Abstract
Purpose
To assess the impact of obesity and population attributes on the circadian pattern of cardiac autonomic modulation (CAM) in a population-based sample of adolescents.
Methods
We used data from 421 adolescents who completed the follow-up exam in the Penn State Children Cohort study. CAM was assessed by heart rate variability (HRV) analysis of beat-to-beat, normal R–R intervals from a 24-hour ECG, on a 30-minute basis. The HRV indices included frequency-domain (HF, LF, and LF/HF ratio) and time-domain (SDNN, RMSSD, and HR) variables. Nonlinear mixed-effect models were used to calculate a cosine periodic curve, each having three parameters quantifying its circadian period: M (mean levels of the HRV variables), Â (amplitude of the oscillation), and θ (the time of the highest oscillation).
Results
The mean (SD) age was 16.9 (2.2) years, with 54 % male and 77 % white. The mean BMI percentile was 66, with 16 % obese (BMI percentile ≥ 95). Overall, HF (a marker of parasympathetic modulation) gradually increased from the late afternoon, reached peak amplitude around 3 a.m., and then decreased throughout the daytime until late afternoon. In contrast, obesity had adverse effects on all circadian parameters. The age, sex and race showed varying differences on the CAM circadian parameters. The adjusted means (95 %Cls) of M, Â, and θ for HF were 5.99 (5.79–6.19), 0.77 (0.66–0.89), 3:15 (2:15–4:15) a.m., and 6.21 (6.13–6.29), 0.66 (0.61–0.70), 2:45 (2:30–3:15) a.m. for obese and non-obese subjects, respectively.
Conclusion
The circadian pattern of CAM can be quantified by the three cosine parameters. Obesity is associated with lower HRV even in young individuals like children/adolescents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases (CVDs) in children are becoming more prevalent in conjunction with the rise in childhood obesity [1]. Obese children are more likely to become obese adults, enhancing their risk of developing CVD. In fact, by the year of 2035, the number of additional cardiovascular events attributable to obesity in adolescence is expected to be >100,000 [2]. In addition, childhood obesity is often accompanied by cardiovascular abnormalities, suggesting the importance of immediate attention to prevent cardiovascular damage at a young age [3].
Heart rate variability (HRV) is used as a noninvasive measurement of cardiac autonomic modulation (CAM) [4]. The balance of sympathetic and parasympathetic modulation regulates HRV. Lower HRV has been associated with CVD morbidity and mortality [5]. In recent years, several epidemiological studies have reported evidence of impaired CAM in obese children, finding a reduction of parasympathetic activity of HRV in the obese as compared to the non-obese [6–10]. Nevertheless, previous findings from our group also demonstrated an impaired CAM towards sympathetic overflow and reduced parasympathetic modulation in obese children [9]. However, these studies examined the effect of obesity on the overall mean levels of HRV without studying its impact on the circadian pattern of HRV.
The circadian pattern of HRV has been described in adults [11–15] and children [16–22]. It can be quantified using a cosine periodic regression model consisting of three cosine function parameters: mean (M), mean levels of a HRV index; amplitude (Â), amplitude of oscillation; and acrophase (θ), the clock time of the highest oscillation. Lack of circadian variation of HRV has been associated with increased risk to cardiovascular events in adults [13]. Previous studies have reported the cosine pattern of HRV in children and young adults at the group-level time-specific average of HRV index [15, 16]. However, they did not account for between-subjects variability or provide reference ranges, e.g., 95 % (CIs) of the cosine parameters. To our knowledge, there is no study examining the effect of obesity on the circadian pattern of HRV, using the cosine function parameters M, Â, and θ, in adolescents from the general population.
Thus, this study has three objectives: to obtain quantitative measures of the HRV circadian pattern and their normative ranges for the three cosine parameters, to evaluate the influence of population attributes on the circadian pattern of HRV, and to examine the effect of obesity on the circadian pattern of HRV in a population-based sample of adolescents.
