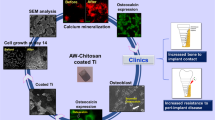
Abstract
Attempts are ongoing to improve the surface properties of dental implants by application of different coatings, aiming to enhance osseointegration, and decrease the adverse effects of titanium and its alloys used in dental implants. Coating of implant surface with hydroxyapatite (HA) is one suggested strategy for this purpose due to its high biocompatibility and similar structure to the adjacent bone. This study aimed to quantify the release of silver ions and expression of osteogenic genes by MC3T3-E1 cells cultured on nano-HA and silver/strontium (Ag/Sr)-coated titanium plates via the electrochemical deposition method. Plates measuring 10 × 10 × 0.9 mm were fabricated from Ti-6Al-4 V alloy, and polished with silicon carbide abrasive papers before electrochemical deposition to create a smooth, mirror-like surface. After applying homogenous nano-HA coatings with/without silver/strontium on the surface of the plates, the composition of coatings was confirmed by energy-dispersive X-ray spectroscopy (EDS), and their morphological properties were analyzed by scanning electron microscopy (SEM). The coated specimens were then immersed in simulated body fluid (SBF), and the concentration of released sliver ions was quantified by spectroscopy at 7–14 days. The MC3T3-E1 osteoblastic cell line was cultured in osteogenic medium for 7–14 days, and after RNA extraction and cDNA synthesis, the expression of runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and osteopontin (OPN); osteogenic genes was quantified by polymerase chain reaction (PCR) using SYBR Green Master Mix kit. The expression of genes and the released amount of silver ions were compared between the two groups using the Mann–Whitney U test. The two groups were not significantly different regarding silver ion release at 14 days (P > 0.05). However, silver ion release was significantly higher from nano-HA coatings with silver/strontium at 7 days (P = 0.03). The difference in expression of RUNX2 (P = 0.04), OPN (P = 0.04), and OCN (P = 0.03) genes was also significant between nano-HA coating groups with and without silver/strontium at 7 days, and the expressions were higher in nano-HA with silver/strontium group, but this difference was not significant at 14 days. Addition of silver and strontium to specimens coated with nano-HA increased the release of silver ions within the non-toxic range, and enhanced the expression of osteogenic genes particularly after 7 days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osseointegration refers to the direct contact of vital bone with the functional implant surface without the interference of fibrous tissue, and is the main primary prerequisite for the long-term success of dental implants. Chemical composition and surface roughness of dental implants are two critical factors affecting their osseointegration [1].
Titanium and its alloys are most suitable for use in implant dentistry due to optimal biocompatibility, insignificant corrosion, and high mechanical resistance. However, titanium and its alloys do not have antibacterial properties [2,3,4]. Since osseointegration refers to mutual interactions between the host bone and implant surface, implant surface modifications can promote osseointegration by enhancing the interactions of biological fluids and cells and facilitating bone healing around dental implants. Recently, nano-scale implant surface modifications were suggested for this purpose, hypothesizing that nano-structures may be able to increase the surface energy and subsequently enhance osseointegration by improving matrix protein absorption, and migration and proliferation of osteoblasts [5].
Hydroxyapatite (HA) has been used for coating of dental implants due to its optimal osteoconductivity and having a chemical structure resembling that of natural bone. The HA molecules present in bone are in nano-crystalline form. Thus, it is believed that nano-HA may be able to create biocompatible surfaces capable of optimal integration with bone tissue [6, 7]. However, nano-HA coatings enhance the adhesion of proteins and cells, and can also increase bacterial colonization, which increases the risk of bacterial infection of the peri-implant tissues and subsequent development of peri-implantitis [8].
Silver (Ag) has strong antibacterial activity and low cytotoxicity in long-term use [9]. Despite the strong antibacterial activity of silver, it can be cytotoxic in concentrations higher than a certain threshold [8]. Addition of a secondary chemical agent such as strontium (Sr) can neutralize the cytotoxicity of silver while preserving its antibacterial activity. Strontium particles not only have osteogenic properties and improve implant osseointegration, but also can make silver ions tolerable to cells [10,11,12].
