Abstract
This study aimed to determine the association between the progressive contraction of the posterior pharyngeal wall and dysphagia in postoperative patients with tongue cancer. A videofluoroscopic swallowing study (VFSS) was performed in 34 patients after tongue cancer surgery. Images were analyzed using a two-dimensional video measurement software. Cases in which the processes on the posterior pharyngeal wall moved downward from the 2nd to 4th vertebral regions were defined as “normal type”, other cases were defined as “abnormal type”. Twenty-four patients showed normal movement of the posterior pharyngeal wall, whereas 10 patients showed the abnormal type. The results showed that there was a significant difference in dysphagia scores between the postoperative swallowing type and swallowing dysfunction score. This implies that dysphagia is related to the movement of the posterior pharyngeal wall after tongue cancer surgery. Furthermore, the extent of resection and stage were significantly different between the normal and abnormal groups in the posterior pharyngeal wall movement. There was also a significant difference between the two groups in terms of the following: whether the tongue base was included in the excision range (p < 0.01), whether neck dissection was performed (p < 0.01), or whether reconstruction was not performed (p < 0.01). VFSS results showed that posterior pharyngeal wall movement was altered after surgery in patients with tongue cancer who had severe dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysphagia is a major functional impairment that frequently occurs after oral cancer surgery. The degree and symptoms of postoperative dysphagia depend on the site and extent of resection, while the degree of impairment depends on the extent of resection [1]. Moreover, Logemann et al. [2] reported that in extensive resections, more problems occur during the pharyngeal and oral phases. Fujiu et al. [3] reported an increase in the anterior protrusion of the posterior pharyngeal wall during swallowing in patients with postoperative oral cancer. Pauloski et al. [4] examined pharyngeal clearance due to contact between the tongue base and posterior pharyngeal wall in irradiated postsurgical oral and oropharyngeal cancer patients. Posterior pharyngeal wall movement and dysphagia in postoperative patients with oral cancer have been reported.
One of the causes of bolus residue is that the contractile muscles of the pharynx are impaired and do not perform sufficient progressive contraction of the pharyngeal wall [5]. As the bolus enters the pharynx, the superior, middle, and inferior constrictor muscles are activated sequentially to narrow and shorten the pharynx, contributing to the progressive contraction in the posterior pharyngeal wall that aid in bolus propulsion into the esophagus [6]. The contraction of the pharynx is the most important mechanism for transporting a bolus of food in the pharynx, and when this is disturbed, the bolus tends to remain in the piriform fossa [7]. As reported in our previous studies, posterior pharyngeal wall movement may also affect swallowing function in postoperative oral cancer patients; however, this has not been adequately studied [8].
Various reference points and measurement methods have previously been reported for analyzing fluoroscopic swallowing [4, 9,10,11,12,13,14,15,16]. However, the measured values vary considerably even in the same patient because of the difficulty of standardizing the patient’s position during examinations. Therefore, we report a new analytical method to achieve the reproducibility of a patient’s position by introducing a time axis into the perspective image analysis of the posterior pharyngeal wall. The movement of the posterior pharyngeal wall was classified as normal or abnormal, and the validity of the evaluation was reported in our previous study [8]. In a report examining the movement of the posterior pharyngeal wall in 13 patients with oral cancer, all patients were classified as normal before surgery. In the normal type, the processes on the posterior pharyngeal wall moved downward from the 2nd to 4th vertebral regions. This is consistent with the reported physiologically normal dynamics of the pharyngeal wall during swallowing [5, 15]. These data can be viewed as a visualization of the normal progressive contraction of the posterior pharyngeal wall.
Several studies have shown that the tongue is the most prevalent subsite in oral cancer [17]. Oral tongue functions in speech and articulation, as well as mastication, oral hygiene, and the oral phase of swallowing [18]. Therefore, it was hypothesized that tongue cancer patients may be an appropriate cohort to examine postoperative swallowing function and movement of the posterior pharyngeal wall. This study aimed to determine the association between progressive contraction of the posterior pharyngeal wall and dysphagia in postoperative patients with tongue cancer using videofluoroscopic swallowing study (VFSS) and sequential image analysis.
