Abstract
One of the goals of oral healthcare management is to manage dry mouth. Thus, moisturizers containing antimicrobial ingredients, such as hinokitiol (HT), are applied to the oral mucosa after oral care. In this study, we investigated the preventive effect of HT against the growth of Candida albicans (C. al) and its synergistic effect when combined with miconazole (MCZ), an oral treatment for candidiasis. As the concentration of HT increased, the length and percentage of germ tubes (GT) decreased. Larger inhibition circles were observed for MCZ concentrations of 2.0 and 4.0 μg/disc compared to the HT medium without HT. The increased inhibitory effect was observed in both aerobic and anaerobic cultures. This suggests that the production of reactive oxygen species (ROS) by C. al cells increased with the combination of HT and MCZ. The length and percentage of GT increased, whereas the amount of ROS decreased when ROS scavengers were used in combination with the drug. HT led to morphological changes that inhibited the GT associated with pathogenic C. al, exhibited a complementary action against MCZ, and showed a possible association with hydrogen peroxide and superhydroxy anion radicals. These effects suggest that HT is a promising candidate for inhibiting C. al. In conclusion, HT demonstrated a prophylactic effect by inhibiting C. al and a synergistic effect with MCZ, a drug used to treat oral candidiasis. HT may also be useful for suppressing the onset and reducing the severity of oral candidiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reducing disease outbreaks and preventing serious illnesses have recently been the focus in healthcare. Oral hygiene management has been actively implemented in hospitals and care facilities to prevent respiratory complications, such as aspiration pneumonia and ventilator-associated pneumonia, as well as oral mucositis in long-term care for older adults, chemotherapy, and bone marrow transplant patients [1,2,3,4,5,6,7]. One of the goals of oral hygiene management is to manage dry mouth; thus, moisturizers are applied to the oral mucosa after rinsing. Moisturizers include antibacterial ingredients, such as hinokitiol (HT), which are used to treat terminally ill patients with severe dry mouth, older immunosuppressed patients, and bedridden patients in long-term care [8,9,10,11].
HT is a tropolone monoterpene obtained from Chamaecyparis obtusa var. formosana (Formosan cypress). HT has multiple pharmacological effects, such as anti-inflammatory, antioxidant, antitumor, antibacterial, and antiviral properties [12,13,14,15,16,17]. Therefore, HT is incorporated in oral hygiene products against periodontitis prevention, food preservation, and cosmetics and has remarkable biological safety features. Particularly, nearly all the commercial oral hygiene products containing HT are fixed-dose combinations with the other antimicrobial agents dispersed in a base material, such as 4-isopropyl-3-methlphenol [18]. All these mixed drugs (gel or liquid agents) contain base agents (ointment or suppository) without exceptions. Bianchi et al. demonstrated the usefulness of the commercial oral hygiene products, particularly gel agents containing amine fluoride against caries, periodontitis, and peri-implantitis [19]. However, analyzing the active principal component of the fixed-dose combination is very challenging. Further, we noted that the loss of gel substrate with antimicrobial agent on the mouse tongue mucous membrane might enhance Candida albicans (C. al) adhesion in experimental oral candidiasis mice. Thus, we first investigated the antifungal effects of HT alone or the combination with miconazole (MCZ) because the precise mechanism of the inhibition and interaction of the antifungal effects of HT remains uncertain.
Oral candidiasis is an opportunistic infection primarily caused by Candida albicans (C. al). The infection is not limited to the oral cavity and can spread to the upper respiratory tract and bloodstream, leading to a deep-seated mycosis. This condition is refractory, has a high mortality rate, and can significantly affect the life expectancy of patients [20, 21]. Salivary gland dysfunction, drugs, dentures, diabetes mellitus, malignancy, and immunosuppressed states predispose individuals to oral candidiasis. Oral candidiasis has a favorable prognosis with proper antifungal therapy, although attaining asepsis for mycological eradication poses a challenge [20, 22]. Furthermore, oral candidiasis can present with various clinical manifestations, rendering it susceptible to misdiagnosis, difficulty in treating the underlying host cause, and risk of relapse and poor control with the inappropriate use of antifungal agents, decreased drug sensitivity, and prevalence of azole-resistant strains over time [22, 23]. Prioritizing the prevention of oral Candida infections is crucial because of the risk of oral candidiasis transitioning to deep-seated mycosis [24]. Moreover, Wu et al. reported important findings concerning the association between C. al induced cerebral mycosis and Alzheimer’ disease [25]. Therefore, HT could potentially act as an efficient prophylactic agent. However, the basic research information concerning HT is not adequate to consider its use for preventing oral candidiasis from progressing to deep mycosis in immunocompromised and high-risk patients in the acute phase of hospitalization. Therefore, this study aimed to examine the preventive effects of HT on C. al and its effectiveness when administered alongside miconazole (MCZ), a treatment for oral candidiasis.