Several techniques have been introduced for coating of implant surfaces with bioceramics to improve their osseointegration and antibacterial properties, such as plasma spray, electrophoretic deposition, and electrochemical deposition. The plasma spray method is most commonly used for coating of implant surfaces; however, the high temperature of the process can cause degradation of HA. Moreover, this method is not suitable for complex, multi-dimensional structures. In electrophoretic deposition, a high voltage is used, which causes anodic polarization of metal and increases the risk of corrosion of its surface, and can subsequently affect the attachment of HA crystals. Electrochemical deposition is another practical coating technique with increasing popularity. The advantages of this technique include enabling the deposition of different coatings on metal surfaces, simple and fast process, low cost, and applicability for coating of multi-dimensional and complex implant surfaces [13].
In previous studies, the titanium surfaces were coated with HA containing silver and strontium separately and together, and the results showed that these coatings had antibacterial and osteogenic properties [14,15,16,17]. However, to the best of the authors’ knowledge, the efficacy of a nano-HA coating containing silver/strontium applied by the electrochemical deposition method has not been previously investigated.
Considering the advantages of nano-HA composition with antibacterial ions, this study aimed to quantify the concentration of released silver ions and osteoblastic gene expression by MC3T3-E1 cells cultured on nano-HP with/without silver/strontium-coated titanium plates via the electrochemical deposition method as the second step of characterization of this new implant coating which was conceptualized and optimized earlier by the second author and her team (Azadeh Esmaeil Nejad, Hanieh Nojehdehian, Amir Pasha, and Negin Nikmanesh) that showed acceptable structure and biocompatibility (Article in press).
Materials and methods
Specimen preparation
As proposed in the prevoius project, the optimized 10 × 10 × 0.9 mm plates were fabricated from Ti-6Al-4 V alloy (Loterios Timet company, Gerenzano, Italy) using a water jet (Robofil 2000, Charmilles technologies, Kaiserslautern, Germany) and polished with 400- to 4000-grit silicon carbide abrasive papers (Starcke, Melle, Germany) to create a smooth and mirror-like surface. The plates were then cleaned in an ultrasonic bath containing acetone, alcohol, and distilled water in separate steps, each for 10 min. To eliminate the superficial oxide layer, the plates were etched in a mixture of 20% nitric acid (TAT Chem, Tehran, Iran) and 2% hydrofluoric acid (TAT Chem, Tehran, Iran) in 10:1 ratio for 1.5 min. Eventually, the plates were dried at room temperature [18].
The plates in the test group received a homogenous coating of nano-HA, strontium and silver, which was confirmed by energy-dispersive X-ray spectroscopy (EDS), and scanning electron microscopy (SEM) in a previous study by the second author and her team. The plates in the control group received a homogenous coating of nano-HA, which was also confirmed by EDS, and SEM. The exclusion criterion for the test group was coatings with particles other than nano-HA, strontium, and silver (formation of new compounds such as AgO or Ag2O, indicating the participation of silver in new compounds instead of its participation in the structure of HA). The exclusion criterion for the control group was coatings with particles other than nano-HA.
Electrochemical deposition of nano-HA silver/strontium coating
For deposition of coating, first, the titanium plates were ultrasonicated in 70% ethanol for 10 min, and were then immersed in acetone for 10 min. Next, they were rinsed with distilled water, ultrasonicated in 20% HNO3 for 1.5 min, and rinsed with distilled water. The plates were then sonicated again in 2% hydrofluoric acid for 1.5 min and rinsed with distilled water. Afterwards, one side of the plates was cleaned and etched, and nail varnish was applied on this side to provide insolation against the electric current. To apply HA coating, the plates were prepared by potentiometry (0.1 mA, 1.2 V for 1500 s using a stirrer) and immersion in 0.5% solution of HA, 0.3% polyacrylic acid polymer, and 0.01 molar KNO3. To apply silver/strontium on the plates coated with nano-HA, potentiometry with − 10 V constant voltage was used for 180 s [13]. For this purpose, first, a solution containing 0.08 mM silver nitrate and 0.16 mM strontium nitrate was prepared, which was found to be the best mixture solution via the optimization procedure carried out in the previous step and then silver/strontium coating was deposited by potentiometry. The plates coated with HA by the electrochemical deposition method (devoid of silver and strontium) served as the control group (Fig. 1).
