Abstract
Plants’ ability to sense and respond to gravity is a unique and fundamental process. When a plant organ is tilted, it adjusts its growth orientation relative to gravity direction, which is achieved by a curvature of the organ. In higher, multicellular plants, it is thought that the relative directional change of gravity is detected by starch-filled organelles that occur inside specialized cells called statocytes, and this is followed by signal conversion from physical information to physiological information within the statocytes. The classic starch statolith hypothesis, i.e., the starch accumulating amyloplasts movement along the gravity vector within gravity-sensing cells (statocytes) is the probable trigger of subsequent intracellular signaling, is widely accepted. Acharya Jagadish Chandra Bose through his pioneering research had investigated whether the fundamental reaction of geocurvature is contractile or expansive and whether the geo-sensing cells are diffusedly distributed in the organ or are present in the form of a definite layer. In this backdrop, a microscopy based experimental study was undertaken to understand the distribution pattern of the gravisensing layer, along the length (node–node) of the model plant Alternanthera philoxeroides and to study the microrheological property of the mobile starch-filled statocytes following inclination-induced graviception in the stem of the model plant. The study indicated a prominent difference in the pattern of distribution of the gravisensing layer along the length of the model plant. The study also indicated that upon changing the orientation of the plant from vertical position to horizontal position there was a characteristic change in orientation of the mobile starch granules within the statocytes. In the present study for the analysis of the microscopic images of the stem tissue cross sections, a specialized and modified microscopic illumination setup was developed in the laboratory in order to enhance the resolution and contrast of the starch granules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant organs are capable of sensing various vectorial stimuli, e.g., light, gravity, touch and the like (Bose 1919; Haberlandt 1900; Morita et al. 2010). Among these, gravity is one of the most important stimuli because the direction and the magnitude of gravity are almost constant on the surface of the earth. When a plant organ is tilted, it adjusts its growth orientation relative to gravity direction, which is achieved by a curvature of the organ. Growth reorientation is the result of a differential cell elongation rate between the two sides of organs undergoing primary growth (Lopez et al. 2014). In higher, multicellular plants, it is thought that the relative directional change of gravity is detected by starch-filled organelles that occur inside specialized cells called statocytes, and this is followed by signal conversion from physical information to physiological information within the statocytes (Morita et al. 2010). Subsequently, the signal is transmitted to the neighbouring cells and other tissues, which leads to differential cell growth between the lower and upper flanks of the responsive organ. Graviception is therefore essential in the control of the posture and determination of growth direction (Moulia et al. 2011).
With respect to gravity sensing, the classic starch statolith hypothesis, i.e., starch accumulating amyloplasts movement along the gravity vector within gravisensing cells (statocytes) is the probable trigger of subsequent intracellular signalling, is widely accepted (Haberlandt 1900; Morita et al. 2010; Nemec 1900). Several lines of experimental evidence have demonstrated that starch is important but not essential for gravity sensing and have suggested that it is reasonable to regard plastids (containers of starch) as statoliths. Although the word statolith means sedimented stone, actual amyloplasts are not static but instead possess dynamic movement (Morita et al. 2010). A recent study has demonstrated that these gravisensing cells, i.e., the statoliths behave more like an active granular liquid, rather than behaving like a stagnant material (Berut et al. 2018). Gravity perception is crucial to plants not only for growth and postural control but also for development and adaptation of plants to various environmental changes (Levernier et al 2021; Takahashi et al. 2021). To date several studies, ranging from experimental to developing computational models to understand graviception in plants have been conducted. Some experimental studies have also focused on understanding the indispensable role of inclination on initiation of graviception induced stem curvature (Chauvet et al. 2016). Following multiple revisions of the starch statolith hypothesis by scientists worldwide, Bose (1919) for the first time investigated whether the fundamental reaction of geocurvature is contractile or expansive and whether the geo-sensing units are diffusedly distributed in the organ or are present in the form of a definite layer. Studies in the past have established that the statocytes are located in different organs of the plants (Pouliquen et al. 2017). In coleoptiles, they are located in a thin layer near conducting tissues. In roots, statocytes are present within a few columns of cells located in the central root cap, called the columella (Su et al 2020). Several lines of experimental findings in the recent past have reported that in Arabidopsis and maize plant shoots the statocytes appear to be located in the starch sheath, an endodermal cell layer that surrounds the vasculature (Edelman 2018; Strohm et al. 2013). Studies in etiolated bean seedlings have shown that a starch sheath layer is present around the vascular stem tissue (Verbelen et al 1985).In this backdrop, a microscopy based experimental study was undertaken using Alternanthera philoxeroides (Mart). Griseb. as the model plant to microscopically investigate the location of statocytes in the stem and correspondingly understand the distribution pattern of the gravisensing layer (starch sheath layer), along the length (node–node). The present study also aims at understanding the microrheological property of the mobile starch granules within the statocytes following inclination-induced graviception in the stem of the model plant. Since the starch granules are mobile in nature, the dynamics of the starch granules over time i.e., the time required for re-orientation of the starch granules within the statocytes, following inclination has also been explored in the present study.