Materials and methods
Range of MCZ concentration settings used with HT
A preliminary experiment was conducted to investigate the minimum inhibitory concentrations (MIC50) of HT (Fujifilm Wako Pure Chemicals Corporation, Osaka, Japan) using the microliquid dilution method.
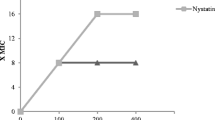
The dose response curve obtained by measurements (MIC50 = 7 μg/mL) performed 6 times is presented in Fig. 1a. Our study aimed to investigate the synergistic pharmacological effects of HT and MCZ (Sigma-Aldrich Co., LLC, St. Louis, MO, USA) against the ability of C. al (IFM40009:ATCC48130) to switch between yeast and hyphal growth forms (dimorphism). Therefore, the concentrations of HT alone that do not inhibit C. al cell proliferation were preferred for use in this study, because MIC50 or higher concentrations of HT may mask the fungicidal effects of MCZ using the both agents. Moreover, considering oral adaptation in clinical practice, the drug concentration (above MIC50) will be decreased due to dissolution and diffusion by saliva with the passage of time; thus, the effects of sub-MIC50 HT against C.al should be investigated. Figure 1b showed the dose response curve of MCZ alone or with 3.0 μg/mL HT obtained by measurements performed thrice. The synergistic effects were observed at the concentration area of MCZ (0.5–4 μg). Thus, we used HT (0.625–4.0 μg/mL) and MCZ (0.5–4.0 μg/mL) in this study.
Concentration setting range for HT and MCZ when HT and MCZ are used together. The concentration setting range for HT was set at 0.625–4 µg/mL, which is not related to cell proliferation against the C. al 40009 strain. *MIC50 = 7.0 μg/mL **HT concentration setting range (0.625–4 µg/mL) In the same way, for MCZ antifungal activity when HT and MCZ were used together, the concentration setting range for MCZ was set at 0.5–4 µg/mL, where the synergism was achieved*: MCZ concentration adjustment range (0.5–4.0 µg/mL). HT, hinokitiol; MCZ, miconazole
Change in germ tube (GT) formation following HT treatment
A loopful of C. al cells were inoculated into 100 mL PYG medium (1% peptone, HIPOLY PEPTON, 2% d(+)glucose, and 0.5% yeast extract, DRIED YEAST, Fujifilm Wako Pure Chemicals Corporation, Osaka, Japan) and incubated in a water bath with shaking (24 h, 37 °C, 140 rpm). After 24 h, the cells harvested from the PYG medium were washed with distilled water by centrifuging three times (5 min, 3000 rpm). The cells were then transferred into 100 mL sterile distilled water and shaken (4 h, 37 °C, 140 rpm) to starve the C. al cells. After starvation, the C. al cells were collected. The cell suspension was adjusted to achieve a final cell concentration of 5 × 106 cells/mL using a turbidimeter (Vi-Spec II PHOTO METER, Kyokuto Pharmaceutical Industries, Tokyo, Japan).
The cell suspension was mixed with 5 mL N-acetyl-D-glucosamine (Glc-NAc)-HEPES buffer (pH 6.8) to induce GT and divide it into microtubes. The experimental group contained 1.0, 2.0, 2.5, and 3.0 μg/mL of HT in each microtube, and the control group comprised sterile purified water in each microtube. The experimental and control cells were incubated at 37 °C with slow shaking (60 rpm), and a portion of the cells was collected at 0, 1, 2, 3, and 24 h. The collected GTs were fixed in 10% formalin and observed under a fluorescence microscope (all-in-one optical microscope; BZ-800; KEYNENCE, Osaka, Japan). When observed by fluorescence microscopy, the cells were stained with Fungiflora Y fluorescence stain for fungi (Fungiflora Y®, Trust Medical, Sendai, Japan).
The outcome measures were changes in GT length and ratio after 24 h, as measured using an all-in-one optical microscope. The experiment was repeated five times with different concentrations of HT; the representative GT formation following HT treatment (24 h) is shown in Fig. 2 and Tables 1 and 2.