Methods
Study population
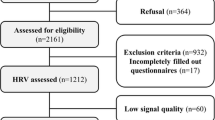
We used available data from 421 adolescents who completed the follow-up examination of the Penn State Children Cohort (PSCC) study. The recruitment and examination procedures for the baseline study have been published elsewhere [18]. During 2010–2013, a follow-up examination for 700 participants from phase II of PSCC was conducted. Among these subjects, 421 of them completed the follow-up with a response rate of 60 % and a mean follow-up time of 7.7 years. The loss to follow-up was mainly due to subjects moving out of central Pennsylvania. However, no major difference in baseline characteristics was observed between subjects who did or did not participate. During the study period, participants were evaluated overnight in the Clinical Research Center at the Pennsylvania State University College of Medicine (COM) for the following: a complete physical examination; a whole body dual-energy X-ray absorptiometry; a high-fidelity Holter ECG monitor to measure beat-to-beat ECG for 39 hours; a 9-hour fixed-time PSG recording; and overnight fasting blood, saliva, and urine samples. After being released from the overnight stay, they were given the following: an 8-day actigraph; a set of questionnaires about habitual behavior; and an activity log. The study protocol was approved by the Pennsylvania State University COM IRB. Written informed consents were obtained from participants and their parents if the participant was a minor (<18 years old).
Classification of obesity
Participants were weighted with a standard weight scale during their visit. Standing height without shoes was measured with a standard height scale. Body mass index (BMI) was calculated by dividing weight (kg) by height (m2), and age- and sex-adjusted BMI percentile was calculated based on the 2000 CDC Growth Charts. For this particular analysis, obesity was defined as a binary variable (non-obese = BMI percentile < 95th, and obese = BMI percentile ≥ 95th).
Continuous ECG recording
A high-fidelity (sampling frequency 1,000 Hz) 12-lead HScribe Holter System (Mortara Instrument, Inc., Milwaukee, WI) was used for 39-hour beat-to-beat ECG data collection. All recordings started between 5 p.m. and 7 p.m. The standardized operation procedures for the PSCC study were followed in the data collection, offline processes, HRV analysis, and interpretation processes. We followed the Holter ECG data collection and analysis procedures previously described [19], but using a 39-hour recording instead of a 24 hour recording.
CAM measures
Out of the 39-hour Holter beat-to-beat ECG data, the first 24 hours (7 p.m. of day-1–7 p.m. of day-2) were used for analysis. The normal beat-to-beat RR interval data were divided into 30-minute segments of RR data. Thus, each individual provided 48 segments of 30-minute RR data. The RR data for HRV analysis were processed according to current recommendations [19]. The time- and frequency-domain HRV analyses were performed on the normal RR interval data if their total length was > 22.5 min (75 % of original data), using the HRV Analysis Software v1.1 [20]. For the frequency-domain HRV analysis we used Fast Fourier Transformation (FFT) previously described [14]. The following HRV indices were calculated as measures of CAM: high-frequency range (0.15–0.40 Hz, HF), low-frequency range (0.04–0.15 Hz, LF), the ratio of LF to HF (LF/HF), standard deviation of all RR intervals (SDNN, ms), and the square root of the mean of the sum of the squares of differences between adjacent RR intervals (RMSSD, ms). For statistical analysis, HF and LF were logarithmic transformed.
Covariables
A standardized questionnaire was used for the following individual-level information: demographic variables; medication uses; and physician diagnosed chronic disease history. For the purpose of this paper, age was used as a continuous variable; gender (female = 0 and male = 1) and race (non-Hispanic white = 1 and non-white = 0) were used as binary variables.