The motivation behind selecting A. philoxeroides as the model plant for the present study, was that this invasive clonal plant can form dense stands through clonal propagation that expel almost all the other species in aquatic ecosystems (Schooler 2012). Clonal integration has been considered to be an important factor for the growth, spread and invasion of this invasive species in different habitat conditions. Studies showed that clonal integration can facilitate the colonization and growth of ramets under stressful conditions, and improve the tolerance of individual ramets to a variety of mechanical and physical forces like gravity (You et al 2016). Moreover, Alligator weed (A. philoxeroides) has a strong ability to adapt to potassium deficiency (LK) stress. Studies have shown that in A. philoxeroides after LK treatment there was a rise in concentration of serine/threonine-protein phosphatase 2A (PP2A), a multifunctional regulator in metabolic enzyme activities, hormone and cell cycle progression in the roots of the plant. Rashotte et al. have reported that PP2A plays vital roles for auxin transport, gravity response, and lateral development in A. philoxeroides and Arabidopsis (Li et al 2019). Understanding the pattern of distribution of the starch sheath layer throughout the length of this model plant may provide a correlation between the region of curvature and continuity of the starch sheath layer which in turn, may further provide insights into the role of the starch sheath layer in inducing geocurvature in the case of A. philoxeroides. This might also facilitate understanding of the probable influence of graviception in clonal invasion patterns of A. philoxeroides in the near future. A specialized and modified microscopic illumination setup was developed in the laboratory for the microscopical image analyses. The stem tissue sections have been viewed under both commercially available microscopes having standard illumination optics and also under the lab-built microscopic illuminator system, for procuring microscopic images of higher optical resolution. The significance of the present study is two-fold. Firstly, studying the mechanistic basis of graviception in plants will aid in the generation of novel strategies to develop biological sensors in the near future. Biological sensors display a wide range of strategies that combine sensitivity and robustness to cope with a fluctuating environment. Studies in recent past have focused on understanding how the gravisensing mechanism of plants can function as a reliable clinometer (Berut et al. 2018). Secondly, understanding the correlation between microscopic localization of the gravisensing starch sheath layer and the points of curvature along the length of the model plant A. philoxeroides at the macroscopic level may contribute to plant model based space biology research pertaining to development of living in space (Takahashi et al. 2021). The concept of utilizing the mechanism of plant graviception for better understanding of life in space is not new. Studies conducted during the late twentieth century had focused on development and modification of spaceflight experimental conditions for monitoring the influence of gravity vector on plant growth inside the space shuttle (Halstead 1987; Jira et al. 1998).
Materials and methods
Experimental procedure
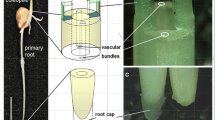
For the present study, the plant A. philoxeroides was collected from the campus garden of the Indian Statistical Institute, Kolkata. For studying the microrheological property, a total of 7 plants, each having 9 nodes, of more or less similar dimensions were chosen. Similarly, for understanding the pattern of distribution of the starch sheath layer another 7 plants, each having 9 nodes were selected. In order to find out the time required for the mobile starch granules to reorient to the new lower cell wall after inclination, another set of 6 plants each having 9 nodes were used. Before starting the experiment all the plants were housed in a plant acclimatization chamber for 4 days in order to adapt the plants with the laboratory growing condition. For the microscopic studies, tissue sections were obtained from the stem of A. philoxeroides and stained with iodine solution. In the present study, microscopic tissue sections were obtained from specified regions of the plant stem. The internodal regions of the plant stem were divided equally into three longitudinal segments (Fig. 1)—Segment A: the upper most region (the region immediately below the preceding node), Segment B: the intermediate region and Segment C—the lower most region (the region immediately above the succeeding node). Before obtaining tissue sections for microscopic examination the orientation of the plant was changed from the vertical to horizontal position. Following this, the tissue sections were prepared and then viewed under the microscopes. The microscopic images of the tissue sections were captured and then subjected to image processing.
