing parental history of hypertension with self-reports, including lack of details (e.g., age of onset, maternal vs paternal) and the tendency for underdiagnosis. Combined with the limited sample size of this study, the most conservative interpretation of this data would be to consider the responses to CPT and parental history of hypertension a spurious relationship that requires further investigation.
Clinically, the results of this study serve as a basis for further investigation into the relationship between sympathetic neural activity and pain regulation. The identification of variability in the neurophysiological components of normal, adaptive pain processing allows for the logical progression to proceed with this type of experimentation in abnormal, pathological pain processing. For example, examining the overlap of the pathological states of these two systems, i.e. cardiovascular dysfunction and maladaptive pain, with the methodology used in this study, would provide clinically relevant data. For instance, alterations in the sympathetic nervous system have been reported in complex regional pain syndrome [29]. Additional investigation into direct recordings of sympathetic nerve activity during stress may better elucidate the contributions of the sympathetically mediated pain in this and other chronic pain pathologies. With the current push by the scientific and medical community for the diagnosis and management of pain based on mechanisms, and not symptoms [16, 52, 56], further research using this technique is warranted and may provide specific targets for therapeutic interventions.
Conclusions
The present study reveals interindividual variability in initial sympathetic neural responses to a CPT in healthy men and women. Furthermore, the two divergent initial MSNA response profiles are significantly different in the reported pain to the CPT, as well as peak sympathetic and BP responses. To our knowledge, this is the first study to find these distinctive MSNA patterns, especially initially, and to link them to pain perception. The mechanisms responsible for the interindividual variability in MSNA and how it relates to pain process and hereditary risk of hypertension remains unclear and requires further investigation. Regardless, this study provides clear evidence that pain perception is tightly tied to neurophysiological responses during a noxious stimulus, which may have important clinical implications.
References
al’Absi M, Buchanan T, Lovallo WR (1996) Pain perception and cardiovascular responses in men with positive parental history for hypertension. Psychophysiology 33:655–661
al’Absi M, Buchanan TW, Marrero A, Lovallo WR (1999) Sex differences in pain perception and cardiovascular responses in persons with parental history for hypertension. Pain 83:331–338
Armstrong HG, Rafferty JA (1950) Cold pressor test follow-up study for seven years on 166 officers. Am Heart J 39:484–490
Bijur PE, Latimer CT, Gallagher EJ (2003) Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med 10:390–392
Bragdon EE, Light KC, Girdler SS, Maixner W (1997) Blood pressure, gender, and parental hypertension are factors in baseline and poststress pain sensitivity in normotensive adults. Int J Behav Med 4:17–38
Bruehl S, Chung OY (2004) Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev 28:395–414
Burns JW, Quartana PJ, Bruehl S, Janssen I, Dugan SA, Appelhans B, Matthews KA, Kravitz HM (2015) Chronic pain, body mass index and cardiovascular disease risk factors: tests of moderation, unique and shared relationships in the study of women’s health across the nation (SWAN). J Behav Med 38:372–383
Burton AR, Birznieks I, Bolton PS, Henderson LA, Macefield VG (2009) Effects of deep and superficial experimentally induced acute pain on muscle sympathetic nerve activity in human subjects. J Physiol 587:183–193
Calhoun DA, Mutinga ML (1997) Race, family history of hypertension, and sympathetic response to cold pressor testing. Blood Press 6:209–213
Carroll D, Davey Smith G, Sheffield D, Willemsen G, Sweetnam PM, Gallacher JE, Elwood PC (1996) Blood pressure reactions to the cold pressor test and the prediction of future blood pressure status: data from the Caerphilly study. J Hum Hypertens 10:777–780
Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG (2001) Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol 1985(91):1679–1686
Curry TB, Charkoudian N (2011) The use of real-time ultrasound in microneurography. Auton Neurosci Basic Clin 162:89–93