Materials and methods
Participants
This study was approved by the ethics committee of the School of Dentistry, Aichi Gakuin University, Nagoya, Japan (approval number: 2). A total of 34 patients (26 men and 8 women) who were diagnosed with tongue squamous cell carcinoma and underwent surgery were examined. The mean age of the participants was 59.5 ± 14.6 years. Patients who received chemotherapy or radiation therapy before or after surgery were excluded from this study. A VFSS was conducted to evaluate swallowing dysfunction at Aichi Gakuin University Dental Hospital and a general hospital (Table 1). According to the classification of the Union for International Cancer Control, tumors were classified as T1 in 10 cases, T2 in 16 cases, T3 in 6 cases, and T4 in 2 cases. Meanwhile, lymphatic metastases were classified as N0 in 23 cases, N1 in 6 cases, N2b in 4 cases, and N2c in 1 case. For the over-all staging, there were 9 patients (26.5%) in stage I, 14 (41.2%) in stage II, 6 (17.6%) in stage III, and 5 (14.7%) in stage IV. For the extent of resection, there were 19 cases of partial glossectomy, 11 of hemiglossectomy, and 4 of subtotal glossectomy. Neck dissections included eight radical neck dissections, seven functional neck dissections, and one supraomohyoid neck dissection. Reconstruction was performed in four patients using the rectus abdominis myocutaneous flap and nine patients using the pectoralis major myocutaneous flap.
VFSS
VFSS was performed 23.0 (9.0–39.0) days (median, interquartile range) after surgery. Patients were instructed to sit in a VFSS chair (MK-102; Tomomi-Koubou Co. Ltd., Shimane, Japan) and swallow in a state similar during normal feeding, without fixing the head with ear rods or bands. The X-ray fluoroscopy system used was DCW-30A (Toshiba Medical Systems Corporation, Tokyo, Japan). The swallowing sample was 50 ml of 50% w/v barium sulfate (Baritogen Deluxe; Fushimi Laboratory Co. Ltd., Kagawa, Japan) mixed with a thickener (Throsoft Liquid 12 g/pac; Kissei Pharmaceutical Co. Ltd., Nagano, Japan). One spoonful of the sample (approximately 5 ml) was held in the patient's mouth for each swallow, and swallowing was started with the signal of the examiner. The sample, given by the examiner, was ingested by the patient using the spoon. Patients who could not be ingest the sample from the oral cavity to the pharynx were injected with the sample into the tongue base with a syringe and to instructed to begin swallowing once it flowed into the pharynx. This method was performed thrice for each test. If less than 5 g of sample passed through the pharynx due to multiple swallows or oral residues, the swallowing time with the highest amount of sample passing through the pharynx was selected for analysis. In this study, the shape and length of the bolus did not affect the results because the order of movement of the posterior pharyngeal wall at the set point was considered.
Image analysis
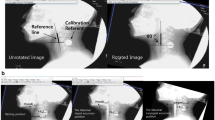
Image analysis was performed according to the procedures reported by Goto et al. [8]. The cervical spine was set as a reference as it is the closest reference to measure the motion (amount of change) of the posterior pharynx wall mucosa that moves from top to bottom as the sample passes through, and because it is considered as a reference object that does not significantly shift with the posterior pharynx wall mucosa when the sample is swallowed.
The lateral view of the VFSS image was recorded on a hard disk video recorder (AX300H; NEC Corporation, Tokyo). The storage format was MPEG2 (CBR), video bit rate was 8 Mbps, and resolution was 720 × 480. The videos recorded on the hard disk video recorder were imported into a personal computer and analyzed using the 2D video measurement software, Move-tr/2D 7.0 (Library Co., Ltd., Tokyo). The frame rate of the videos was 29.97 frames/s. The midpoints (A, B, C) between the upper end of the anterior surface of the 2nd, 3rd, and 4th cervical vertebrae and the lower end were plotted on each frame of the lateral view. The points of intersection of a line perpendicular to the line connecting the upper and lower ends of the cervical vertebrae and passing through A, B, and C with the anterior border of the posterior wall of the pharynx were defined as A', B', and C', respectively. The distances (thickness) between A-A', B-B', and C–C' were measured as a, b, and c, respectively (Figs. 1 and 2). Analysis started when the velum touched the pharyngeal wall and ended when it passed through the esophageal opening at the posterior end of the sample. For the evaluation of the swallowing pattern, the continuous changes in thickness (a, b, c) were analyzed in time series and the maximum values (amax, bmax, and cmax) were defined (Fig. 3). If the thickest value was observed on two or more frames, the last frame was used. If the maximum thickness (amax, bmax, cmax) was observed in the order of amax → bmax → cmax from top to bottom, it was defined as the “Normal” type. “Abnormal” type is defined when the timings of amax and bmax are the same (amax and bmax → cmax) or reversed (bmax → amax → cmax). When different patterns were shown in one test (during 3 swallows), the one showing the same pattern twice was determined as the swallowing type of the test. These analyses were performed by a radiologist (MG).