Image showing the specific segments of the stem of A. philoxeroides from where the microscopic tissue sections were obtained. a Different segments of the intermodal region (Node 7–8) from where microscopic tissue sections were prepared. Segment A–The upper most region, immediately below the preceding node, Segment B: The intermediate region and Segment C–The lower most region, immediately above the succeeding node. b Image showing the point of curvature (node-internode junction) at the Segment C
Induction of ‘Graviceptive’ curvature
In order to initiate graviceptive geocurvature in the stem of A. philoxeroides a specialized inclined plane setup was developed in the laboratory (Fig. 2). The plants were positioned in the horizontal plane, i.e., at an angle of 180º using the inclined plane setup for a maximum of 5 min. Following this, tissue sections were obtained from the specified regions along the plant stem and viewed under both, the modified microscopic illumination setup developed in the laboratory and also under standard commercial microscopes.
The microscopic setup
The Modified Microscopic Illuminator System: Since the size of the starch granules when viewed under a microscope are too small, a specialized microscopic illumination set up has been developed in the laboratory using a cost-effective technique (Fig. 3). The set up primarily consists of two segments—Optical part and Electronic part. The optical part was made up of a plano-concave lens of focal length (f) = 4 mm and diameter = 4.2 mm. The aperture value is 3.2 mm. The Electronic part consists of a white light emitting diode (LED) as the light source, a switch, battery and a complementary metal oxide semiconductor (CMOS) sensor connected to an eye-piece. The intensity of the LED was controlled through pulse width modulation technique via the Arduino microcontroller, connected to a 10 k potentiometer. This set up enhances the image quality from two aspects—by increasing the resolution and the contrast of the starch granules as observed from the microscopic tissue cross sections. The set up thus provides an optimal uniform illumination across the microscopic field of view. They act to gather light from the microscope's light source and concentrate it into a cone of light that illuminates the starch sheath layer. The microscopic images captured under this developed illumination system were then subjected to computational analysis through image processing.
The high-resolution microphotographs captured with the aid of the customized microscopic illuminator system developed in the laboratory allowed detailed investigation of the rheological sedimentation property and dynamics of the amyloplast filled starch granules within the statocytes. The speciality of this lab-built microscopic illuminator system is that microscopic specimens can be viewed at a magnification as high as 1000x, without even using the oil-immersion principle.
Microscopic Observation: For microscopic observations of the tissue sections, three microscopes, depending on the purpose of the study, were selected—compound microscope (Co. Leica Microsystems), stereo microscope (Co. Radiso Engineering and Marketing) and another compound microscope (Co. O.F.D. M-1811) integrated with the modified illuminator system developed in the laboratory. For identifying the starch granules, the tissue sections were stained with Iodine solution (Parker et al. 2021).
Analysis of the microscopic images using image processing
In the present study, identification of the starch sheath layer, orientation of the starch granules within the statocytes with respect to gravity, and cell wall of the statocytes has been carried out using three steps in digital image processing. These are namely, image enhancement, image segmentation and object recognition. In the very first step of the work the image has been enhanced with a view to make the region of interest (RoI) more prominent compared to other features in the image. For this purpose both image smoothing and image sharpening filters have been applied simultaneously. The first filter has been applied to overcome small noisy particles present in the image and the later filter has been implemented to sharpen the RoI. For smoothing the image, Gaussian filter has been used as it provides for a much smoother process than the classical low-pass filters.
In the later stage, Ideal Highpass Filter (IHPF) has been used for sharpening the image in the frequency domain. This has been done with a view to enhance and highlight the edges and fine details in the image. Basically the filter eliminates low-frequency components and brings out high-frequency elements from an image.
The ideal high pass filter (IHPF) allows all the frequencies outside of a circular mask of radius d0 from the origin of the frequency plane without attenuation and eliminates all the frequencies within the circular mask. This d0 is the cut off frequency between H(x, y) = 1 and H(x, y) = 0.
