Abstract
This study investigated the effects of acute footshock stress (FS) on the occurrence of rhythmic masticatory muscle activity (RMMA) during sleep in guinea pigs. Animals were prepared for chronic recordings from electroencephalogram, electrooculogram and electromyograms of neck and masseter muscles. The signals were recorded for six hours on the two successive days: the first day with stress-free condition (non-FS condition) and the second day with acute FS (FS condition). Sleep/wake states and RMMA were scored visually. Sleep variables and the frequency of RMMA occurring during non-rapid eye movement (NREM) sleep were compared during 6-h periods between the two conditions. Compared to non-FS condition, the amount of total sleep and NREM sleep significantly reduced during 2 h following the acute FS in the FS condition. Similarly, the frequency of RMMA significantly increased during 2 h following the acute FS for the FS condition compared to non-FS condition. During 2–6 h after FS in the FS condition, sleep variables and the frequency of RMMA did not differ from those without FS in the non-FS condition. These results suggest that acute experimental stress can induce transient changes in sleep–wake states and the occurrence of RMMA in experimental animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep bruxism (SB) is characterized by the excessive rhythmic masticatory muscle activity (RMMA) occurring predominantly in non-rapid eye movement (NREM) sleep. Among various factors, stress has been commonly believed as a risk or causative factor for SB. In humans, many studies have attempted to show the associations between stress and daily variations of masticatory muscle activity during sleep period [1,2,3,4,5]. However, those studies failed to demonstrate clear and consistent associations. Those studies did not measure and assess sleep states (i.e., electroencephalogram, EEG) in response to stress although stress can influence sleep architecture and increase arousals.
Experimental animals showed a state-dependent modulation of masticatory muscle electromyogram (EMG) activity during sleep states [6]. In addition, previous studies showed that the balance in the time distribution between sleep and wakefulness was modified by the acute experimental stress in animals [7]. RMMA, typically seen in patients with sleep bruxism, was found to occur during NREM sleep in naturally sleeping guinea pigs [8]. Therefore, animal model can be used to assess the stress-related response of RMMA during sleep. Previous studies have been conducted to assess the physiological and behavioral effects of acute stress in experimental animals, as animal study has the advantage to control the level of stress loading and to minimize the inter-individual difference of stress response in daily life. Those studies showed that the amount of different vigilance states is modified by the experimental stress in animals [7, 9, 10]. However, masticatory muscle activity has been rarely investigated in relation to vigilance states. Therefore, this study aimed to investigate the changes in the occurrence of rhythmic masticatory muscle activity during sleep and the association with the alteration of sleep induced by acute footshock stress (FS) in freely moving guinea pigs.
Materials and methods
Experiments were carried out on nine adult male albino guinea pigs (Hartley) weighing 550–650 g. All experimental procedures were approved by the animal research ethics committee of the Osaka University Graduate School of Dentistry.
Surgery was performed under ketamine (40 mg/kg, i.p.) and xylazine (40 mg/kg, i.m.) anesthesia with a premedication of atropine (0.04 mg/kg, i.p.). Electrodes for EEG, electro-occulogram and EMGs from the dorsal neck muscle and the unilateral masseter (MAS) muscle were installed as reported previously [6]. Fourteen days were allowed for the animals to recover from surgical intervention and adapt to the recording conditions.
Recordings were made during the two consecutive days for 6 h during a light period (10:30–16:30). During the recording, an animal was housed in the recording acrylic chamber (14 × 25 × 28 cm) with a footshock grid floor (8 mm). Footshock stress was induced by the electric shocks (intensity: 1 mA and duration: 0.5 s) [7, 9, 10]. Electric shocks were not delivered on the first day while they were delivered on the second day between 10:00 and 10:30 with an inter-stimulus interval of 30 s. A lightweight shielded cable connected the multiple pins on the animal’s head to the multi-channel slip-ring. Animals had free access to food and water during recordings.