The figure indicates how to determine midpoint using real VFSS images. The second cervical vertebra is more triangular shape than other cervical vertebrae, but the anterior part has a bone with a considerable thickness; hence, that part was used for measurement.
The midpoints (A, B, C) between the upper end of the anterior surface of the 2nd, 3rd, and 4th cervical vertebrae and the lower end are plotted on each frame of the lateral view. The points of intersection of a line perpendicular to the line connecting the upper and lower ends of the cervical vertebrae and passing through A, B, and C with the anterior border of the posterior wall of the pharynx were defined as A', B', and C', respectively. The distances (thickness) between A-A', B-B', and C–C' were measured as a, b, and c, respectively.
There is no thickening of the posterior pharyngeal wall before swallowing (a), but during swallowing it thickens at the second intervertebral space (a).
Normal type: The thickest values (amax, bmax, and cmax) were observed in the following order: amax → bmax → cmax.
Abnormal type: The amax and bmax were observed in the same frame, followed by the cmax, or the amax and bmax were observed out of sequence (bmax → amax → cmax).
Analytical methods
The relationship between the postoperative swallowing type and swallowing dysfunction score was determined. A radiologist (EA) and an oral surgeon (SW) with more than 10 years of experience in interpreting VFSS images assessed the level of swallowing dysfunction using the VFSS images obtained. These evaluators were not those who assessed pharyngeal wall movement. Three parameters—residual sample amount in the epiglottic vallecula and piriform sinus, and aspiration amount—were examined to evaluate the level of functional impairment. It was graded using a 4-point scale (0–3): a score of 0 corresponded to no rest or no aspiration, 1 corresponded to a small amount of sample observed, and 2 and 3 points corresponded to medium and large amounts of sample observed, respectively (maximum 9 points). Two evaluators assessed each patient separately and averaged their scores to determine the dysfunction score. The determined score was compared to the postoperative swallowing type (normal or abnormal). The Wilcoxon signed rank-sum test and Fisher’s exact test were used to analyze the results, and statistical significance was set at p < 0.05. Factors related to the postoperative swallowing type were analyzed using logistic multiple regression analysis. Multiple regression analysis was conducted only for factors that were significantly different in the univariate analysis. The statistical software program JMP 16.1.0(SAS Institute) was used for analysis.
Results
A total of 102 swallowing VFSS images were obtained from 34 patients. In the no-VFSS file, any patient that showed marked neck torsion disturbed the analysis of the lateral view. In all cases, swallowing was voluntarily started, and no patient started swallowing reflexively when the sample flowed into the pharynx.
Image analysis
Among the samples, 24 patients were classified under the normal type, while 10 patients were in the abnormal type (Table 2). In the normal type, all three swallows were of normal pattern in 20 cases. In the other four cases, the abnormal pattern emerged 1 in 3 times while the subject was swallowing. The four cases included the same (amax and bmax → cmax) in two cases and the reverse (bmax → amax → cmax) in two cases. In the abnormal type, there was no trend in the combination of the same (amax and bmax → cmax) and reversed (bmax → amax → cmax) swallows; however, in 6 out of 10 cases, the first swallow was inverse (bmax → amax → cmax).
Out of the 102 sets of VFSS files, 75 showed normal activity. Meanwhile, 27 files showed abnormal activity, seven of which were the same (amax and bmax → cmax), 12 were reversed (bmax → amax → cmax) swallow, and three were showed no movement.
