Abstract
The guided bone regeneration (GBR) technique is often applied to provide sufficient bone for ideal implant placement. The objective of this study was to evaluate whether GC membrane®, which has already been used for guided tissue regeneration (GTR), can also be available for GBR. Twenty-three implants in 18 patients were evaluated in the study. All patients underwent implant placement with GBR using GC membrane®. Cone-beam computed tomography was performed at 13–30 weeks after surgery and the amount of augmented bone was assessed. The implant stability quotient (ISQ) was measured at the second operation to evaluate implant stability. Although wound dehiscence was observed at 4 of 23 regions (17.4%), all wounds closed quickly without any events by additional antibiotic administration. GBR-induced bone augmentation of 0.70–2.56 mm horizontally and 0–6.82 mm vertically. Only 0.18 mm of bone recession was observed at 16–24 months after implant placement. GBR with GC membrane® induced sufficient bone augmentation, leading to successful implant treatment. The present results suggest that GC membrane® is available not only for GTR, but also for GBR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental implant treatment is one of the reliable treatment strategies for oral rehabilitation of the edentulous alveolus. However, the placement of an implant at the ideal position and direction is often difficult at the edentulous region, because the alveolar bone recedes with time after tooth loss, especially on the labial/buccal side [1]. In such cases, bone augmentation, including bone transplantation and guided bone regeneration (GBR), is necessary to improve the outcome of dental implant treatment both functionally and esthetically. GBR is more popular because of its reliability and need for less intervention.
A resorbable or non-resorbable membrane is essential for GBR to prevent soft tissue invasion at the bone augmentation site [2, 3]. It was reported that a non-resorbable membrane shows more bone regenerative ability than a resorbable membrane because of its persistent blocking effect on soft tissue invasion [4, 5]. However, a non-resorbable membrane has some disadvantages compared with a resorbable membrane. First, a non-resorbable membrane requires an additional surgical intervention for its removal after an appropriate period. Second, there is a risk of infection through membrane exposure resulting from wound dehiscence. Meanwhile, a resorbable membrane has the great advantage of not requiring removal. However, resorbable membranes made from porcine or bovine collagen, comprising most commercially available membranes [6], have an insufficient space-forming period of 2–3 months [7, 8] Thus, we focused on a bioabsorbable synthetic material as a GBR membrane.
GC membrane® (GC Corporation, Tokyo, Japan) is composed of polylactide-co-glycolide acid (PLGA), a bioabsorbable synthetic polymer. This membrane has already been used clinically for guided tissue regeneration (GTR), and provided favorable outcomes with no epithelial invasion or severe complications including infection [9, 10]. The objective of GTR is to regenerate periodontal tissue, including cementum, periodontal ligament and bone, around a natural tooth [11]. Conversely, the objective of GBR is to regenerate bone tissue around a dental implant [7, 12]. This study aimed to access the efficacy of GC membrane® for GBR.
Materials and methods
This single cohort clinical study was approved by the Clinical Research Ethics Committee of Nagasaki University Hospital (Approval No. 14052640).
Patients
The study included patients who required simultaneous dental implant placement with alveolar ridge augmentation. All patients agreed to have dental implant treatment with GBR using GC membrane® according to our protocol, and provided written informed consent to participate in the study. Patients who were heavy smokers (more than 10 per day), abused alcohol, or suffered from uncontrolled diabetic disease, hypertension, and other systematic diseases treated by bisphosphonates or denosumab were excluded from the study.
GBR procedure
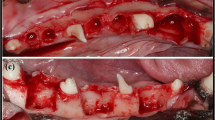
Autologous bone was collected by scraping the surrounding cortical bone with a harvesting tool (Safescraper®; META, Reggio Emilia, Italy) and transplanted onto the exposed implant body with platelet-rich plasma (PRP) after implant placement. PRP was prepared from the patient’s own peripheral blood by centrifugation in a Medifuge® (Silfradent Srl, Santa Sofia, Italy). The transplanted bone was covered with GC membrane®, and the wound was primarily closed without any tension (Fig. 1). Patients were prescribed sitafloxacin (100 mg) for 5 days.
Evaluation
The following patient characteristics were assessed.
-
1.
Sex and age.
-
2.
Bone augmented region.
-
3.
Type of dental implant system.
-
4.
Clinical symptoms including inflammation (gingival swelling, redness, recession, dehiscence, pus discharge) during the healing period.
-
5.
Horizontal and vertical bone augmentation at the second operation (Fig. 2).