Wakefulness, NREM sleep and REM sleep were determined for 10-s epochs as previously reported [6]. The percentages of these states during the two-hour recording periods were calculated based on the number of the scoring epochs. Sleep episodes were classified into NREM-REM episodes with NREM sleep followed by REM sleep and NREM episodes followed by wakefulness. The frequency of sleep episodes per hour was calculated. The durations of NREM and REM sleep were also calculated by the number of the NREM or REM epochs in each sleep episode. RMMA was scored as the masseter EMG events including at least three consecutive phasic bursts during NREM sleep [8] (Fig. 1A, B, C). These episodes were counted separately if the inter-burst interval was > 3 s. The time from the start of recording to the first sleep episodes was also measured. The frequency during NREM sleep per hour of the two-hour recording periods was calculated and the duration of RMMA was measured. Power spectral analyses were performed by computing fast Fourier transforms on 10-s epochs with a cosine window tapering. Artifacts were rejected by visual inspection and analyses were performed on artifact-free epochs. The median power of the delta frequency band (0.5–4.5 Hz) was assessed for each animal.
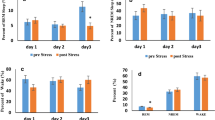
Rhythmic masticatory muscle activity (RMMA) during non-rapid eye movement (NREM) sleep and the changes after acute footshock stress (FS). (A) Chewing during wakefulness. (B, C) Examples of RMMA during NREM sleep. EEG electroencephalogram; EOG electrooculogram; NE neck electromyogram; and MA: masseter electromyogram. Vertical bar: 0.5 mV; horizontal bar: 1 s. (D) Time-course change of the frequency of RMMA for six hours. **: p < 0.01. (E) Time-course changes of the duration of RMMA episodes for six hours. Dashed lines: non-FS; solid lines: FS. Data are presented as mean ± SEM
For statistical analyses, sleep and oromotor variables were quantified for each 2-h recording section. All data were pooled for each animal. Data were presented by mean ± standard error. Two-way repeated-measure ANOVAs were done to assess time-related changes of the variables with post hoc paired t tests between the FS and control conditions. Pearson correlation analysis was done by comparing the difference of the frequency of RMMA and sleep variables during the first two hours after the start of the recording. Statistical significance was determined by p < 0.05.
Results
The frequency of RMMA per hour of NREM sleep showed a significant condition effect (p = 0.006) while neither interaction between condition and time (p = 0.307) nor time effect was found (p = 0.075) (Fig. 1d). The frequency of RMMA per hour of NREM sleep during the first two hours was significantly higher in the FS condition (12.5 ± 1.7 times/hr) than in the non-FS conditions (7.9 ± 1.3 times/hr) (p = 0.005) (Fig. 1d). After that, it did not differ between FS and non-FS. In the first two hours, the difference in the number of RMMA episodes between the two conditions was marginal (CTL: 4.8 ± 1.3 times; FS: 7.0 ± 3.8 times, p = 0.07; paired t test). In addition, for non-FS condition, within the first two hours, approximately one-third of RMMA episodes (34.5 ± 18.7%) were found to occur in the first one hour and 65.5 ± 18.7% in the following one hour (p = 0.001; paired t test). However, in FS condition, no difference was found for the number of RMMA episodes between the first (41.9 ± 34.2%) and the following (58.1 ± 34.2%) one hour of the first two hours after the start of recording. The duration of RMMA episode did not show any significant difference between the two conditions (Condition effect: p = 0.218; Time effect: p = 0.233; Interaction: p = 0.815) (Fig. 1e).
After FS, animals showed a significant delay in starting sleep in comparison to non-FS condition: the first sleep episode appeared at 1056.7 ± 1014.7 (60–2760) sec after the start of recording (i.e., after FS) in FS condition while it did at 202.2 ± 396.1 (0–1200) sec in non-FS condition (p = 0.04, paired t test). In the FS condition, the percentages of wakefulness showed significant condition effect (p = 0.022) and interaction between time and group effects (p = 0.002). The percentage of wakefulness during the first two hours was significantly higher in the FS conditions (69.3 ± 3.0%) than in non-FS condition (53.9 ± 1.8%; p = 0.002) (Fig. 2A). Therefore, total sleep time showed significant decrease during the first two hours from the first day to the second day, i.e., non-FS vs. FS conditions (p = 0.002) (Fig. 2B). During sleep, the percentage of NREM sleep showed significant interaction between condition and time (p = 0.003) and condition effect (p = 0.013): it was significantly lower for the FS (28.8 ± 2.8%) than non-FS (40.8 ± 1.7%) (p = 0.002) for the first two hours (Fig. 2C). The changes of the RMMA frequency during the first two hours from non-FS to FS conditions were not correlated with those of sleep variables. No statistical difference was found for the percentage of REM sleep for six hours between the two conditions (condition effect: p = 0.419; Time effect: p = 0.3; interaction: p = 0.095) (Fig. 2D).