Statistical analysis
Mean (SD) and proportions were used to describe the study population. After excluding individuals who did not have sufficient ECG data, 388 individuals contributed up to 48 segments of 30-minute RR interval data. Thus, we analyzed 18,624 segments in total. A two-stage analysis was performed to assess the relationship of obesity and population attributes on the circadian pattern of HRV. At stage 1, for each individual, we fit the HRV data based on all 48 segments to a cosine periodic regression model [21] using nonlinear least squares:
where M i = average of HRV of the ith subject, A i = amplitude of HRV of the ith subject around M i , t = time-specific segment order number (one unit of t = 30 min, with t = 1 indicating 7 p.m.–7:30 p.m., 2 indicating 7:30 p.m.–8 p.m., etc.), T = 48 segments, θ i = lag from the reference time point (7 p.m.) to the time of the zenith of the cosine curve fit to the data of the ith subject, and ε i = error term of the ith subject. Thus, from the above cosine model, we obtained the estimated individual-level cosine periodic regression parameters (M, Â, and θ) to quantify the periodicity of the HRV variables. At stage 2, we used random-effect meta-analyses to obtain overall estimates of the three parameters and 95 % (CIs) to test the associations [22]. To visualize the gender difference on these three parameters, we plotted the predicted HF based on gender. We also plotted the predicted HF in the entire sample, and stratified by obesity status to illustrate the impact of obesity. For the analysis on the effect of obesity on each cosine parameters of HRV, six adolescents with type 2 diabetes were excluded. All analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, N.C. USA).
Results
Characteristics of study population
From the PSCC study follow-up, 390 participants completed the Holter recording for 39 hours, two were excluded due to insufficient ECG data for the HRV analysis. The effective sample size for this study was 388 children (92 % of the original 421 children). The characteristics of the study population as well as stratification by gender are presented in Table 1. The prevalence of obesity was 16 %. The mean (SD) BMI percentile was 66 (28). All HRV indices, with the exception of HR, were lower in females than males.
Circadian pattern of HRV variables
The overall means of the three cosine parameters and their 95 % (CIs) for each of the HRV indices from the entire sample are presented in Table 2. The acrophases of HF and RMSSD were similar (around 3 a.m.), and that of LF and SDNN were similar (around 6 a.m.). The acrophases of LF/HF and HR were similar (around 1 p.m.). The tests of the amplitude (Hu: Â = 0) were highly significant for each HRV variable, suggesting a significant circadian variation.
Population attributes and HRV circadian pattern
The association between each cosine parameter and population attributes is given in Table 3. Older age was significantly associated with higher M for LF/HF and lower M for HR; with higher Âs of HF, LF/HF, RMSSD; and with higher θ for LF/HF and HR. The between-gender comparison of the Ms indicated no significant gender difference, except for LF, SDNN, and HR. The M was significantly higher in males than in females for LF and SDNN, and lower for HR. For between-gender comparisons of the Âs, males showed higher fluctuation amplitude of LF and SDNN. The between-gender comparison of the θs indicated no significant gender difference, except for HF. To visualize the gender influence on the HRV circadian pattern, we presented a circadian curve of HF by gender in Fig. 1, which clearly illustrated that for both genders HF gradually increases from late afternoon throughout the evening, reaching the highest oscillations and then gradually decreasing throughout most of the daytime until late afternoon. However, males had later θ of HF, about 1 h later than females. Non-Hispanic whites had significantly higher M for LF, and higher θ of SDNN, suggesting that their θ of SDNN was significantly delayed by over 1 h when compared to non-whites. No significant ethnicity difference in the Âs of any HRV variable was found.
Obesity and HRV circadian pattern
The adjusted means of the cosine parameters and their 95 % (CIs) for each HRV index from the entire sample as well as stratified by obesity status are presented in Table 4. In general, obesity was significantly associated with lower M of HF, LF, SDNN, and higher HR; and marginally related to higher LF/HF. No significant association was found on RMSSD. Obesity was significantly associated with higher  of LF and marginally associated with higher  of HF and with lower θ of HR, suggesting that θ was 30 min earlier in obese than non-obese adolescents.
Figure 2 presents the circadian patterns of HF from the entire population, as stratified by obesity status and adjusted by major covariables. The predicted circadian pattern of HF in the entire sample showed the parasympathetic modulation gradually increasing from late afternoon throughout the evening, reaching θ at 3 a.m., and gradually decreasing throughout most of the daytime until late afternoon. For obesity status, it shows the same patterns on the parasympathetic modulation as the entire sample. Obese adolescents had later θ in HF, about 30 min later than non-obese adolescents, even after adjusting for major covariables.