Change in GT formation after 24 h as a function of HT concentration. The final cell concentration in the bacterial solution was adjusted to 5 × 106 cells/mL for starved C. al. The cells were mixed with 5 mM N-acetyl-d-glucosamine (Glc-NAC)-HEPES buffer (pH 6.8), which induces GT, divided into experimental and control groups, collected over time, and fixed in 10% formalin. Cells were stained with Fungiflora Y stain and observed under a fluorescence microscope, which showed concentration-dependent inhibition of GT formation. GT, germ tube; HT, hinokitiol
Evaluation of the combined effects of HT and MCZ using the disc diffusion method
Growth inhibition tests were performed using the upper (1.0% soft agar) and lower (1.5% base hard agar) layered PYG agar plate. The yeast cells grown in liquid PYG medium with shaking (24 h, 37 °C, 140 rpm) were harvested. The cell solution was added into 50 °C 25 mL melting soft agar containing 3.0 μg/mL HT, and then poured into the prepared 25 mL 1.5% base agar containing 3.0 μg/mL HT using 100 mL square petri dish (final cell concentration of the upper soft agar was adjusted to 2 × 105 cells/mL). For control, the same two-layered agar plates without HT were prepared. Drug resistance test filter papers containing 2.0 μg/disc MCZ and 4.0 μg/disc MCZ were placed on the plates and incubated at 37 °C under different culture conditions (normal aerobic and anaerobic cultures using AnaeroPac, Mitsubishi Gas Chemical Co., Tokyo, Japan), and inhibition circles were observed after 48 h.
This experiment was repeated five times with similar results; the representative inhibition circles obtained in the experiment are shown in Fig. 3a–d.
Change in the formation of MCZ inhibition circles in a medium containing 3.0 µg/mL HT by the disk diffusion method. Two-layer plates were prepared with HT 3.0 µg/mL added to soft agar in the upper layer and hard agar in the lower layer (The inhibition circle was larger for HT). HT, hinokitiol; MCZ, miconazole
HT- and MCZ-induced reactive oxygen species (ROS)
ROS production assay was performed using highly sensitive DCFH-DA (Dojin Chemical Laboratory Co., Tokyo, Japan) with reference to the manufacturer’s instruction. The cell pellets contained approximately 2 × 105 cells for ROS production prepared in the aforementioned PYG culture. The working solution (DCFH-DA dye diluted with tenfold dilution of the loading buffer) was added to the cell pellet in each microtube. The reaction mixture was incubated at 37 °C for 30 min to absorb the dye into the cells and centrifuged to collect the stained cells. The cells were resuspended in sterile Hank’s balanced salt solution without phenol red (HBSS) (Fujifilm Wako Pure Chemicals Co. Osaka, Japan) as a control. For experimental groups, 3.0 μg/mL HT, 0.5 μg/mL MCZ alone, and a combination of both drugs were prepared. The samples in the microtube were incubated at 37 °C for 1 h with shaking. Then, 50 μL of the samples was added to 10 wells in a 96-well plate for fluorescence microscopy and measured using a SpectraMax iD3 multimode microplate reader (MOLECULAR DEVICE, San Jose, USA) at an excitation wavelength of 490 nm and fluorescence wavelength of 540 nm.
Moreover, the effects of antioxidant butylated hydroxyanisole (BHA) on ROS production and GT formation were investigated using 1 mM BHA following the same experimental systems mentioned above.
The ROS experiments and the effect of BHA on ROS production were performed a total of six times, and the statistical analysis of the measured results is shown in Figs. 4 and 5.
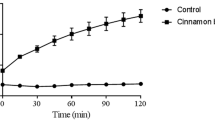
ROS production of C. al cells by HT and MCZ. A bacterial solution with a final cell concentration of 2 × 105 cells/mL was used to incorporate the fluorescent dye for ROS detection into the cells, and each group was prepared and measured using a multiple reader at an excitation wavelength of 490 nm and a fluorescence wavelength of 540 nm. Immediately after the measurement, the cells were mixed with 1 mM BHA and measured in the same manner. Each group was subjected to the Steel–Dwass test. Differences in maternal means (with correspondence) were determined before and after BHA administration in each group (*P < 0.05, **P < 0.01; n = 6). BHA, butylated hydroxyanisole; HT, hinokitiol; MCZ, miconazole; ROS, reactive oxygen species
The effect of BHA on GT formation is shown in Fig. 6 and Tables 3 and 4, which show the number and percentage of GTs and GT formation after a typical HT treatment (24 h) obtained in one experiment.
Statistical analysis
Statistical analysis was performed using Excel statistics (Bell Curve). Data are presented as means ± standard errors of mean. Significant differences between the experimental and control results are indicated by p values < *0.05 or **0.01. GT length and ROS production were compared using the Steel–Dwass test. Further, the GT ratio after adding BHA was analyzed using the difference of mother means (with correspondence).