Changes of sleep variables during recording. Percentage of wakefulness (A) and sleep periods (B). Percentage of NREM sleep (NR) (C) and REM sleep (R) (D). Duration of NREM sleep (E) and REM sleep (F). Cortical delta (δ) power calculated by power spectral analysis (G). Dashed lines: non-FS; solid lines: FS. Data are presented as mean ± SEM. **: p < 0.01
Frequency of total sleep episodes did not differ between the conditions for six hours (condition effect: p = 0.634; time effect = 0.649; interaction: p = 0.163). There was no significant interaction between condition and time (p = 0.05) for the frequency of NREM-REM sleep episodes (condition effect: p = 0.36; time effect: p = 0.965). Mean duration of NREM sleep did not show significant interaction between condition and time (p = 0.225, Fig. 2E) and that of REM sleep did not (p = 0.819, Fig. 2F). Power spectral analyses of delta EEG power during NREM sleep showed no significant difference between the two conditions for six hours (condition effect: p = 0.184; time effect: p = 0.287; interaction: p = 0.074, Fig. 2G).
Discussion
There is an inter-individual difference in the association between stress and sleep-related masticatory muscle activity in the daily life in humans [1, 11, 12]. However, little studies have done to investigate the association between masticatory muscle activities during sleep and stress-related sleep changes in humans. This study assessed sleep states and the occurrence of RMMA after acute FS in freely moving guinea pigs. After acute FS, sleep periods significantly decreased while waking periods increased. Following FS, the frequency of RMMA significantly increased. The results showed that acute FS was followed by the transient changes in the sleep–wake patterns as well as in the occurrence of RMMA during NREM sleep in guinea pigs.
This study recorded sleep and wakefulness on the two consecutive days. On the first day without FS, sleep variables, such as the percentage of sleep/wake periods, fell within a range that was reported by the previous studies in guinea pigs [6, 13, 14]. In the following day, acute FS was applied during 30 min before the recordings. Stimulus intensity used in this study was in a similar range to that in the previous studies [7, 9, 10]. The results showed that, during the first two hours after acute FS, the amount of waking periods was significantly increased and that of sleep was decreased. These results were consistent with those of the previous studies in which the amount and the occurrence of sleep periods were varied after FS. In rats, acute FS decreased REM sleep rather than NREM sleep [15,16,17] while both NREM and REM sleep decreased during 6 h after FS [9]. In mice, acute FS reduced NREM sleep by 20% and REM sleep by 3% for two hours after FS [10]. The discrepancy in the effects of FS can be due to the stimulus duration and intensity, and experimental protocol. In addition, our study showed that the first sleep episode appeared significantly later after the start of recording in FS condition compared to the non-FS condition. However, the previous and present studies consistently reported that transient changes of sleep following acute FS were reversible: the decrease of sleep and increase of wakefulness were recovered 2 h after acute FS in this study [18, 19]
RMMA during NREM sleep was found to occur more frequently during 2 h after FS than during 2-h period corresponding the previous day, i.e., non-FS condition. Thus, the decrease in the occurrence of sleep states and the increase of wake states after FS were associated with the increase of RMMA in guinea pigs. However, the changes of RMMA frequency was not correlated with those of sleep variables. The results would support the previous findings that inter-individual variability in the changes of sleep and masticatory muscle activities in response to stress in humans [1,2,3,4,5, 7, 9, 10]. In addition, the duration of RMMA episodes did not differ between the two conditions, suggesting that the initiation of RMMA was exaggerated during NREM sleep after FS. RMMA episodes in guinea pigs can occur in association with a slight decrease of delta EEG power and cardiac activation during sleep [8]. When RMMA was experimentally induced by electrical stimulation to the pyramidal tract during NREM sleep, the period was associated with a lower delta EEG activity than the period without no RMMA responses [20]. Therefore, the increase of RMMA can be associated with the enhanced arousal activity during NREM sleep after acute FS. Previous studies showed that the changes of sleep and awake periods after acute FS were associated with the increased activity of catecholaminergic and monoaminergic system [21, 22]. The changes in the activity of catecholaminergic and monoaminergic systems can also play roles for the increase of RMMA after FS since they are known to have direct projections to central pattern generator networks [23,24,25] and facilitate the excitability of trigeminal motoneurons and central pattern generator network [26,27,28,29,30]. In addition, limbic system such as the amygdala might be another candidate for the increased occurrence of RMMA after acute FS [21, 22, 31, 32] since it was found to trigger RMMA [33, 34]. The results would suggest the possibility that the increase of RMMA after acute FS is a secondary stress response of brainstem networks contributing to the arousal and RMMA genesis. The above neural systems need to be investigated for clarifying the association between sleep physiology and RMMA genesis after acute stress.