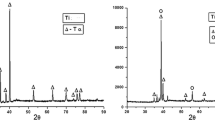
Confirming the coating by EDS and SEM
The chemical composition of nano-HA strontium/silver coatings was evaluated by EDS, and their morphological properties were assessed by SEM (Fig. 2).
Silver ion release test
To quantify the amount of released silver ions, each coated plate was immersed in 50 mL of simulated body fluid (SBF; Fanavaran, Yazd, Iran) [19], with a composition of 142.0 mmol/L Na+, 5.0 mmol/L K+, 1.5 mmol/L Mg2+, 2.5 mmol/L Ca2+, 147.8 mmol/L Cl−, 4.2 mmol/L HCO3, 1.0 mmol/L HPO42−, and 0.5 mmol/L SO42− with a pH of 7.4 ± 0.2 at 36.5 °C, and the concentration of silver ions in the solution was measured after 7–14 days using inductively-coupled plasma atomic emission spectroscopy (AA240FS; Varian, USA). The measurements were repeated in triplicate.
Cell culture
Osteoblast progenitor cells (MC3T3-E1 osteoblastic cell line) were obtained from the Research institute of Dental Sciences, (Shahid Beheshti University of Medical Sciences). Titanium plates sterilized with 70% alcohol and placed in 24-well plates in two groups. Three repetitions were considered for each group. Next, 10,000 cells were cultured on each titanium plate. The seeded cells were incubated in standard Dulbecco’s modified Eagle’s medium and 15% fetal bovine serum for 48 h. Next, the culture medium was changed to osteogenic medium (Ready-to-use; Bioidea, Iran), and the cells were cultured in osteogenic medium for 7–14 days.
Gene expression analysis
At 7–14 days, the cells were detached from the titanium plates using trypsin and a cell scraper. RNA extraction was performed using RNAx Plus kit (Cinnagen, Iran). Next, cDNA was synthesized using Mo-MuLV RT Master Mix kit (Cinnagen, Iran). After cDNA synthesis, the expression of osteogenic-related genes including runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and osteopontin (OPN) osteogenic genes was measured using SYBR Green Master Mix kit (Ampliquon, Denmark), and real-time RT-PCR (Roche, France), at 7–14 days. Table 1 presents the primer sequences for the abovementioned genes. The gene expression results were analyzed by delta CT formula. B-actin was used as the housekeeping gene. All analyses were repeated in triplicate.
The measures of central dispersion for the ratio of expression of RUNX2, OPN, and OCN genes, and the amount of silver ions released from the nano-HA coatings with and without silver/strontium into the SBF at 7–14 days were calculated and reported. The ratio of expression of genes and the amount of released silver ions were compared between the test and control groups using the non-parametric Mann–Whitney test since the data were not normally distributed.
Results
Expression of osteogenic genes
Table 2 shows the mean ratio of expression of osteogenic genes in the two groups at 7–14 days. According to the non-parametric Mann–Whitney U test, significant differences were noted in the ratio of expression of RUNX2 (P = 0.04), OPN (P = 0.04), and OCN (P = 0.03) between the two groups of nano-HA coatings with and without silver/strontium at 7 days, and the expressions of osteogenic markers were significantly higher in nano-HA coating with silver/strontium group. However, no significant difference was noted in the expression ratio of RUNX2 (P = 0.7), OPN (P = 0.2), and OCN (P = 1.0) between the two groups of nano-HA coatings with and without silver/strontium at 14 days.
Release of silver ions
Table 3 presents the mean and standard deviation of the amount of silver ions released from the nano-HA coatings with/without silver/strontium at 7–14 days. According to the results of non-parametric Mann–Whitney U test, no significant difference was noted between the two groups in release of silver ions at 14 days (P = 0.15). However, the difference in this respect was significant at 7 days, and the amount of released silver ions was higher in nano-HA with silver/strontium group (P = 0.03).
Discussion
This study aimed to quantify the concentration of released silver ions and osteoblastic gene expression by MC3T3-E1 cells cultured on titanium plates coated with nano-HA with/without silver/strontium via the electrochemical deposition method.