Results
Effect of HT on GT formation
The length and percentage of GTs decreased after 24 h in 2.0, 2.5, and 3.0 μg/mL HT. In 3.0 μg/mL HT, GT formation was completely inhibited and remained in yeast form. However, 1.0 μg/mL HT produced no inhibitory effect on the length and percentage of GTs (Fig. 2, Tables 1, 2).
Changes in growth inhibition circle using the disc diffusion method
We used the double-layered agar method to form a clear distinct growth inhibition circle and set the aerobic or anaerobic culture conditions, considering the environments of the oral cavity and biofilm of C. al. In both anaerobic and aerobic cultures, the growth inhibition circles of 2.0 and 4.0 μg/disc MCZ on 3.0 μg/mL HT additional agar (Fig. 3a, b) were larger than those on HT non-additional agar (Fig. 3c, d). Regarding the change in growth inhibition circle formation between aerobic and anaerobic cultures, 2.0 and 4.0 μg/disc MCZ in anaerobic culture (Fig. 3b) resulted in larger growth inhibition circles than those in aerobic cultures (Fig. 3d). In addition, anaerobic cultures (Fig. 3a, b) formed growth inhibition circles with well-defined margins.
ROS production and changes in GT formation
HT and MCZ induced ROS production in C. al cells. Significantly higher ROS levels were produced at HT 3.0 + MCZ 0.5 μg/mL compared to those in the control, HT 3.0 μg/mL alone, and MCZ 0.5 μg/mL alone. ROS production in C. al cells before and after BHA treatment was significantly reduced (Fig. 4). However, the difference before and after BHA treatment was not statistically significant in each group (Fig. 5). Compared to BHA alone, the combination of BHA and HT resulted in an increase in GT length and percentage (Fig. 6, Tables 3, 4).
Discussion
Adhesion of C. al to epithelial cells is involved in the early stages of the pathogenesis of oral candidiasis. As C. al develops into mycelial form in single or multi-species biofilm, the hypha presses into the epithelial cell membrane and forms invasion pockets. It then produces candidalysin (peptide toxin), which causes epithelial inflammatory reactions and cellular damage [26,27,28]. Therefore, considering prevention of oral candidiasis, it is important to prevent this early stage [29]. HT has been shown to be effective in inhibiting C. al growth; however, it was unclear which growth form was inhibited under the sub-MIC of HT [30]. The present study showed that HT inhibited, in a concentration-dependent manner, the morphological change from yeast form to GT (the model of dimorphism), which is involved in the early stage of oral candidiasis. Recently, Ohara et al. reported similar results to ours; thus, the inhibition of GT formation by HT is an important morphological finding for preventing the onset of initial biofilm formation and is followed by oral candidiasis [18].
In the present experiment, the inhibition circle formed by MCZ 2.0 and 4.0 µg/disc on HT 3.0 µg/mL-supplemented medium was greater in aerobic and anaerobic cultures than that formed by MCZ 2.0 and 4.0 µg/disc on HT-free medium, suggesting that the addition of HT 3.0 µg/mL complements the antifungal effect of MCZ. MCZ has been reported to induce ROS production in C. al, and ROS production is important for the antifungal activity of MCZ. Moreover, low concentrations of HT can promote iron-catalyzed ROS production and enhance DNA damage [31,32,33,34]. In view of this, we measured the amount of ROS in HT and MCZ alone and in HT and MCZ together and found that the amount of ROS significantly increased when HT and MCZ were combined, suggesting that HT, which complements MCZ action, was related to ROS. However, when HT was combined with BHA, which is used as an antioxidant and preservative in food and feed additives, ROS levels were significantly reduced, and no significant difference in ROS levels was observed before and after BHA administration. Superhydroxy anion radicals have been reported to have an excellent ability to remove these radicals [34, 35]. The highly sensitive DCFH-DA ROS assay kit used in this experiment detects ROS, such as singlet oxygen, superoxide anion radicals, hydrogen peroxide, and hydroxyl radicals. These results suggest that ROS, particularly hydrogen peroxide and superoxide anion radicals, were associated with HT action complementary to MCZ and may have acted together in each group.
MCZ decreases ROS production and antifungal activity when co-administered with antioxidants [36]. In the present study, HT and MCZ alone and HT and MCZ together resulted in lower ROS levels when administered with BHA, suggesting that HT may have also reduced antifungal activity. When BHA alone and HT and BHA in combination were administered to GT, which is considered a pre-stage of pathogenic C. al, GT grew better when HT and BHA were combined. This suggests that, as in the case of MCZ, the co-administration of HT with an antioxidant effect reduces the ability to produce ROS and its antifungal activity.