Humans have polyphasic sleep with a sequence of several NREM–REM sleep cycles in a night, while sleep of guinea pigs is monophasic with behavioral wakefulness between sleep cycles. Despite structural difference of sleep between humans and guinea pigs, human sleep studies have shown that negative emotions before sleep can disturb deep sleep such as the increase of intermittent awakenings and the delay of the start of sleep [18, 35]. Thus, the decrease in the occurrence of sleep states and the increase of wake states after FS in guinea pigs can correspond to the sleep disturbance after stress experience in humans. Nonetheless, in humans, the roles of stress or stress-related response of sleep on the genesis of RMMA have not been clearly demonstrated with sleep and masticatory EMG recordings [1, 11, 12]. Therefore, cautions are needed to apply the results of the present study in animals for the stress-related responses of RMMA during NREM sleep in humans.
Conclusions
This study investigated the after-effects of acute FS on sleep states and the occurrence of RMMA during NREM sleep. The results suggest that acute experimental stress can induce transient changes in sleep–wake states along with the occurrence of RMMA in experimental animals. Therefore, our results suggest that acute stress can be a candidate factor transiently affecting the RMMA genesis in naturally sleeping guinea pigs.
References
Pierce CJ, Chrisman K, Bennett ME, Close JM. Stress, anticipatory stress, and psychologic measures related to sleep bruxism. J Orofac Pain. 1995;9:51–6.
Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain. 2009;23:153–66.
Rao SM, Glaros AG. Electromyographic correlates of experimentally induced stress in diurnal bruxists and normals. J Dent Res. 1979;58:1872–8.
Clark GT, Rugh JD, Handelman SL. Nocturnal masseter muscle activity and urinary catecholamine levels in bruxers. J Dent Res. 1980;59:1571–6.
Manfredini D, Fabbri A, Peretta R, Guarda-Nardini L, Lobbezoo F. Influence of psychological symptoms on home-recorded sleep-time masticatory muscle activity in healthy subjects. J Oral Rehabil. 2011;38:902–11.
Kato T, Masuda Y, Kanayama H, et al. Heterogeneous activity level of jaw-closing and -opening muscles and its association with arousal levels during sleep in the guinea pig. Am J Physiol Regul Integr Comp Physiol. 2010;298:R34-42.
Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008;32:99–117.
Kato T, Toyota R, Haraki S, et al. Comparison of rhythmic masticatory muscle activity during non-rapid eye movement sleep in guinea pigs and humans. J Sleep Res. 2018;27:e12608.
Adrien J, Dugovic C, Martin P. Sleep-wakefulness patterns in the helpless rat. Physiol Behav. 1991;49:257–62.
Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003;147:193–202.
Watanabe T, Ichikawa K, Clark GT. Bruxism levels and daily behaviors: 3 weeks of measurement and correlation. J Orofac Pain. 2003;17:65–73.
van Selms MK, Lobbezoo F, Wicks DJ, Hamburger HL, Naeije M. Craniomandibular pain, oral parafunctions, and psychological stress in a longitudinal case study. J Oral Rehabil. 2004;31:738–45.
Ibuka N. Ontogenesis of circadian sleep-wakefulness rhythms and developmental changes of sleep in the altricial rat and in the precocial guinea pig. Behav Brain Res. 1984;11:185–96.
Tobler I, Franken P. Sleep homeostasis in the guinea pig: similar response to sleep deprivation in the light and dark period. Neurosci Lett. 1993;164:105–8.
Kant GJ, Pastel RH, Bauman RA, et al. Effects of chronic stress on sleep in rats. Physiol Behav. 1995;57:359–65.
Palma BD, Suchecki D, Tufik S. Differential effects of acute cold and footshock on the sleep of rats. Brain Res. 2000;861:97–104.