SEM micrographs of the surface of specimens prior to coating revealed lines created by the polishing process. Also, the homogenous HA/Ag/Sr coating was evident on the surface of Ti-6Al-4 V alloy. In general, the coating was uniform and homogenous, and the particles had a uniform pattern of distribution on the alloy surface. Moreover, condensed rod-shaped nanoparticles with a mean width of 35 nm and mean length of 203.3 nm had covered the surface. These observations indicated optimal coating process and selection of influential factors such as concentration, temperature, time, and voltage. On back-scattered FE-SEM images, silver and strontium nanoparticles were seen in the form of separate islands on the surface of HA coating. Considering the uniformity and homogeneity of the coatings in this study, this technique can be used as a practical and precise method in similar future studies. It has been reported that heterogeneity in distribution of crystals on the coated surfaces can lead to local defects or a reduction in overall percentage of osseointegration in dental implant treatments [20].
Silver ion release from HA coatings into a physiological environment plays an important role in their antibacterial activity. Release of silver ions into SBF is quantified by spectroscopy, and if the concentration of released ions exceeds 1.6 ppm, the respective coating would be considered toxic [21]. Accordingly, the designed and deposited coatings in the present study were not toxic. Erdem and Turkoz (2019) evaluated the pattern of silver ion release from the structure of silver-reinforced HA products under in vitro conditions, and demonstrated that the amount of released silver ions from all products was limited, and the products did not show any sign of degradation. Thus, they confirmed non-toxicity of nano-HA products containing certain concentrations of silver, which was in accordance with the present results [22], although they synthesized the nano-HA and silver compounds by the deposition method, which was different from our methodology.
In the present study, the amount of silver ions released from the nano-HA coatings with silver/strontium into the SBF was higher than the corresponding value in nano-HA coating without silver/strontium group at both 7 (mean value of 0.06 ppm versus 0.01 ppm) and 14 (mean value of 0.04 ppm versus 0.01 ppm) days. However, this difference was only significant at 7 days. Thus, the amount of silver ions released from nano-HA coatings with silver/strontium decreased over time while this value remained constant in nano-HA coating without silver/strontium group. Also, this value was insignificant and negligible in the latter group. Considering the fact that the amount of silver ions released from nano-HA coatings with silver/strontium was also very low (0.04 ppm), it may be concluded that the designed nano-HA coatings containing silver/strontium were compatible in the respective ratios, and can be safely used in dental treatments [22].
Release of silver ions from nano-HA plus silver/strontium coatings can be related to the oxidation of silver following reaction with water, and conversion of silver to its ionic form [19]. Release of silver ions into SBF is closely correlated with the antimicrobial properties of the coatings. The minimum concentration of silver ions for induction of antibacterial properties is 0.1 ppm as reported in the literature, while its cytotoxic concentration is 1.6 ppm [23]. Zhu et al. (2009) evaluated the release of silver ions from the antimicrobial HA coatings and reported that the release of silver ions decreased over time, and reached a plateau at 7–14 days [24]. In the present study, release of silver ions from nano-HA coatings containing silver/strontium was very small, and reached a plateau at 7 days; also, reuptake of silver ions occurred by day 14 [24]. These findings were in agreement with the results of Zhu et al. (2009) to some extent. However, they coated the titanium specimens with HA by the vacuum plasma spray technique, which was different from our methodology.
In the process of deposition of HA on the titanium surface in SBF, some silver ions are trapped in the new apatite layer and thus, the release of silver ions is prevented. Chen et al. (2010) reported high amounts of silver ions released into SBF in primary phases of immersion, which can be beneficial for prevention of bacterial growth in early postoperative phases [22]. In their study, the concentration of silver in SBF decreased after the early phases and reached a plateau at 336 h. Also, they applied silver-containing HA coating by the coprecipitation or plasma spray technique and reported that ion release from the coatings deposited by the coprecipitation technique was higher than that from the coatings applied by the plasma spray technique.