Circadian curves of estimated HF for overall population and by obesity status. Thick line Overall (adjusted for age, race, and sex): β(M) = 6.17; β(A) = 0.67; β(θ) = 03:00, all p < 0.05. By Obesity (adjusted for age, race, and sex): Dashed line No: β(M) = 6.21; β(A) = 0.66; β(θ) = 02:45. Dashed dotted line Yes: β(M) = 5.99; β(A) = 0.78; β(θ) = 03:15. p value for the difference: P_ M = 0.02; P_ A = 0.06; P_ θ = 0.36
Discussion
This is the first study to define the circadian pattern of HRV and provide its normative ranges in a population-based sample of adolescents. Furthermore, we demonstrated that male and older adolescents have delayed a circadian pattern of HRV, while ethnicity appears to play a less relevant role. Importantly, we show that adolescent obesity is associated with an overall impairment of HRV as well as with a shift in its circadian pattern. These findings support the need to address obesity in earlier stages of life to prevent CVD.
We first quantitatively describe the HRV circadian pattern using a cosine periodic regression model, and obtain the normative range of the three cosine parameters. Our results shown in Table 2 demonstrated that the circadian patterns of adolescents can be captured by these cosine parameters. In our population, HF, LF, SDNN, and RMSSD variables follow similar circadian pattern (Fig. 2)—increasing in the evening, reaching the acrophase highest level early morning, and then decreasing during the morning until reaching the lowest levels in the afternoon. On the other hand, LF/HF and HR variables showed an opposite diurnal pattern—decreasing during the evening, reaching the lowest levels after midnight, then increasing in the morning until reaching the acrophase in the afternoon, and then turning downwards again. In addition, the tests of non-zero amplitude, suggesting a significant day–night circadian variation, were highly significant for every HRV variable.
The circadian pattern of CAM in healthy adults has been previously quantitatively described using the three cosine parameters [14, 15]. To our knowledge, there is one previous study in children that examined the circadian pattern of HRV as a cosine function [16]. They identified a significant circadian variation in HRV from late infancy, characterized by a rise during sleep. However, they used time-specific averages from the sample and estimated the cosine parameters from there. In contrast, we used a two-stage analysis, allowing us to capture the individual variation of HRV over time per participant and the between-individual differences in the circadian variation at any time point, and to provide 95 % (CIs) for each cosine parameter. Compared to adults [14], adolescents have higher M levels of HRV indices, except for LF/HF, indicative of a healthier CAM profile, approximately 30 min earlier θ on parasympathetic HRV indices, and around 1-h delay of the θ on the sympathetic HRV indices. Our findings can be used to pursue studies on the CAM effects of various CVD risk factors (e.g., obesity).
We examine the influences of the population attributes on the cosine parameters of HRV. Our findings suggest that males have significantly higher Ms of LF and SDNN, lower Ms of HR, higher  of HF and SDNN, and delayed θ (1 h) in HF than females. Available reports in adolescents on gender differences on HRV are controversial, reporting females with significantly lower [23, 24] or greater [25, 26] values of HRV than males. These inconsistences in the findings from different studies could be due to confounding factors such as data collection, HRV recording time, hormonal differences between genders, and menstrual cycle in females. However, similar to our findings, a study in adolescents found that males have higher SDNN than females [24]. To our knowledge, we are the first group examining the influence of gender on  and θ in adolescents. These findings are similar to previous report from our group showing that middle-age males have higher  of HRV than females, and that the θ for LF/HF is significantly delayed by about 3 h in males [14]. Furthermore, in our population, the impact of older age on higher LF/HF and lower HF Ms is consistent with previous studies in children [24, 26] and adults [14]. With increased age we found higher  for HF and RMSSD, and higher θ for LF/HF and HR. No data in adolescent are available on the effect of age on Â, and θ. We did not find significant ethnic effects on the cosine parameters; however, θ for SDNN was significantly delayed by 1 h in non-Hispanic whites as compared to non-whites. The influence of ethnicity on HRV in children has been inconsistent [23, 24, 26], with studies reporting that African American youth have lower HRV [23], Asians have higher LF/HF [26], or that there are no significant ethnic differences [27]. Although we were not able to find any significant ethnic differences on  and θ, we are the first group analyzing the ethnicity and these parameters on adolescents.