D’Antono B, Ditto B, Rios N, Moskowitz DS (1999) Risk for hypertension and diminished pain sensitivity in women: autonomic and daily correlates. Int J Psychophysiol 31:175–187
Delius W, Hagbarth KE, Hongell A, Wallin BG (1972) General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84:65–81
Ditto B, France J, France CR (1997) Risk for hypertension and pain sensitivity in women. Int J Behav Med 4:117–130
Dworkin RH, Bruehl S, Fillingim RB, Loeser JD, Terman GW, Turk DC (2016) Multidimensional diagnostic criteria for chronic pain: introduction to the ACTTION-American Pain Society Pain Taxonomy (AAPT). J Pain 17:T1–T9
Edwards L, McIntyre D, Carroll D, Ring C, France CR, Martin U (2003) Effects of artificial and natural baroreceptor stimulation on nociceptive responding and pain. Psychophysiology 40:762–769
El Sayed K, Macefield VG, Hissen SL, Joyner MJ, Taylor CE (2016) Rate of rise in diastolic blood pressure influences vascular sympathetic response to mental stress. J Physiol 594:7465–7482
Fayaz A, Ayis S, Panesar SS, Langford RM, Donaldson LJ (2016) Assessing the relationship between chronic pain and cardiovascular disease: a systematic review and meta-analysis. Scand J Pain 13:76–90
Fazalbhoy A, Birznieks I, Macefield VG (2012) Individual differences in the cardiovascular responses to tonic muscle pain: parallel increases or decreases in muscle sympathetic nerve activity, blood pressure and heart rate. Exp Physiol 97:1084–1092
France C, Adler PS, France J, Ditto B (1994) Family history of hypertension and pain during blood donation. Psychosom Med 56:52–60
France CR (1999) Decreased pain perception and risk for hypertension: considering a common physiological mechanism. Psychophysiology 36:683–692
Ghione S (1996) Hypertension-associated hypalgesia. Evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension 28:494–504
Godfrey KM, Strachan E, Dansie E, Crofford LJ, Buchwald D, Goldberg J, Poeschla B, Succop A, Noonan C, Afari N (2014) Salivary cortisol and cold pain sensitivity in female twins. Ann Behav Med Publ Soc Behav Med 47:180–188
van Hecke O, Hocking L, Torrance N (2017) Chronic pain, depression and cardiovascular disease linked through a shared genetic predisposition: analysis of a family-based cohort and twin study. PloS One 12:1–19
Incognito AV, Doherty CJ, Lee JB, Burns MJ, Millar PJ (2018) Interindividual variability in muscle sympathetic responses to static handgrip in young men: evidence for sympathetic responder types? Am J Physiol Regul Integr Comp Physiol 314:R114–R121
Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research [Online]. National Academies Press (US). http://www.ncbi.nlm.nih.gov/books/NBK91497/. Accessed 30 Aug 2017
Kasagi F, Akahoshi M, Shimaoka K (1995) Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension 1979(25):71–76
Knudsen LF, Terkelsen AJ, Drummond PD, Birklein F (2019) Complex regional pain syndrome: a focus on the autonomic nervous system. Clin Auton Res 29:457–467
Kregel KC, Seals DR, Callister R (1992) Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol 454:359–371
Lara-Cinisomo S, Grewen KM, Girdler SS, Wood J, Meltzer-Brody S (2017) Perinatal depression, adverse life events, and hypothalamic–adrenal–pituitary axis response to cold pressor stress in latinas: an exploratory study. Women’s Health Issues 27:673–682
Latremoliere A, Woolf CJ (2009) Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10:895–926
Maixner W, Gracely RH, Zuniga JR, Humphrey CB, Bloodworth GR (1990) Cardiovascular and sensory responses to forearm ischemia and dynamic hand exercise. Am J Physiol 259:R1156–R1163
Matsukawa T, Gotoh E, Uneda S, Miyajima E, Shionoiri H, Tochikubo O, Ishii M (1991) Augmented sympathetic nerve activity in response to stressors in young borderline hypertensive men. Acta Physiol Scand 141:157–165
Matthews KA, Woodall KL, Allen MT (1993) Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension 1979(22):479–485
McIver TA, Kornelsen J, Stroman PW (2018) Diversity in the emotional modulation of pain perception: an account of individual variability. Eur J Pain 22:319–332
Menkes MS, Matthews KA, Krantz DS, Lundberg U, Mead LA, Qaqish B, Liang KY, Thomas CB, Pearson TA (1989) Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension 1979(14):524–530
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474