From the results of these experiments, we observed that HT suppressed the yeast-to-hyphal transformation of C. al and complemented the antifungal activity of MCZ by producing ROS. Farnesol, a quorum sensing molecule synthesized by C. al, acts as a negative regulator of morphogenesis, inhibits the yeast-to-hyphal transformation of C. al, and is associated with reactive oxygen species. Farnesol, a common molecule with antibacterial properties among monoterpenes and other fungi, is involved in biofilm formation and acts as a virulence factor. In particular, 200–300 mM concentrations of farnesol exhibit antifungal activity, whereas 40 mM, the physiologically active concentration, exhibits stress inhibitory activity; this duality depends on the concentration [37]. Since HT is a cyclic monoterpene with antimicrobial activity, it is possible that it acts similarly to farnesol, and it is necessary to further investigate the mechanism of action of HT against the dimorphism of C. al and its relationship with farnesol.
Jin et al. presented the results of a detailed study of HT on C. al concerning the chelating effects of HT particularly against Fe2+. They revealed that HT inhibited the enzyme activities of mitochondrial complexes I and II in C. al with the generation of ROS. As a result, ATP generation of C. al was reduced by down regulation of the Ras 1—cAMP—PKA pathway. This down regulation might depress the Efg 1—inducing gene expressions, which are important for GT formation [38]. Since ROS of eukaryotic origin are produced from mitochondrial complexes I and III, when the mitochondrial function is impaired, the ROS production from C. al under the treatment of HT observed in our study may be attributed to mitochondrial complexes I inhibition by HT as previously reported. Komaki et al. revealed that the fungicidal effects of HT were decreased under complete anaerobic conditions using the RPMI 1640 medium containing 10 mg/mL sodium sulfate (Na2SO3 + O2 = 2Na2SO4). This result demonstrates involvement of the fungicidal effect of HT in the respiration systems of C. al. However, they reported that 5 μg/mL HT did not inhibit the respiration system and ATP production of C. al did not decrease compared with the control; thus, they suggested that the phenomenon was caused by the C. al respiration signal branched at Co-enzyme Q to the ATP production system and cAMP activation system [30]. We also found the diameter of the inhibitory circle treated with 100-μg HT/disk under complete anaerobic conditions using 10-mg/mL sodium sulfate containing PYD medium after 48 h to be shorter than that of aerobic conditions (data not shown). In contrast, HT revealed the fungicidal effects for C. al, such as amphotericin B, to be distinctly different from fungistatic fluconazole under aerobic conditions, from the degree of clarity of inhibition circle edges after 48 h using disk plate method (data not shown). Based on these results, HT may have unknown lethal action points. Thus, further studies on the fungicidal mechanism of HT against C. al are warranted.
We have previously performed C. al slide cultures on cornmeal agar, a nutrient-poor medium that relies primarily on the respiratory system for growth. When a single yeast-like cell is placed in the center of a slide, it initially grows in a yeast-like form under aerobic conditions; however, as oxygen is consumed and the culture becomes anaerobic, mycelia grows from the edge cells of the small yeast-like colony. The tips of the mycelia extend to the edge of the slide, where they come into contact with air, and when they reach the aerobic edge, they proliferate again in a yeast-like form [39]. Although we cannot directly relate the results of the experimental culture to growth in vivo, our finding that the combination of HT and MCZ inhibited both the aerobic and anaerobic growth of C. al may be of interest in clinical applications, particularly in the prevention of oral candidiasis.
The results of this experiment showed that C. al can act in a mixed aerobic and anaerobic environment in the oral cavity and that synergistic effects were observed using HT, which has fewer side effects, in combination with known antifungal agents. Meanwhile, new agents and combinations are being developed in recent years due to the increase in resistant strains of C. al. These findings provide useful information for clinical applications. However, there are still many unknowns regarding C. al studies when HT is combined with other antifungal agents and the basic clinical aspects of HT. Our goal is to elucidate these in the future.
Conclusion
In this study, the inhibition of GT formation by HT on C. al is an important morphological finding that reduces C. al pathogenicity; HT in combination with MCZ was found to produce ROS in C. al cells and has a complementary effect. These findings suggest that HT may effectively prevent the development of oral candidiasis.
Data availability
Data available within the article or its supplementary materials.
References
Kuramoto C, Watanabe Y, Tonogi M, Hirata S, Sugihara N, Ishii T, Yamane GY. Factor analysis of oral healthcare for acute hospitalized patients in Japan. Geriatr Gerontol Int. 2011;11:460–6. https://doi.org/10.1111/j.1447-0594.2011.00709.x. (Epub 2011 May 18. PMID: 21592269).