Based on recent reports that childhood obesity increases the risk of CVD, we further examined the effect of obesity on the three cosine parameters. After adjustment for major covariables, obesity was significantly associated with lower Ms for HF, LF, and SDNN, and higher Ms for LF/HF and HR. Our results are consistent with previous reports studying CAM in obese children, indicating a significant reduction in HRV [7–9]. In terms of the circadian pattern of HRV, obesity was significantly associated with higher  of LF, and the θ of HR in obese adolescents was 30 min earlier than in non-obese adolescents. Furthermore, Fig. 2 demonstrates that obese adolescents have later θ than non-obese adolescents. To our knowledge, the effects of obesity in healthy adolescents on the circadian  and θ of HRV have not been reported in the literature, making this the first study examining the effects of childhood obesity on these parameters of HRV. This is of importance since we know that impaired CAM is a predictor of CVD morbidity and mortality in adults [28–30]. Thus, evaluating these parameters of CAM could help in the early identification of children/adolescents at risk of CVD.
To obtain the normal circadian pattern of HRV in our sample of adolescents, we excluded individuals with/or at risk for diabetes from the obesity and cosine parameters analysis. Therefore, the generalizability of our data to individuals with diabetes may be limited. Another limitation is that we cannot establish a temporal relationship from our data. Thus, a longitudinal study is needed to investigate the effect of obesity on the circadian parameters of CAM. Also, we did not account for the influence of hormones on gender differences. In addition, for this particular analysis, we did not account for central obesity measurements like waist circumference and waist-to-hip ratio. However, several strengths are worth mentioning. The 24-h ECG data was acquired at daytime and nighttime. In humans, the autonomic activity shows a circadian rhythm towards a prevalence of parasympathetic activity during the night and a prevalence of sympathetic activity during the day. Therefore, the HRV indices collected are representative of day–nighttime HRV. Distinguished from previous publication, which primarily analyzed short-time HRV variables in children [19], we utilized cosine parameters to fit individual-level models to 24 h of HRV data to get individual-level M, Â and θ, of each HRV variable. We were able to collect a long duration of continuous ECG recording, and successfully acquired HRV analysis on 92 % of the follow-up PSCC sample.
In this population-based sample of healthy adolescents, the HRV can be quantified not only by the mean HRV values, but also by the circadian pattern of HRV through cosine periodic parameters like Â, and θ. The HRV variables exhibit a distinct circadian rhythm over a 24-h period. Furthermore, population attributes are associated with the HRV cosine circadian parameters. Finally, our data showed that general obesity is associated with lower HRV even in young individuals like children/adolescents; demonstrating that obesity-associated alterations in cardiovascular autonomic activity are already occurring in healthy young adolescents.
References
Crowley DI, Khoury PR, Urbina EM, Ippisch HM, Kimball TR (2011) Cardiovascular impact of the pediatric obesity epidemic: higher left ventricular mass is related to higher body mass index. J Pediatr 158(709–714):e1
Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L (2007) Adolescent overweight and future adult coronary heart disease. N Engl J Med 357:2371–2379
Koopman LP, McCrindle BW, Slorach C et al (2012) Interaction between myocardial and vascular changes in obese children: a pilot study. J Am Soc Echocardiogr 25(401–410):e1
Kleiger RE, Stein PK, Bigger JT (2005) Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 10:88–101
Liao D, Barnes RW, Chambless LE, Simpson RJ Jr, Sorlie P, Heiss G (1996) Population based study of heart rate variability and prevalent myocardial infarction—The ARIC Study. J Electrocardiol 29:189–198
Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM (2013) Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol 62(15):1309–1319