Relationship between the postoperative swallowing type and swallowing dysfunction score
The dysfunction scores were 1.0 (0–4.0) (Table 1). A significant difference was found in the scores between patients with normal type 0 (0–1.8) and abnormal type 4.0 (4.0–5.0) (p < 0.01). As the swallowing dysfunction score increased, the percentage of abnormal type increased in the swallowing type (Table 3).
Relationship between the postoperative swallowing type and methods of surgery (Table 4).
In the normal group, 17/24 (70.8%) patients underwent partial resection. The extent of resection and stage was significantly different between the normal and abnormal groups. There was also a significant difference between the two groups in terms of the following: whether the tongue base was included in the excision range (p < 0.01), whether neck dissection was performed (p < 0.01), or whether reconstruction was not performed (p < 0.01). However, there were no significant differences for these items in the multivariate analysis.
Discussion
Despite being small sample size, the results of this study are based on an image analysis of the swallowing patterns of each case in detail and divided into normal and abnormal in the posterior pharyngeal wall movement. The results showed that there was a significant difference in dysphagia scores between the postoperative swallowing type and swallowing dysfunction score. This implies that dysphagia is related to the movement of the posterior pharyngeal wall after tongue cancer surgery.
The inclusion of the tongue base in the resection area was more frequent in the abnormal than in the normal group. Previous reports that examined the relationship between surgical details and dysphagia reported that the extent of resection, including the tongue base, was associated with decreased swallowing function [4, 19, 20]. Aspiration was observed at a significantly higher rate in patients who underwent hemi- or total glossectomy than in those who underwent partial glossectomy [21]. McConnel et al. [1] reported that in cases of dysfunction after tongue cancer surgery, swallowing function is relatively good if more than one-half of the tongue base remains after partial glossectomy of the movable portion of the tongue or hemiglossectomy. The results of this study are similar to those of previous reports, which showed that dysphagia was more frequent when the tongue base was included in the resection area.
The more extensive the resection area, the more problems occur in the pharyngeal phase in addition to oral phase impairments [2]. There was a significant difference between the extent of resection and the swallowing type of the posterior pharyngeal wall. Similar to the findings of previous reports, there was an association between pharyngeal phase impairment and the extent of resection. However, few reports have examined the relationship between oral cancer and the movement of the posterior pharyngeal wall. Fujiu et al. [3] studied the motion of the posterior pharyngeal wall in postoperative patients with oral cancer and reported a 30% increase in the anterior protrusion of the posterior pharyngeal wall during swallowing in 6/11 patients 3 months postoperatively. It was concluded that the anterior protrusion of the posterior pharyngeal wall compensated for the anterior shift of the tongue base due to tongue resection. McConnel et al. [22] used manofluorography under VFSS to test swallowing pressure and reported that the maximum swallowing pressure produced in the pharynx propagates sequentially from above to below, creating a clearance force that handles pharyngeal residue. Sufficient contact time between the tongue base and posterior pharyngeal wall is important for producing sufficient pharyngeal pressure [4]. Pharyngeal constriction, set into motion by the swallow response, is critical in generating the forces and pressure necessary for guiding the bolus efficiently to the upper esophageal sphincter. The wave begins when the tongue base makes firm contact with the posterior pharyngeal wall. The mechanical force of the tongue and the pressure increase created by the decrease in the supra-bolus area serve to propel the bolus downward. The segmental and sequential contraction of the pharyngeal constrictors follow along the tail end of the bolus, acting to guide and clear the residue that remains in the pharynx [23]. Thus, the mechanical force of the tongue and the segmental and sequential contraction of the pharyngeal constrictors are important. The posterior movement of the tongue is impeded by the extensive resection of the tongue base and reconstruction with a flap. In addition, resection and reconstruction change the shape of the tongue base and pull or compress the pterygomandibular raphe. The superior constrictor extends back from the pterygomandibular raphe to the posterior pharyngeal raphe. The superior, middle, and inferior constrictor muscles terminate at the middle of the posterior pharyngeal wall, creating a seam called the posterior pharyngeal raphe [23]. It is possible that the movement of the posterior pharyngeal wall was changed by affecting the segmental and sequential contraction of the pharyngeal constrictors by pulling or compressing the pterygomandibular raphe. These two factors may have caused pharyngeal residue and aspiration.