Chen R, Irving M, Wright FA, Cunich M. Evaluation of health workforce models addressing oral health in residential aged care facilities: a systematic review of the literature. Gerodontology. 2020;37:222–32. https://doi.org/10.1111/ger.12475.
Izumi M, Akifusa S. Tongue cleaning in the elderly and its role in respiratory and swallowing functions: Benefits and medical perspectives. J Oral Rehabil. 2021;48:1395–403. https://doi.org/10.1111/joor.13266. (Epub 2021 Oct 13. PMID: 34612518).
Winning L, Lundy FT, Blackwood B, McAuley DF, El Karim I. Oral health care for the critically ill: a narrative review. Crit Care. 2021;25:353. https://doi.org/10.1186/s13054-021-03765-5. (PMID: 34598718, PMCID: PMC8485109).
Cheng KKF, Molassiotis A, Chang AM, Wai WC, Cheung SS. Evaluation of an oral care protocol for the prevention of chemotherapy-induced oral mucositis in pediatric patients with cancer. Eur J Cancer. 2001;37:2056–63. https://doi.org/10.1016/S0959-8049(01)00098-3. (PMID: 11597384).
Salvador P, Azusano C, Wang L, Howell D. A pilot randomized controlled trial of an oral care intervention to reduce the severity of mucositis in stem cell transplant patients. J Pain Symptom Manag. 2012;44:64–73. https://doi.org/10.1016/j.jpainsymman.2011.08.012. (Epub 2012 Jun 5. PMID: 22672917).
Kuriyama K, Sohda M, Watanabe T, Saito H, Yoshida T, Hara K, Sakai M, Kim M, Asami T, Yokoo S, Kuwano H, Shirabe K, Saeki H. Resistance to preoperative oral care is associated with postoperative pneumonia after esophageal cancer surgery. Anticancer Res. 2021;41:1507–14. https://doi.org/10.21873/anticanres.14909. (PMID: 33788743).
Sakuramoto A, Hasegawa Y, Sugahara K, Komoda Y, Hasegawa K, Hikasa S, Kurashita M, Sakai J, Arita M, Yasukawa K, Kishimoto H. A new paste for severe stomatitis in patients undergoing head-and-neck cancer radiotherapy and/or chemotherapy with oral appliances. BMC Cancer. 2018;18:245. https://doi.org/10.1186/s12885-018-4017-2. (PMID: 29499657, PMCID: PMC5834906).
Suzuki H, Furuya J, Matsubara C, Aoyagi M, Shirobe M, Sato Y, Tohara H, Minakuchi S. Comparison of the amount used and the ease of oral care between liquid and gel-type oral moisturizers used with oral care simulators. Int J Environ Res Public Health. 2022;19:8158. https://doi.org/10.3390/ijerph19138158. (PMID: 35805817, PMCID: PMC9266061).
Wu TY, Liu HY, Wu CY, Chen HC, Huang ST, Chen PH. Professional oral care in end-of-life patients with advanced cancers in a hospice ward: improvement of oral conditions. BMC Palliat Care. 2020;19:181. https://doi.org/10.1186/s12904-020-00684-0. (PMID: 33246449, PMCID: PMC7697385).
Matsumura M, Shigeishi H, Su CY, Nishimura R, Ohta K, Sugiyama M. High rate of oral candida detection in dependent elderly Japanese older people. Geriatrics. 2020;5:21. https://doi.org/10.3390/geriatrics5010021. (PMID: 32213908, PMCID: PMC7151096).
Hiyoshi T, Domon H, Maekawa T, Yonezawa D, Kunitomo E, Tabeta K, Terao Y. Protective effects of hinokitiol against periodontal bone loss in mice with ligature-induced periodontitis. Arch Oral Biol. 2020;112: 104679. https://doi.org/10.1016/j.archoralbio.2020.104679. (Epub 2020 Feb 7. PMID: 32062102).
Lin HC, Wang CC, Wu CF, Lin YH, Lee WC, Chen PJ, Chang YU, Su YC. Hinokitiol inhibits the viability of oral squamous carcinoma cells by inducing apoptosis and autophagy. Anticancer Res. 2023;43:1167–73. https://doi.org/10.21873/anticanres.16262. (PMID: 36854527).
Hoang BX, Han B. A possible application of Hinokitiol can be used as a natural zinc ionophore and anti-infective agent for the prevention and treatment of COVID-19 and other viral infections. Med Hypotheses. 2020;145:110333. https://doi.org/10.1016/j.mehy.2020.110333. (Epub 2020 Oct 5. PMID: 33045596, PMCID: PMC7534793).