Tonhajzerova I, Javorka M, Trunkvalterova Z et al (2008) Cardio-respiratory interaction and autonomic dysfunction in obesity. J Physiol Pharmacol 59(6):709–718
Vanderlei LC, Pastre CM, Freitas IF Jr, Godoy MF (2010) Analysis of cardiac autonomic modulation in obese and eutrophic children. Clinics 65:789–792
Rodriguez-Colon SM, Bixler EO, Li X, Vgontzas AN, Liao D (2011) Obesity is associated with impaired cardiac autonomic modulation in children. Int J Pediatr Obes 6:128–134
Lazarova Z, Tonhajzerova I, Trunkvalterova Z et al (2009) Baroreflex sensitivity is reduced in obese normotensive children and adolescents. Can J Physiol Pharmacol 87:565–571
Li X, Shaffer ML, Rodríguez-Colón SM et al (2011) Systemic inflammation and circadian rhythm of cardiac autonomic modulation. Auton Neurosci 162:72–76
Rodríguez-Colón SM, Li X, Shaffer ML et al (2010) Insulin resistance and circadian rhythm of cardiac autonomic modulation. Cardiovasc Diabetol 9:85
Kardelen F, Akcurin G, Ertug H, Akcurin S, Bircan I (2006) Heart rate variability and circadian variations in type 1 diabetes mellitus. Pediatr Diabetes 7:45–50
Li X, Shaffer ML, Rodriguez-Colon S et al (2011) The circadian pattern of cardiac autonomic modulation in a middle-aged population. Clin Auton Res 21(3):143–150
Nakagawa M, Iwao T, Ishida S et al (1998) Circadian rhythm of the signal averaged electrocardiogram and its relation to heart rate variability in healthy subjects. Heart 79:493–496
Massin MM, Maeyns K, Withofs N, Ravet F, Gerard P (2000) Circadian rhythm of heart rate and heart rate variability. Arch Dis Child 83:179–182
Bilan A, Witczak A, Palusiński R, Myśliński W, Hanzlik J (2005) Circadian rhythm of spectral indices of heart rate variability in healthy subjects. J Electrocardiol 38(3):239–243
Bixler EO, Vgontzas AN, Lin HM et al (2008) Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension 52(5):841–846
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93:1043–1065
Niskanen JP, Tarvainen MP, Ranta-Aho PO, Karjalainen PA (2004) Software for advanced HRV analysis. Comput Methods Programs Biomed 76(1):73–81
D’Negri CE, Marelich L, Vigo D et al (2005) Circadian periodicity of heart rate variability in hospitalized angor patients. Clin Auton Res 15:223–232
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Faulkner MS, Hathaway D, Tolley B (2003) Cardiovascular autonomic function in healthy adolescents. Heart Lung 32(1):10–22
Wang X, Thayer JF, Treiber F, Snieder H (2005) Ethnic differences and heritability of heart rate variability in African- and European American youth. Am J Cardiol 96(8):1166–1172
Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ (2004) Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol 93:381–385
Reed KE, Warburton DE, Whitney CL, McKay HA (2006) Differences in heart rate variability between Asian and Caucasian children living in the same Canadian community. Appl Physiol Nutr Metab 31(3):277–282
Franke WD, Lee K, Buchanan DB, Hernandez JP (2004) Blacks and whites differ in responses, but not tolerance, to orthostatic stress. Clin Auton Res 14:19–25
Liao D, Sloan RP, Cascio WE et al (1998) The multiple metabolic syndrome is associated with lower heart rate variability – The ARIC Study. Diabetes Care 21:2116–2222
Dekker JM, Crow RS, Folsom AR et al (2000) Low heart rate variability in a two minute rhythm strip predicts risk of coronary heart disease and mortality from several causes—The ARIC Study. Circulation 102:1239–1244
Liao D, Carnethon MR, Evans GW, Cascio WE, Heiss G (2002) Lower Heart Rate Variability Is Associated with the Development of Coronary Heart Disease in Individuals with Diabetes - The ARIC Study. Diabetes 51:3524–3531
Acknowledgments
The authors gratefully acknowledge the adolescents and their parents who participated in this study. This project was supported by National Institutes of Health (NIH) Grants: 1 R01 HL 097165, R01 HL63772, R21HL087858, and the Penn State CTSI Grant UL Tr000127. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez-Colón, S., He, F., Bixler, E.O. et al. The circadian pattern of cardiac autonomic modulation and obesity in adolescents. Clin Auton Res 24, 265–273 (2014). https://doi.org/10.1007/s10286-014-0257-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-014-0257-7