Fig. 2 Bone augmentation. The augmented bone was horizontally measured at the implant platform (HW0) and at 1 mm (HW1), 3 mm (HW3), and 5 mm (HW5) below the implant platform, and vertically measured from the platform to the original crestal alveolar bone (VH2). The impacted implant length in the original bone (VH1) was also measured
The augmented bone was evaluated by cone-beam computed tomography (CBCT) after the healing period (mean 16.1 weeks; range 13–30 weeks). The second operation was then performed. Because several factors, including overloading and infection, influence the bone around dental implants [13] following attachment of the superstructure, the augmented bone was assessed before the second operation to determine the bone augmentation effect of GBR using the GC membrane® in this study. Based on previous reports [14, 15], the horizontal width (HW) of the augmented bone by GBR was measured. The CBCT images were reconstructed by SimPlant Pro 15.0 software (Materialise, Dentsply Implants, Mölndal, Sweden) and the HW values were evaluated by the cross-sectional findings in the middle of the implant. The perpendicular line to the implant body axis was defined as the horizontal line. The labial/buccal bone width was measured horizontally at four levels: implant platform level (HW0), and 1, 3, and 5 mm below the implant platform (HW1, HW3, and HW5, respectively). The vertical height of the lingual impacted implant in the original bone (VH1) was measured. The vertical height of the augmented bone (VH2) was defined as the distance from the top of the augmented bone to the original crestal alveolar bone. VH2 represented the amount of the implant exposure from the original bone after implant placement.
-
6.
Vertical bone recession on the latest X-ray film in the follow-up period.
To evaluate the vertical bone resorption, the distance from the implant platform to the alveolar crest was measured on the latest X-ray film during the follow-up period (16–24 months after implant placement). The distance was corrected by the implant length on each film.
-
7.
Implant stability quotient (ISQ) at the second operation.
The ISQs of all implants were measured to evaluate implant stability using the Osstell ISQ® (Osstell, Gothenburg, Sweden) at the second operation. The relationships between ISQ and torque value at implant placement, VH1, VH2, and healing period were assessed.
Results
Patients
Seven males (mean age: 58.4 years; range 46–67 years) and 11 females (mean age: 56.0 years; range 41–72 years) were included in the study. Five dental implants were embedded in the maxilla and 18 were embedded in the mandible. Twenty one of 23 implants (91.3%) were embedded in premolar/molar regions (Table 1). Four implant systems were applied (Table 2).
Postsurgical progress
Transient wound swelling was observed in most cases after implant placement with GBR, but no swelling was maintained for more than 2 weeks. Although wound dehiscence causing membrane exposure was observed at 4 of 23 regions (17.4%), infection, leaking of autologous bone, and implant exposure were not observed, and all wounds closed within 4 weeks without any events with additional preventive administration of antibiotics. The ISQ at the second operation was not dependent on the transient wound dehiscence. The final restoration was achieved in all cases and there was no implant exposure during the follow-up period (16–24 months). No symptoms of infection, such as pus discharge, swelling, redness, and pain, were observed in all cases.
Horizontal and vertical bone augmentation
HW0 was 0.70 ± 0.60 mm and there was no exposure of implant threads. HW1, HW3, and HE5 were 1.00 ± 0.58, 1.74 ± 1.54, and 2.56 ± 1.90 mm, respectively. VH1 was 2.6–11.0 mm (mean: 6.5 mm) and VH2 was 0–6.82 mm (mean 3.52 mm). The ratio of exposed and embedded implant length was also calculated. VH2/VH1 was 0–1.97 (mean 0.71). VH2/(VH1 + VH2) was 0–0.66 (mean 0.36), meaning that 36% of the implant body was exposed from the original bone (Table 3).
ISQ
The ISQ was measured for 19 of 23 implants at the second operation, and the mean value was 78.7 (range 69–85). Even the lowest ISQ (69) was higher than 62.6, as the value considered to reflect osseointegration [16]. The ISQ was not dependent on the torque value at implant insertion in the 18 implants measured for both ISQ and torque (Fig. 3). The ISQ was also independent from the implant system (Table 2), VH1, VH2 (Fig. 4), and healing period (> 13 weeks; Fig. 5).
Vertical bone recession on X-ray films
The vertical bone recession was evaluated on X-ray films taken at 16–24 months after the implant insertion with GBR. Twenty two of 23 implants could be evaluated. The distance from the implant platform to the distal and medial crests of the alveolar bone was 0–1.5 mm (mean 0.18 mm).