Ye J, Xu YF, Lou LX, Jin K, Miao Q, Ye X, Xi Y. Anti-inflammatory effects of hinokitiol in human corneal epithelial cells: an in vitro study. Eye. 2015;29:964–71. https://doi.org/10.1038/eye.2015.62. (Epub 2015 May 8. PMID: 25952949, PMCID: PMC4506343).
Domon H, Hiyoshi T, Maekawa T, Yonezawa D, Tamura H, Kawabata S, Yanagihara K, Kimura O, Kunitomo E, Terao Y. The antibacterial activity of hinokitiol against both antibiotic-resistant and -susceptible pathogenic bacteria was predominant in the oral cavity and upper airways. Microbiol Immunol. 2019;63:213–22. https://doi.org/10.1111/1348-0421.12688. (PMID: 31106894).
Jayakumar T, Liu CH, Wu GY, Lee TY, Manubolu M, Hsieh CY, Yang CH, Sheu JR. Hinokitiol inhibited the migration of A549 lung cancer cells via suppression of MMPs, induction of antioxidant enzymes, and apoptosis. Int J Mol Sci. 2018;19:939. https://doi.org/10.3390/ijms19040939. (PMID: 29565268, PMCID: PMC5979393).
Ohara H, Odanaka K, Shiine M, Hayasaka M. Antimicrobial effect of oral gel containing hinokitiol and 4-isopropyl-3-methlphenol against intraoral pathogenic microorganisms. PLoS ONE. 2023. https://doi.org/10.1371/journal.pone.0283295.
Bianchi S, Fantozzi G, Bernardi S, Antonouli S, Continenza MA, Macchiarelli G. Commercial oral products and implant collar surfaces: scanning electron microscopy observations. Can J Dent Hyg. 2020;54:26–31.
Morgan R, Tsang J, Harrington N, Fook L. Survey of hospital doctors’ attitudes and knowledge of oral conditions in older patients. Postgrad Med J. 2001;77:392–4. https://doi.org/10.1136/pmj.77.908.392.
Deepa A, Nair BJ, Sivakumar T, Joseph AP. Uncommon opportunistic fungal infections of the oral cavity: a review. J Oral Maxillofacial Pathol. 2014;18:235–43. https://doi.org/10.4103/0973-029X.140765. (PMID: 25328305, PMCID: PMC4196293).
Lu S-Y. Oral candidosis: pathophysiology and best practices for diagnosis, classification, and successful management. J Fungi. 2021;7:555. https://doi.org/10.3390/jof7070555.
Pierce CG, Srinivasan A, Uppuluri P, Ramasubramanian AK, López-Ribot JL. Antifungal therapy with an emphasis on biofilms. Curr Opin Pharmacol. 2013;13:726–30. https://doi.org/10.1016/j.coph.2013.08.008. (Epub 2013 Sep 4. PMID: 24011516, PMCID: PMC3795934).
Katagiri H, Fukui K, Nakamura K, Tanaka A. Systemic hematogenous dissemination of mouse oral candidiasis is induced by oral mucositis. Odontology. 2018;106:389–97. https://doi.org/10.1007/s10266-018-0366-1. (Epub 2018 May 24. PMID: 29797142, PMCID: PMC6153985).
Wu Y, Du S, Bimler LH, Mauk KE, Lortal L, Kichik N, Griffiths J, Osicka R, Song L, Polsky K, Kasper L, Sebo P, Weatherhead J, Knight JM, Kheradmand F, Zheng H, Richardson JP, Hube B, Naglik JR. Toll-like receptor 4 and CD11b expressed on microglia coordinate eradication of Candida albicans cerebral mycosis. Cell Rep. 2023;42:1–18. https://doi.org/10.1016/j.celrep.2023.113240.
Mogavero S, Sauer FM, Brunke S, Allert S, Schulz D, Wisgott S, Jablonowski N, Elshafee O, Krüger T, Kniemeyer O, Brakhage AA, Naglik JR, Dolk E, Hube B. Candidalysin delivery to the invasion pocket is critical for host epithelial damage induced by Candida albicans. Cell Microbiol. 2021;23:e13378. https://doi.org/10.1111/cmi.13378. (Epub 2021 Jul 20. PMID: 34245079; PMCID: PMC8460606).
Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–28. https://doi.org/10.4161/viru.22913. (Epub 2013 Jan 9. PMID: 23302789; PMCID: PMC3654610).
Lohse MB, Gulti M, Jhonson AD, Noble CJ. Development and regulation of single-and multi-species Candida albicans biofilms. Nat Rev Microbiol. 2018;16:19–31. https://doi.org/10.1038/nrmicro.2017.107.