Fielding et al. (2012) demonstrated that silver ions released from HA plus silver coatings were attached to alkaline phosphatase with areas of high-affinity metal ions and changed its functional stability. However, these areas showed a better performance with strontium (Sr2 +) ions and did not change the functional stability [25]. Therefore, strontium can compete with silver for attachment to specific sites of cellular function [26]. It appears that reinforced HA coatings with low contents of strontium and silver can have non-toxic properties and prevent bacterial proliferation while enhancing cell proliferation.
The nano-HA particles used in titanium coatings can reportedly increase osteoblastic proliferation, adhesion and calcium deposition [27]. However, the performance of metal implants coated with HA depends on their physicochemical and biological properties. The implant surface should be able to provide adequate support for osteoblastic synthesis of new bone, and at the same time, dental implants should be supported by the host defense mechanisms for optimal osseointegration and survival, and prevention of bacterial attachment. HA serves as a biocompatible ceramic, and silver serves as an antimicrobial agent commonly used in dental prosthetics and surgical instruments [28]. The antimicrobial activity of silver is exerted by damaging the bacterial cell wall and subsequent bacterial cell death [29]. However, optimal amounts of silver should be incorporated in HA coatings to achieve adequate antimicrobial activity while minimizing cytotoxicity.
In this study, to assess the release of silver ions, each coated specimen was immersed in 50 mL of SBF at 36.5 °C, and the ion concentration was measured after 7 and 14 days. SBF is an electrolyte solution that simulates the ionic balance, composition, and pH of human plasma [30]. When HA is immersed in SBF for long periods of time, excess calcium phosphate may deposit from SBF on the surface of HA. Deposition of calcium phosphate from SBF in vitro indicates the surface activity of HA to enhance biological mineralization.
The nano-HA + silver/strontium coatings often contain Al, P, Cl, Ca, Ti, V, Sr and Ag; among which, elements such as P and Ca are primarily derived from carbonated HA while Sr and Ag are derived from replacement of Ca2+ ions in HA crystals with Ag+ and Sr2+. Thus, these coatings are suitable for cell proliferation due to the presence of minerals such as Sr in HA. The EDS results also indicated the formation of Ag/Sr HA coatings on the surface of titanium plates [15].
In this study, the ratio of expression of RUNX2, OPN, and OCN genes in nano-HA + silver/strontium coatings was significantly higher than that in nano-HA without silver/strontium group at 7 days; however, this difference was not significant at 14 days. At 7 days, the expression of RUNX2, OPN, and OCN was 1.47%, 2.0%, and 1.2%, respectively in nano-HA coating plus silver/strontium group while these values were 1.0%, 1.0% and 1.0%, respectively in nano-HA coating without silver/strontium group. The ratio of expression of genes decreased over time, and the difference in this respect was no longer significant between the two groups at 14 days. Decreased expression of osteogenic genes at 14 days compared with 7 days may indicate that the genes had already exerted their specific effects and had no additional effect at day 14. On the other hand, the time of effect (early/late) of each gene is also important in the process of osteogenesis, and these genes might have an early effect on osteogenesis. Thus, further investigations are required to assess the expression of osteogenic genes at time points earlier than 7 days in more details. On the other hand, early events in the process of osseointegration including accumulation of adhesion proteins and osteoblastic precursors, and the inductive effects of such surface coatings on improvement of initial events can all affect the results.
In the present study, no significant difference was noted in percentage of expression of OCN gene in the two coating groups at 14 days; however, this difference was significant at 7 days. Also, the expression of RUNX2, OPN, and OCN genes remained constant (1.0%) in nano-HA without silver/strontium group at both time points.
Huang et al. (2017) reported an increase in production of OCN by prolonging the cellular incubation time in all samples [15]. In this study, HA coating containing silver/strontium showed maximum OCN expression compared with other samples after 14 days. At the same time, mineral ions released from nano-HA coatings containing silver/strontium such as silver and strontium ions can induce osteoblastic differentiation.