Discussion
GBR with GC membrane® led to successful augmentation of sufficient alveolar bone comparable to previous reports of GBR with non-resorbable membranes [3]. However, GC membrane® has an advantage over non-resorbable membranes, as it does not require removal because of its bio-absorbability. The present study indicates that GC membrane® is applicable not only for GTR, but also for GBR.
GBR is an efficient and popular bone augmentation technique [17] with non-resorbable membranes, such as polytetrafluorethylene, or resorbable membranes, such as collagen or polyethylene glycol (PEG) [3]. McGinnis et al. [18] reported that augmented bone by GBR with a resorbable membrane exhibited unpredictable resorption. However, it was also reported that GBR with a resorbable membrane had almost the same bone augmentation efficacy as GBR with a non-resorbable membrane [3]. Our study on GBR with GC membrane® achieved sufficient bone augmentation leading to successful oral rehabilitation after dental implant placement, thereby supporting the efficacy of this resorbable membrane. GC membrane® is composed of PLGA, a material that is bioabsorbable because its structure is susceptible to hydrolysis. GC membrane® has been primarily used for GTR, and provided good clinical outcomes [9]. Specifically, Yamanouchi et al. [9]. applied GC membrane® for GTR in 60 patients, finding that the pocket depth recovered by approximately 3 mm at 3 months and the depth was maintained at 6 months after GTR.
While collagen membranes, which are mainly derived from animal products, have been widely used, bioabsorbable polymers such as PLGA and PEG have recently been focused upon [19, 20]. According to the cited studies, the bone augmentation by GBR with both materials was comparable to that by GBR with collagen. GBR with GC membrane® induced 0.70 mm of horizontal bone formation at the implant platform level in the present study. Moreover, the augmented bone at 1, 3, and 5 mm below the implant platform was 1.00, 1.74, and 2.56 mm, respectively. This amount of bone augmentation was equal to not only GBR with other resorbable membranes [5, 17, 19], but also GBR with a non-resorbable membrane [5]. The ISQ was 78.7 at the second operation performed at 13–30 weeks after GBR in the present study. This finding indicates that all implants acquired sufficient osseointegration. Moreover, the ISQ was not dependent on the height of the embedded implant in the original bone (VH1), the height of implant exposure from the original bone (VH2) at the first operation, or VH2/VH1 + VH2. These results suggest that the augmented bone had sufficient quality to acquire osseointegration.
We used autologous bone with PRP, which may enhance bone formation and could have led to the favorable results in the present study. As a weakness of the study, there was no control without GC membrane®. Therefore, we cannot conclude that GC membrane® is essential for alveolar bone augmentation using autologous bone with PRP. However, bone augmentation was reported to be induced by autologous bone graft with a GBR membrane [21]. Thus, it is reasonable that the autogenous bone graft including PRP together with GC membrane®, as a GBR membrane, led to the satisfying outcomes in the present study. At least, GC membrane® did not hamper the bone augmentation by autologous bone with PRP.
No symptoms of infection around the implant were found in all cases, and it is noteworthy that there were even no signs of infection in the four cases with occurrence of wound dehiscence. It is possible that GC membrane® may have resistance to infection. The augmented bone was maintained on the implant at 13–30 weeks after GBR with GC membrane® according to the CBCT findings, and only 0.18 mm of bone resorption was observed radiologically. Recession of gingival margins and exposure of implant threads were not observed clinically during the follow-up period (16–24 months). These observations indicate that the augmented bone was functionally maintained because the bone was incorporated into the original bone during the remodeling cycle. Therefore, it is expected that the bone surrounding the implant will remain stable in the future.
Conclusions
GBR with GC membrane® constructed from PLGA could supply sufficient bone augmentation for implant treatment and the augmented bone was maintained for a long period, resulting in successful implant treatment. The present study suggests that GC membrane® is useful not only for GTR, but also for GBR.
References
Tanaka K. A comparison between the upper and lower jaws of the alveolar bone changes due to the extraction of frontal teeth. Shika Kiso Igakkai Zasshi. 1989;31:148–83 (in Japanese).
Hermann JS, Buser D. Guided bone regeneration for dental implants. Curr Opin Periodontol. 1996;3:168–77.
Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthod Res. 2013;57:3–14.
Konstantinidis I, Kumar T, Kher U, Stanitsas PD, Hinriches JE, Kotsakis GA. Clinical results of implant placement in resorbed ridges using simultaneous guided bone regeneration: a multicenter case series. Clin Oral Invest. 2015;19:553–9.