In the posterior pharyngeal wall movement, the frequency of neck dissection was significantly higher in the abnormal type than in the normal type group. Many of the cases in which neck dissection was performed had advanced primary lesions, and many of them were accompanied by the excision of the tongue base. Son et al. [21] reported that male gender, extensive tumor resection, higher node stage, and more extensive lymph node dissection were major risk factors for aspiration in tongue cancer patients. The report states that lymph node metastasis results in pharyngeal dysphagia because it implies a more advanced disease that requires more extensive resection. Hirai et al. [24] studied neck dissection and swallowing function and found that swallowing function after cervical dissection was altered by the anterior and inferior displacement of the hyoid bone at rest and in the highest position, decreased distance of the hyoid bone movement during swallowing, and increased laryngeal penetration compared with preoperative observations. The middle constrictor originates in a narrow band on the greater horn of the hyoid bone and courses back to the posterior pharyngeal raphe just below the superior constrictor [23]. Because of the displacement of the hyoid bone, neck dissection may have affected the motion of the posterior pharyngeal wall. The relationship between neck dissection and the dysphagia of the posterior pharyngeal wall is unclear from previous studies. However, it cannot be ruled out that neck dissection affects the movement of the posterior pharyngeal wall.
As the dysphagia score increased, the percentage of abnormal swallowing types increased. Compared to the relationship between dysphagia score and swallowing type, dysphagia score increased as the stage increased, and abnormal type tended to increase in swallowing type. As the stage increased, the extent of resection became wider and the surgical invasiveness increased, which may have contributed to the increase in dysphagia score and abnormal swallowing type. We observed one case with abnormal type and 0 dysphagia score in stage II and another case with normal type and 7 dysphagia score in stage IV. Not only surgical invasion, but also other factors can be considered. For a patient with abnormal swallowing type despite zero swallowing dysfunction score, association with preoperative swallowing type may exist, but preoperative evaluation was not performed in this study. In a previous report [8], we examined preoperative and postoperative posterior pharyngeal wall motion in 13 head and neck cancer patients. Preoperative posterior pharyngeal wall motion was normal in all patients and was not evaluated preoperatively in this study. We believed that preoperative swallowing types would not affect postoperative swallowing types. However, further studies are needed to examine factors other than surgical invasion, including preoperative swallowing type. This study was limited by the small number of instances, variability in resection range, wide range of days for VFSS, and fewer advanced stages of cases (III or IV). Nevertheless, the results showed that posterior pharyngeal wall movement was altered after surgery in patients with tongue cancer who had severe dysphagia by the VFSS. Further studies with a more detailed analysis and a larger number of patients are necessary.
Patients with tongue cancer and severe dysphagia had altered posterior pharyngeal wall movement postoperatively. It was not possible to clarify whether the movement of the posterior pharyngeal wall was related to pharyngeal residue or aspiration. However, patients with severe dysphagia show abnormal movements in the posterior pharyngeal wall as well. Therefore, posterior pharyngeal wall movement and dysphagia may be related. This condition is not sustained and may be in the process of healing; therefore, it may be possible to restore it back into normal in future. For further studies, it will be necessary to track the course of cases showing the abnormal type and verify whether the changes are reversible.
Change history
09 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10266-022-00740-6
References
McConnel FM, Logemann JA, Rademaker AW, Pauloski BR, Baker SR, Lewin J, Shedd D, Heiser MA, Cardinale S, Collins S, Graner D. Surgical variables affecting postoperative swallowing efficiency in oral cancer patients: a pilot study. Laryngoscope. 1994;104:87–90.
Logemann JA, Bytell DE. Swallowing disorders in three types of head and neck surgical patient. Cancer. 1979;44:1095–105.
Fujiu M, Logemann JA, Pauloski BR. Increased postoperative posterior pharyngeal wall movement in patients with anterior oral cancer: preliminary findings and possible implications for treatment. Am J Speech Lang Pathol. 1995;4:24–30.
Pauloski BR, Logemann JA. Impact of tongue base and posterior pharyngeal wall biomechanics on pharyngeal clearance in irradiated postsurgical oral and oropharyngeal cancer patients. Head Neck. 2000;22:120–31.