Majima T, Ito-Kuwa S, Nagatomi R, Nakamura K. Study of the carriage of Candida sp in dental students and staff—identification of Candida sp and background survey. Oral Sci Int. 2013;29:30–4. https://doi.org/10.1016/S134-8643(13)00029-3.
Komaki N, Watanabe T, Ogasawara A, Sato N, Mikami T, Matsumoto T. Antifungal mechanism of hinokitiol against Candida albicans. Biol Pharm Bull. 2008;31:735–7.
Qin M, Shao B, Lin L, Zhang ZQ, Sheng ZG, Qin L, Shao J, Zhu BZ. Molecular mechanisms underlying the unusual biphasic effects of the natural compound hinokitiol on iron-induced cellular DNA damage. Free Radic Biol Med. 2023;194:163–71. https://doi.org/10.1016/j.freeradbiomed.2022.11.042. (Epub 2022 Dec 5 PMID: 36476568).
Murakami K, Ohara Y, Haneda M, Tsubouchi R, Yoshino M. Pro-oxidant action of hinokitiol: hinokitiol-iron-dependent generation of reactive oxygen species. Basic Clin Pharma Tox. 2005;97:392–4. https://doi.org/10.1111/j.1742-7843.2005.pto_214.x. (PMID: 16364055).
Francois IEJA, Cammue B, Borgers M, Ausma J, Dispersyn G, Thevissen K. Azoles: mode of antifungal action and resistance development. Effect of miconazole on endogenous reactive oxygen species production in Candida albicans. Anti Infect Agents Med Chem. 2006;5:3–13. https://doi.org/10.2174/187152106774755554.
Adjimani JP, Asare P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol Rep. 2015;2:721–8. https://doi.org/10.1016/j.toxrep.2015.04.005. (PMID: 28962407, PMCID: PMC5598521).
Zhang Y, Fang Y, Liang H, Wang H, Hu K, Liu X, Yi X, Peng Y. Synthesis and antioxidant activities of 2-oxo-quinoline-3-carbaldehyde Schiff-base derivatives. Bioorg Med Chem Lett. 2013;23:107–11. https://doi.org/10.1016/j.bmcl.2012.11.006. (Epub 2012 Nov 10. PMID: 23206864).
Tits J, Berman J, Cammue BPA, Thevissen K. Combining miconazole and domiphen bromide results in excess of reactive oxygen species and killing of biofilm cells. Front Cell Dev Biol. 2021;8: 617214. https://doi.org/10.3389/fcell.2020.617214. (PMID: 33553152, PMCID: PMC7858260).
Dižová S, Bujdáková H. Properties and role of the quorum sensing molecule farnesol in relation to the yeast Candida albicans. Pharmazie. 2017;72:307–12. https://doi.org/10.1691/ph.2017.6174. (PMID: 29442016).
Jin X, Zhang M, Lu J, Duan X, Chen J, Liu Y, Chang W, Lou H. Hinokitiol chelates intracellular iron to retard fungal growth by distributing mitochondrial respiration. J Adv Res. 2021;34:65–77. https://doi.org/10.1016/j.jare.2021.06.016.
Aoki S, Ito-Kuwa S, Nakamura K, Vdotto V, Takeo K. Oxygen as a possible tropic factor in hyphal growth of Candida albicans. Mycoscince. 1998;39(3):231–8. https://doi.org/10.1007/BF02464003.
Acknowledgements
We would like to thank Professor Koichi Shinkai for his constructive suggestions regarding statistical analysis.
Funding
This study was supported by JSPS KAKENHI (Grant number: JP23K09470).
Author information
Authors and Affiliations
Contributions
Conceptualization: Akira Tanaka; methodology: Nakamura Kenjirou and Fukui Kayoko; formal analysis and investigation: Nobuchika Takeuchi; writing—original draft preparation: Nobuchika Takeuchi; writing—review and editing: Akira Tanaka, Nakamura Kenjirou, and Fukui Kayoko; funding acquisition: Akira Tanaka, Nakamura Kenjirou, and Fukui Kayoko; resources: Akira Tanaka, Nakamura Kenjirou, and Fukui Kayoko; supervision: Akira Tanaka and Nakamura Kenjiro. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Nobuchika Takeuchi, Kayoko Fukui, Kenjiro Nakamura, and Akira Tanaka declare conflicts of interest associated with this manuscript. Company Name: EN Otsuka Pharmaceutical Co. Ltd.
Ethical approval
No ethical approval was required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takeuchi, N., Fukui, K., Nakamura, K. et al. Studies on the antifungal effects of Hinokitiol on Candida albicans: inhibition of germ tube formation and synergistic pharmacological effects of miconazole. Odontology (2024). https://doi.org/10.1007/s10266-024-00992-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10266-024-00992-4