Strontium coatings are used to inhibit the cytotoxic effects of silver while maintaining its antimicrobial properties. Strontium prevents osteoclastic proliferation, improves new bone formation, and decreases bone loss by stimulating osteoblastic proliferation [31]. Moreover, strontium increases osteogenic cell proliferation and activity of osteoblasts in synthesis of bone matrix. It also increases the alkaline phosphatase activity and simultaneously decreases the production of osteoclastic markers during differentiation of bone marrow cells. It prevents osteoclastic differentiation and decreases the activity of osteoclasts as well [32, 33]. Alkaline phosphatase also serves as a primary osteoblastic differentiation marker [34], and is produced by cells with mineralized extracellular matrix [35]. The activity of alkaline phosphatase decreases when the cells are saturated and also at the beginning of mineralization. On the other hand, strontium induces the osteogenic differentiation of bone marrow mesenchymal stem cells and other progenitor cells [36]. It has been reported that combination of strontium with HA, calcium phosphate, calcium silicate, calcium sulfate, bioglass, and some other compounds can induce bone tissue regeneration and new bone formation and excellent antibacterial properties [37,38,39,40]. Thus, coating of dental implants with strontium may be beneficial for some patients especially those with osteoporosis or osteopenia.
This study described part of the physical and biological assessment of the introduced surface which was prooved to be suitable and compatible for cell adaptation in the previous project.
High cost of coating of specimens, not assessing the long-term cytotoxicity of the coatings for osteoblast-like cells and other cell types, and high risk of contamination of specimens were among the limitations of this study.
The clinicians may choose among several compounds according to the clinical conditions and regenerative requirements of patients with the aim to enhance and sustain bone tissue regeneration and new bone formation [41, 42] Further investigations are still required to improve the surface properties of implants by application of coatings such as nano-HA plus Ag/Sr and enhance the process of osseointegration as such. Also, the proliferation and differentiation of stem cells and their long-term behavior on these surfaces, and the quality of the regenerated hard tissue, bone, and osteoid should all be assessed over time by histomorphometric assessments. The behavior of stem cells on such surfaces is now under investigation by the authors of the present study.
Conclusion
Addition of silver and strontium to specimens coated with nano-HA increased the release of silver ions within the non-toxic range, and enhanced the expression of osteogenic genes particularly after 7 days.
Data availability
The datasets obtained and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- HA:
-
Hydroxyapatite
- Ag/Sr:
-
Silver/Strontium
- EDS:
-
Energy-Dispersive X-ray Spectroscopy
- SEM:
-
Scanning Electron Microscopy
- SBF:
-
Simulated Body Fluid
- RUNX2:
-
Runt-Related Transcription Factor 2
- OCN:
-
Osteocalcin
- OPN:
-
Osteopontin
- PCR:
-
Polymerase Chain Reaction
References
Ambard AJ, Mueninghoff L. Calcium phosphate cement: Review of mechanical and biological properties. J Prosthodont. 2006;15:321–8.
Brunette DM, Tengvall P, Textor M TP, Textor M, Thomsen P. (2013) Titanium in medicine: material science, surface science, engineering, biological responses and medical applications. Springer Sci Bus Media 13–24.
Bernardi S, Bianchi S, Tomei AR, Continenza MA, Macchiarelli G. Microbiological and SEM-EDS evaluation of titanium surfaces exposed to periodontal gel. In Vitro Study Mater (Basel). 2019;12(9):1448.
Kula Z, Semenov M, Klimek L. Carbon coatings deposited on prosthodontic Ni-Cr alloy. Appl Sci. 2021;11(10):4551.
Prodanov L, Lamers E, Domanski M, Luttge R, Jansen JA, Walboomers XF. The effect of nanometric surface texture on bone contact to titanium implants in rabbit tibia. Biomaterials. 2013;34:2920–7.
Bral A, Mommaerats MY. In vivo bio-functionalization of titanium patient-specific implants with nano-hydroxyapatite and other nano-calcium phosphate coatings: a systematic review. J Cranio-maxillofac Surg. 2016;44:400–12.
Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nano-phase metals: Ti, Ti-6Al-4V, and CoCrMo. Biomaterials. 2004;25:4731–9.
Gopi D, Shinyjoy E, Kavitha L. Synthesis and spectral characterization of silver/magnesium co-substituted hydroxyapatite for biomedical applications. Spectrochim Acta Part A Mol Biomol Spectrosc. 2014;127:286–91.
Lv M, Su S, He Y, Huang Q, Hu W, Li D, Fan C, Lee ST. Long-term antimicrobial effect of silicon nanowires decorated with silver nanoparticles. Adv Mater. 2010;22:5463–7.