Vazquez-Palacios G, Velazquez-Moctezuma J. Effect of electric foot shocks, immobilization, and corticosterone administration on the sleep-wake pattern in the rat. Physiol Behav. 2000;71:23–8.
Kim EJ, Dimsdale JE. The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav Sleep Med. 2007;5:256–78.
Vandekerckhove M, Weiss R, Schotte C, et al. The role of presleep negative emotion in sleep physiology. Psychophysiology. 2011;48:1738–44.
Yamada KI, Higashiyama M, Toyoda H, et al. Experimentally induced rhythmic jaw muscle activities during non-rapid eye movement sleep in freely moving guinea pigs. J Sleep Res. 2019;28:e12823.
Liu X, Tang X, Sanford LD. Fear-conditioned suppression of REM sleep: relationship to Fos expression patterns in limbic and brainstem regions in BALB/cJ mice. Brain Res. 2003;991:1–17.
Liu X, Yang L, Wellman LL, Tang X, Sanford LD. GABAergic antagonism of the central nucleus of the amygdala attenuates reductions in rapid eye movement sleep after inescapable footshock stress. Sleep. 2009;32:888–96.
Takeuchi Y, Satoda T, Tashiro T, Matsushima R, Uemura-Sumi M. Amygdaloid pathway to the trigeminal motor nucleus via the pontine reticular formation in the rat. Brain Res Bull. 1988;21:829–33.
Mascaro MB, Bittencourt JC, Casatti CA, Elias CF. Alternative pathways for catecholamine action in oral motor control. Neurosci Lett. 2005;386:34–9.
Mascaro MB, Prosdocimi FC, Bittencourt JC, Elias CF. Forebrain projections to brainstem nuclei involved in the control of mandibular movements in rats. Eur J Oral Sci. 2009;117:676–84.
Stafford IL, Jacobs BL. Noradrenergic modulation of the masseteric reflex in behaving cats II Physiological studies. J Neurosci. 1990;10:99–107.
Ribeiro-do-Valle LE, Metzler CW, Jacobs BL. Facilitation of masseter EMG and masseteric (jaw-closure) reflex by serotonin in behaving cats. Brain Res. 1991;550:197–204.
Shao YP, Sutin J. Noradrenergic facilitation of motor neurons: localization of adrenergic receptors in neurons and nonneuronal cells in the trigeminal motor nucleus. Exp Neurol. 1991;114:216–27.
Nagase Y, Moritani M, Nakagawa S, et al. Serotonergic axonal contacts on identified cat trigeminal motoneurons and their correlation with medullary raphe nucleus stimulation. J Comp Neurol. 1997;384:443–55.
Schwarz PB, Mir S, Peever JH. Noradrenergic modulation of masseter muscle activity during natural rapid eye movement sleep requires glutamatergic signalling at the trigeminal motor nucleus. J Physiol. 2014;592:3597–609.
Nikolaev E, Kaczmarek L, Zhu SW, Winblad B, Mohammed AH. Environmental manipulation differentially alters c-Fos expression in amygdaloid nuclei following aversive conditioning. Brain Res. 2002;957:91–8.
Trentani A, Kuipers SD, te Meerman GJ, et al. Immunohistochemical changes induced by repeated footshock stress: revelations of gender-based differences. Neurobiol Dis. 2003;14:602–18.
Kawamura Y, Tsukamoto S. Analysis of jaw movements from the cortical jaw motor area and amygdala. Jpn J Physiol. 1960;10:471–88.
Nakamura Y, Kubo Y. Masticatory rhythm in intracellular potential of trigeminal motoneurons induced by stimulation of orbital cortex and amygdala in cats. Brain Res. 1978;148:504–9.
Petersen H, Kecklund G, D’Onofrio P, Nilsson J, Akerstedt T. Stress vulnerability and the effects of moderate daily stress on sleep polysomnography and subjective sleepiness. J Sleep Res. 2013;22:50–7.
Acknowledgements
This study was supported by the Japan Society of the Promotion for Science (JSPS; #16H0696) and partially by the JSPS (#18H02965 and #20K18632), and by the funds from the Intractable Oral Disease at Osaka University Graduate School of Dentistry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yano, H., Ueno, Y., Higashiyama, M. et al. After-effects of acute footshock stress on sleep states and rhythmic masticatory muscle activity during sleep in guinea pigs. Odontology 110, 476–481 (2022). https://doi.org/10.1007/s10266-021-00679-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-021-00679-0