Groher ME. Nature of the problem. In: Groher ME, editor. Dysphagia, diagnosis and management. 3rd ed. Boston: Butterworth-Heinemann; 1997. p. 1–214.
Groher ME, Crary MA. Dysphagia: clinical management in adults and children. Maryland Heights, MO: Mosby Elsevier; 2010.
Dejaeger E, Pelemans W, Ponette E, Joosten E. Mechanisms involved in postdeglutition retention in the elderly. Dysphagia. 1997;12:63–7.
Gotoh M, Watanabe S, Ohshige H, Izumi M, Naitoh M, Ariji Y, Oh-iwa I, Shimozato K, Ariji E. Computer-based videofluorographic analysis of posterior pharyngeal wall movement during swallowing in patients with head-and-neck cancer. Oral Radiol. 2009;25:123–8.
Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology. 1992;103:128–36.
Johnson ER, McKenzie SW, Rosenquist CJ, Lieberman JS, Sievers AE. Dysphagia following stroke: quantitative evaluation of pharyngeal transit times. Arch Phys Med Rehabil. 1992;73:419–23.
Johnson ER, McKenzie SW. Kinematic pharyngeal transit times in myopathy: evaluation for dysphagia. Dysphagia. 1993;8:35–40.
McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98:71–8.
Aloysius A, Born P, Kinali M, Davis T, Pane M, Mercuri E. Swallowing difficulties in Duchenne muscular dystrophy: indications for feeding assessment and outcome of videofluroscopic swallow studies. Eur J Paediatr Neurol. 2008;12:239–45.
Nozaki S, Umaki Y, Sugishita S, Tatara K, Adachi K, Shinno S. Videofluorographic assessment of swallowing function in patients with Duchenne muscular dystrophy. Rinsho Shinkeigaku. 2007;47:407–12.
Leonard RJ, Kendall KA, Johnson R, McKenzie S. Swallowing in myotonic muscular dystrophy: a videofluoroscopic study. Arch Phys Med Rehabil. 2001;82:979–85.
Han TR, Kim HR, Kim SJ. Dysphagia development after surgery unrelated to laryngeal andpharyngeal structures. Dysphagia. 2009;24:167–71.
World Health Organization - International Agency for Research on Cancer (IARC). Alcohol drinking. In: IARC monographs on the evaluation of the carcinogenic risks to humans volume 44. United Kingdom: IARC; 1988.
Lam L, Samman N. Speech and swallowing following tongue cancer surgery and free flap reconstruction–a systematic review. Oral Oncol. 2013;49:507–24.
Pauloski BR, Rademaker AW, Logemann JA, McConnel FMS, Heiser MA, Cardinale S, Lazarus CL, Pelzer H, Stein D, Beery Q. Surgical variables affecting swallowing in patients treated for oral/oropharyngeal cancer. Head Neck. 2004;26:625–36.
Kodama N, Kumai Y, Miyamoto T, Matsubara K, Samejima Y, Orita Y. Factors affecting the swallowing dysfunction following oral cancer surgery. Ann Rehabil Med. 2021;45:368–78.
Son YR, Choi KH, Kim TG. Dysphagia in tongue cancer patients. Ann Rehabil Med. 2015;39:210–7.
McConnel FM, Cerenko D, Mendelsohn MS. Manofluorographic analysis of swallowing. Otolaryngol Clin North Am. 1988;21:625–35.
Corbin-Lewis K, Liss JM, Sciortino KL. Examination of the pharyngeal swallow component. In: Clinical anatomy and physiology of the swallow mechanism. New York: Thomson Delmar Learning; 2005. p. 41–66.
Hirai H, Omura K, Harada H, Tohara H. Sequential evaluation of swallowing function in patients with unilateral neck dissection. Head Neck. 2010;32:896–904.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Approval was obtained from the ethics committee of the School of Dentistry, Aichi Gakuin University, Nagoya, Japan (approval number: 2). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Patients signed informed consent regarding publishing their data and photographs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Watanabe, S., Gotoh, M., Naitoh, M. et al. Alterations of posterior pharyngeal wall movement during swallowing in postoperative tongue cancer patients: assessment with a videofluoroscopic swallowing study. Odontology 111, 228–236 (2023). https://doi.org/10.1007/s10266-022-00731-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-022-00731-7