Zhou JH, Li B, Lu SM, Zhang L, Han Y. Regulation of osteoblast proliferation and differentiation by interred spacing of Sr-HA nanorods on microporous titania coating. ACS Appl Mater Int. 2013;5:5358–65.
Wong KL, Wong CT, Liu WC, Pan HB, Fong MK, Lam WM, Cheung WL, Tang WM, Chiu KY, Luk KDK, Lu WW. Mechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetherether-ketone composites. Biomaterials. 2009;30:3810–7.
Roy M, Fielding GA, Beyenal H, Bandyopadhyay A, Bose S. Mechanical in vitro antimicrobial, and biological properties of plasma sprayed silver doped hydroxyapatite coating. ACS Appl Mater Interfaces. 2012;4(3):1341–9.
El-Wassefy NA, Reicha FM, Aref NS. Electrochemical deposition of nano hydroxyapatite-zinc coating on titanium metal substrate. Int J Implant Dent. 2017;3:39–50.
Geng Z, Wang R, Zhuo X, Li Z, Huange Y, Ma L, Cuia Z, Zhu S, Liang Y, Liu Y, Bao H, Li X, Huo Q, Liu Z, Yang X. Incorporation of silver and strontium in hydroxyapatite coating on titanium surface for enhanced antibacterial and biological properties. Mater Sci Eng. 2017;71:852–61.
Huang Y, Zhang X, Zhang H, Qiao H, Zhang X, Jia T, Han S, Gao Y, Xiao H, Yang H. Fabrication of silver- and strontium-doped hydroxyapatite/ TiO2 nanotube bilayer coatings for enhancing bactericidal effect and osteoinductivity. Ceramics Int. 2017;43(1):992–1007.
Fu C, Zhang X, Savino K, Gabrys P, Gao Y, Chaimayo W, Miller BL, Yates MZ. Antimicrobial silver hydroxyapatite composite coatings through two-stage electro-chemical synthesis. J Ceramics. 2016;301:13–9.
Liang Y, Li H, Xu J, Li X, Li X, Yan Y, Qi M, Hu M. Strontium coating by electrochemical deposition improves implant osseointegration in osteopenic models. Exp Ther Med. 2015;9:172–6.
Shimazaki T, Miyamoto H, Ando Y, Noda I, Yonekura Y, Kawano S, Miyazaki M, Mawatari M, Hotokebuchi T. In vivo antibacterial and silver-releasing properties of novel thermal sprayed silver-containing hydroxy-apatite coating. J Biomed Mater Res B Appl Biomater. 2010;92(2):386–9.
Chen Y, Zheng X, Xie Y, Ji H, Ding C, Li H, Dai K. Silver release from silver-containing hydroxyapatite coatings. Surf Coat Technol. 2010;205:1892–6.
Fu C, Savino K, Gabrys P, Zeng A, Guan B, Olvera D, et al. Hydroxyapatite thin films with giant electrical polarization. Chem Mater. 2015;27:1164–71.
Bartmanski M, Cieslik B, Glodowska J, Kalka P, Pawloski L, Pieper M, Zielingski A. Electrophoretic deposition (EPD) of nanohydroxyapatite- nano-silver coatings on Ti13Zr13Nb alloy. Ceramics Int. 2017;43:11820–9.
Erdem U, Turkoz MB. Silver release of Ag (I) doped hydroxyapatite: In vitro study. Microsc Res Tech. 2019;82(7):961–71.
Mirzaee M, Vaezi M, Palizdar Y. Synthesis and characterization of silver doped hydroxyapatite nano-composite coatings and evaluation of their antibacterial and corrosion resistance properties in simulated body fluid. Mater Sci Eng C. 2016;69:675–84.
Zhu Z-Y, Zhang F-Q, Zheng X-B. Study on the slow release of silver ion from silver containing antibacterial HA coating material. Shanghai Kou Qiang Yi Xue. 2009;18(1):66–8.
Fielding GA, Roy M, Bandyopadhyay A, Bose S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyl-apatite coatings. Acta Biomater. 2012;8:3144–52.