Now to extract the RoI from the enhanced image, the image segmentation technique has been applied. This segmentation task has been performed, inspired by a similar recent segmentation problem in solar images, using the parameterized online region-based active contour method (POR-ACM) (Bandyopadhyay et al. 2020a, b(a); Bandyopadhyay et al. 2020a, b(b)). The method has the advantage of faster computational speed and capability of real—time object detection. Also, the method is less mathematically complex than the other existing active contour method (ACM)—based segmentation techniques. The overall formulation for the contour level set updation of the POR-ACM-based segmentation is given by,
Here, in Eq. 1 the term pspf (I(t)) represents the parametric-signed pressure function of an image I at t-th iteration which is denoted by
where, notions α1 and α2 indicate the weight parameter for the average intensity inside and outside the curve respectively. These parameters introduced generate an extra force on the curve so that the curve can locate the actual boundary of RoI. The notions c1 and c2 represent the mean intensity values inside and outside the curve, respectively. The term H in Eq. 1 denotes the unit step or the Heaviside function. Finally, the term \(\phi \)(t) in Eq. 1 indicates the level set energy function of the circular curve at t-th iteration. The final termination criterion for the evolution curve for the POR-ACM is given by the equation:
Laplacian of Gaussian (LoG) based Object Detection: The detection of cell wall of the statocytes is a bit complicated as the intensity distribution of the cell wall is not same throughout the image. In some sections it is too faint having almost the same intensity as that of the image background, while in some parts it is too bright as that of the starch granules and having the same intensity level as that of the granules. Thus to extract this cell wall the LoG filter have been applied. In this case also, first the image has been smoothed using the Gaussian filter to overcome the starch granules with high intensity. Later, Laplacian operator is used over the obtained smoothed image. The overall equation is represented as follows:
The image is convolved with this isotropic LoG filter to locate the zero crossings of the second derivative as the edge of the image. A threshold value has been set for these zero crossings in order to preserve only those zero crossings that exceed the threshold limit.
Object Recognition: During the evaluation process of the curve the POR-ACM detect certain redundant pixels or regions which have similar intensity as that of the RoI. This in turn, increases the error-rate of the detection procedure. Thus, to remove the redundant pixels or regions and to recognize the RoI, a post-processing image morphological operation (closing operation) to identify the unconnected regions has been applied. Here, the areas of the segmented pixels have been considered as the morphological properties for the operation. The final image regions obtained after the post-processing steps are considered as the starch sheath layer. Although there are several Java based free image processing softwares available, like the ImageJ software, in the present study, for detecting the starch sheath layer and the cell walls of the statocytes the proposed computer vision techniques were used because this customized algorithm provides selective removal of redundant pixels and allows more accurate object detection, as compared to ImageJ which primarily uses classical image segmentation algorithms. The problem with classical image segmentation algorithms is that these methods very often wrongly classify image background pixels as the region of interest for the images under consideration (Yuheng and Hao 2017). This problem was addressed using the applied parameterized online region-based active contour method towards a better segmentation of the target regions.
Results
The results of the present study indicated that upon changing the orientation of the plant from the vertical position to the horizontal position (180°) there was a characteristic change in orientation of the mobile starch granules within the statocytes. It was noted that in the case of the horizontal orientation of the plant, the mobile starch granules were re-oriented in the horizontal plane within the statocytes (Fig. 4), unlike that of the vertical position, where the mobile starch granules were found to be oriented in the vertical plane (Fig. 5), as observed from the microscopic tissue sections and the images obtained after the image processing steps.
Images of the microscopic longitudinal section of Segment C region (as shown in Fig. 1) of the A. philoxeroides stem viewed under the lab-built illuminator system, at Vertical orientation of the plant showing the accumulation of the starch granules in the short arm of the Cell wall of Statocyte (Section thickness in the order of 175 µm; Magnification in the order of 1000×). a Longitudinal tissue section viewed using the modified illuminator system integrated with the compound microscope. b to f Images of the tissue section obtained using several image processing methods:– b Binary mask using POR-ACM image segmentation method, c Final output using POR-ACM image segmentation method, d Binary mask using LoG (Laplacian of Gaussian) filter, e— Binary mask using combination of POR-ACM method and LoG filter, f Final output obtained using combination of LoG and POR-ACM
Images of the microscopic longitudinal section of Segment C region (as shown in Fig. 1) of the A. philoxeroides stem viewed under the lab-built illuminator system, at Horizontal orientation of the plant showing the accumulation of the starch granules in the long arm of the cell wall of statocyte (Section thickness in the order of 175 µm; Magnification in the order of 1000×). a Longitudinal tissue section viewed using the modified illuminator system integrated with the compound microscope. b to f Images of the tissue section obtained using several image processing methods:– b Binary mask using POR-ACM image segmentation method, c Final output using POR-ACM image segmentation method, d Binary mask using LoG (Laplacian of Gaussian) filter, e Binary mask using combination of POR-ACM method and LoG filter, f Final output obtained using combination of LoG and POR-ACM
Moreover, it was found that the time required for these mobile starch granules to re-orient in the horizontal plane within the statocytes following change in orientation of the whole plant from the vertical to horizontal position was nearly 5 min (Fig. 6). The difference in migration pattern of the falling starch granules studied from 1 to 5 min, following change in orientation of the plant from vertical to horizontal position has been represented in Table 1.