Moralez G, Jouett NP, Tian J, Zimmerman MC, Bhella P, Raven PB (2018) Effect of centrally acting angiotensin converting enzyme inhibitor on the exercise-induced increases in muscle sympathetic nerve activity. J Physiol 596:2315–2332
Nordin M, Fagius J (1995) Effect of noxious stimulation on sympathetic vasoconstrictor outflow to human muscles. J Physiol 489(Pt 3):885–894
Nordin M, Fagius J, Waldenlind E (1997) Sympathetic vasoconstrictor outflow to extremity muscles in cluster headache. Recordings during spontaneous and nitroglycerin-induced attacks. Headache 37:358–367
Normand E, Potvin S, Gaumond I, Cloutier G, Corbin J-F, Marchand S (2011) Pain inhibition is deficient in chronic widespread pain but normal in major depressive disorder. J Clin Psychiatry 72:219–224
Olsen RB, Bruehl S, Nielsen CS, Rosseland LA, Eggen AE, Stubhaug A (2013) Hypertension prevalence and diminished blood pressure-related hypoalgesia in individuals reporting chronic pain in a general population: the Tromsø study. Pain 154:257–262
Randich A, Maixner W (1984) Interactions between cardiovascular and pain regulatory systems. Neurosci Biobehav Rev 8:343–367
Saccò M, Meschi M, Regolisti G, Detrenis S, Bianchi L, Bertorelli M, Pioli S, Magnano A, Spagnoli F, Giuri PG, Fiaccadori E, Caiazza A (2013) The relationship between blood pressure and pain. J Clin Hypertens 15:600–605
Santa Ana EJ, Saladin ME, Back SE, Waldrop AE, Spratt EG, McRae AL, LaRowe SD, Timmerman MA, Upadhyaya H, Brady KT (2006) PTSD and the HPA axis: differences in response to the cold pressor task among individuals with child vs. adult trauma. Psychoneuroendocrinology 31:501–509
Schobel HP, Ringkamp M, Behrmann A, Forster C, Schmieder RE, Handwerker HO (1996) Hemodynamic and sympathetic nerve responses to painful stimuli in normotensive and borderline hypertensive subjects. PAIN® 66:117–124
Schwabe L, Haddad L, Schachinger H (2008) HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology 33:890–895
Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS (1995) When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 61:277–284
Steptoe A, Marmot M (2005) Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. J Hypertens 23:529–536
Stewart KM, France CR (1996) Resting systolic blood pressure, parental history of hypertension, and sensitivity to noxious stimuli. Pain 68:369–374
Swieboda P, Filip R, Prystupa A, Drozd M (2013) Assessment of pain: types, mechanism and treatment. Ann Agric Environ Med AAEM Spec 1:2–7
Thomas CB, Duszynski KR (1982) Blood pressure levels in young adulthood as predictors of hypertension and the fate of the cold pressor test. Johns Hopkins Med J 151:93–100
Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55:377–391
Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG (1979) Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59:919–957
Vardeh D, Mannion RJ, Woolf CJ (2016) Toward a mechanism-based approach to pain diagnosis. J Pain 17:T50–T69
Victor RG, Leimbach WN, Seals DR, Wallin BG, Mark AL (1987) Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 1979(9):429–436
Villemure C, Bushnell MC (2002) Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain 95:195–199
White DW, Shoemaker JK, Raven PB (2015) Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci Basic Clin 193:12–21
Wood DL, Sheps SG, Elveback LR, Schirger A (1984) Cold pressor test as a predictor of hypertension. Hypertension 1979(6):301–306
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152:S2–15
Woolf CJ, Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288:1765–1769
Zhao Q, Gu D, Lu F, Mu J, Wang X, Ji X, Hu D, Ma J, Huang J, Li J, Chen J, Cao J, Chen C-S, Chen J, Rice TK, He J (2015) Blood pressure reactivity to the cold pressor test predicts hypertension among Chinese adults: the GenSalt study. Am J Hypertens 28:1347–1354
Acknowledgements
We are grateful to the study volunteers for their participation. This study was supported, in part, by an AHA Grant-In-Aid (13GRNT16990064), the Harry S. Moss Heart Trust, and Department of Defense—US Army grant (W81XWH1820012). JPM was supported by a British Heart Foundation Travel Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any financial or personal conflict of interest to disclose.
Rights and permissions
About this article
Cite this article
Huang, M., Yoo, JK., Stickford, A.S.L. et al. Early sympathetic neural responses during a cold pressor test linked to pain perception. Clin Auton Res 31, 215–224 (2021). https://doi.org/10.1007/s10286-019-00635-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-019-00635-7