Geng Z, Cui ZD, Li ZY, Zhu SL, Liang YQ, Liu YD. Strontium incorporation to optimize the antibacterial and biological characteristics of silver-substituted hydroxyl-apatite coating. Mater Sci Eng C. 2016;58:467–77.
Guo X, Gough JE, Xiao P, Liu J, Shen Z. Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. J Biomed Mater Res A. 2007;82:1022–32.
Matsumoto N, Sato K, Yoshida K, Hashimoto K, Toda Y. Preparation and characterization of β-tricalcium phosphate co-doped with monovalent and divalent antibacterial metal ions. Acta Biomater. 2009;5:3157–64.
Song WH, Ryu HS, Hong SH. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J Biomed Mater Res. 2009;88A:246–54.
Hata K, Kokubo T, Nakamura T, Yamamuro T. Growth of a bonelike apatite layer on a substrate by a biomimetic process. J Am Ceram Soc. 1995;78(4):1049–53.
Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42:129–38.
Ni GX, Yao ZP, Huang GT, Liu WG, Lu WW. The effect of strontium incorporation in hydroxyapatite on osteoblasts in vitro. J Mater Sci Mater Med. 2011;22:961–7.
Choudhary S, Halbout P, Alander C, Raisz L, Pilbeam C. Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: involvement of prostaglandins. J Bone Miner Res. 2007;22:1002–10.
Gotoh Y, Hiraiwa K, Nagayama M. In vitro mineralization of osteoblastic cells derived from human bone. Bone Miner. 1990;8(3):239–50.
Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4:3111–23.
Capuccini C, Torricelli P, Sima F. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: in vitro osteoblast and osteoclast response. Acta Biomater. 2008;4:1885–93.
Caverzasio J, Thouverey C. Activation of FGF receptors is a new mechanism by which strontium ranelate induces osteoblastic cell growth. Cell Physiol Biochem. 2011;27:243–50.
Baier M, Staudt P, Klein R. Strontium enhances osseointegration of calcium phosphate cement: a histomorphometric pilot study in ovariectomized rats. J Orthop Surg Res. 2013;8:16.
Pan HB, Zhao XL, Zhang X. Strontium borate glass: potential biomaterial for bone regeneration. J R Soc Interface. 2010;7:1025–31.
Xuejiao Z, Bingbing W, Lifei M, Lei X, Hao Y, Yichao L, Saisai W, Haixia Q, He L, Jingpin L, Yong H. Chemical stability, antibacterial and osteogenic activities study of strontium-silver co-substituted fluorohydroxyapatite nanopillars: a potential multifunctional biological coating. Ceram Int. 2020;17:27758–73.
Bernardi S, Macchiarelli G, Bianchi S. Autologous Materials in Regenerative Dentistry: Harvested Bone, Platelet Concentrates and Dentin Derivates. Molecules. 2020;25(22):5330.
Bianchi S, Mancini L, Torge D, Cristiano L, Mattei A, Varvara G, Macchiarelli G, Marchetti E, Bernardi S. Bio-Morphological Reaction of Human Periodontal Ligament Fibroblasts to Different Types of Dentinal Derivates: In Vitro Study. International Journal of Molecular Sciences. 2021; 22(16):8681.
Acknowledgements
The authors gratefully acknowledge the financial support for this work that was provided by Research Institute for Dental Sciences, Shahid Beheshti University of Medical Sciences.
Funding
This research was supported by Research Institute for Dental Sciences, Shahid Beheshti University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
A.L. and A.E.N. conceived the study; M.R.R. and T.S. performed the experiments; M.N. analyzed the data; A.L. and T.S. wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have declared that No competing interests exist.
Ethics approval and consent to participate
This study was approved by the Research Institute of Dental Sciences (Shahid Beheshti University of Medical Sciences) Ethics Committee (approval number 161).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lafzi, A., Esmaeil Nejad, A., Rezai Rad, M. et al. In vitro release of silver ions and expression of osteogenic genes by MC3T3-E1 cell line cultured on nano-hydroxyapatite and silver/strontium-coated titanium plates. Odontology 111, 33–40 (2023). https://doi.org/10.1007/s10266-022-00747-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-022-00747-z