Shneider D, Weber FE, Grunder U, Andreoni C, Burkhardt R, Jung RE. A randomized controlled clinical multicenter trial comparing the clinical and histological performance of a new, modified polyactide-co-glycolide acid membrane to an expanded polytetrafluorethylene membrane in guided bone regeneration procedures. Clin Oral Impl Res. 2014;25:150–8.
Bunyaratavej P, Wang HL. Collagen membranes: a review. J Periodontol. 2001;72:215–29.
Rodella LF, Favero G, Labanca M. Biomaterials in maxillofacial surgery: membranes and grafts. Int J Biomed Sci. 2011;7:81–8.
Geurs NC, Korostoff JM, Vassilopoulos PJ, Kang TH, Jeffcoat M, Kellar R, Reddy MS. Clinical and histologic assessment of lateral alveolar ridge augmentation using a synthetic long-term bioabsorbable membrane and an allograft. J Periodontol. 2008;79:1133–40.
Yamada S, Matsumoto Y, Takahashi Y, Yamanouchi K, Aoki H, Sato T, Ishikawa T, Hyon SH, Ikada Y. Histopathological study of poly (lactic acid-co-glycolic acid) membranes to guided tissue regeneration in dogs. Jpn Clin Periodontol. 1991;33:396–405 in Japanese.
Yamanouchi K, Nakagawa T, Seida K, Saito A, Yamada S, Hiwatashi K, Setoguchi T, Chuman M, Sueda T. Clinical study on the effect of absorbable membrane applied to guided tissue regeneration technique. Jpn Clin Periodontol. 1994;36:884–94 in Japanese.
Buser D, Brägger U, Lang NP, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Impl Res. 1990;1:22–32.
Chiapasco M, Romeo E, Casentini P, Rimondini L. Alveolar distraction osteogenesis vs. vertical guided bone regeneration for the correction of vertically deficient edentulous ridges; a 1-3-year prospective study on humans. Clin Oral Impl Res. 2004;15:82–95.
Duyck J, Vandamme K. The effect of loading on peri-implant bone: a critical review of the literature. J Oral Rehab. 2014;41:783–94.
Mario MS, Sacha A J, Istvan U, Luigi C, Milena P, Marco T. Horizontal ridge augmentation using GBR with a native collagen membrane and 1:1 ratio of particulated xenograft and autologous bone: A 1-year prospective clinical study. Clin Impl Dent Relat Res. In press.
Le B, Borzabadi-Farahani A, Nielsen B. Treatment of labial soft tissue recession around dental implants in the esthetic zone using guided bone regeneration with mineralized allograft: a retrospective clinical case series. J Oral Maxillofac Surg. 2016;74:1552–61.
Lόpez AB, Martínez JB, Pelayo JL, Carcía CC, Diago MP. Resonance frequency analysis of dental implant stability during the healing period. Med Oral Pathol Oral Cir Bucal. 2008;13:e244–7.
Aimetti M, Romano F, Pigella E, Pranzini F, Debernardi C. Treatment of wide, shallow, and predominantly 1-wall intrabony defects with a bioabsorbable membrane: a randomized controlled clinical trial. J Periodontol. 2005;76:1354–61.
McGinnis M, Larsen P, Miloro M, Beck M. Comparison of resorbable and nonresorbable guided bone regeneration materials: a preliminary study. Int J Oral Maxillofac Implants. 1998;13:30–5.
Jung RE, Benic GI, Scherrer D, Hämmerle CHF. Cone beam computed tomography evaluation of regenerated buccal bone 5 years after simultaneous implant placement and guided bone regeneration procedures—a randomized, controlled clinical trial. Clin Oral Impl Res. 2015;26:28–34.
Ramel CF, Wismeijer DA, Hammerle CH, Jung RE. A randomized, controlled clinical evaluation of a synthetic gel membrane for guided bone regeneration around dental implants: clinical and radiologic 1- and 3-year results. Int J Oral Maxillofac Impl. 2012;27:435–41.
Becker W, Urist M, Becker BE, Jackson W, Parry DA, Bartold M, Vincenzzi G, Georges DD, Niederwanger M. Clinical and histologic observations of sites implanted with intraoral autologous bone grafts or allografts. 15 human case reports. J Periodontol. 1996;67:1025–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kawasaki, T., Ohba, S., Nakatani, Y. et al. Clinical study of guided bone regeneration with resorbable polylactide-co-glycolide acid membrane. Odontology 106, 334–339 (2018). https://doi.org/10.1007/s10266-018-0349-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-018-0349-2