In the present study, after experimental observation of the continuity of the starch sheath layer along the length (node to node) of the plant stem, it was found that there was a prominent difference in the pattern of distribution of the starch sheath layer among the three internodal segments. It was noted that in Segment A (the region immediately below the preceding node) and in Segment B (the intermediate region) there was an absence of the continuous starch sheath layer. However, in the Segment C region (the region immediately above the succeeding node) a prominent and continuous starch sheath layer was detected. It was also observed that the points of curvature in the mature nodes (Node nos. 7 and 8) of the A. philoxeroides plant were at the Segment C regions of the 7–8 internodal segment, as observed from the photographic images of the whole plant (Fig. 7).
Images of the microscopic transverse section of the A. philoxeroides stem under 20× magnification (a–c), 60 × magnification (d–f) and 1000 × magnification (g–i) (stained with iodine solution), showing the difference in distribution pattern of the starch sheath layer along Internode 7–8 (Section thickness in the order of 175 µm). a Segment A region–No continuous starch sheath layer detected by iodine staining and image processing. b Segment B region—No continuous starch sheath layer detected by iodine staining and image processing. c Segment C region–A prominent and continuous single starch sheath layer detected through iodine. d Segment A region–No continuous starch sheath layer detected by iodine staining and image processing. e Segment B region—No continuous starch sheath layer detected by iodine staining and image processing. f Segment C region–A prominent and continuous single starch sheath layer detected through iodine. g Segment A region–No amyloplasts filled starch granules detected by iodine staining and image processing. h Segment B region—No amyloplasts filled starch granules detected by iodine staining and image processing. i Segment C region–Prominent and dense amyloplasts filled starch granules detected through iodine staining and image processing
Discussion
Several lines of experimental findings in the recent past have reported that in plant shoots the statocytes appear to be located in the starch sheath, an endodermal cell layer that surrounds the vasculature (Edelman 2018; Strohm et al. 2013). In the present study, in the case of the plant A. philoxeroides, coinciding results have been found upon microscopic examination of the tissue sections of the stem. From the microscopic tissue cross-sections, it was observed that in the stem of this model plant, the statocytes filled with starch granules were located in the starch sheath layer surrounding the vasculature (Fig. 7d–f). However, a novel phenomenon that has been found in the present work is that there exists a specific pattern of distribution of the starch sheath layer along the length of the plant stem. As evident from (Fig. 7) in the mature node-interndal region, i.e., segment C region (Fig. 1b), there was a continuous starch sheath layer present. However, in the other two segments, B and C (Fig. 1a) no continuous starch sheath layer was localized (Fig. 7a, b). This peculiar distribution pattern of the starch sheath layer along the intermodal regions of the stem of A. philoxeroides coincides with Bose’s experiments on localization of geoperceptive layer in Bryophyllum and Nymphaea by means of electric probe (Bose 1920). Bose (1920) reported that there exists a difference in the anatomical characteristics of the starch sheath layer based on electrical localization and microscopic examination of the geoperceptive layer. Bose (1920) experimentally demonstrated that in the case of Bryophyllum a continuous starch sheath layer was recognized whereas, in Nymphaea the starch sheath was discontinuous and occurred in discrete points separated from each other. In Bryophyllum, there was a continuous starch sheath layer present throughout the shoot of the plant. However, in the flower stalk of Nymphaea the starch sheath was discontinuous and was present in crescents above the vascular bundles, separated from each other. Thus, the concept of existence of a difference in distribution pattern of the starch sheath layer along the length of the plant A. philoxeroides somewhat coincides with Bose’s findings highlighting the variation in distribution pattern of the (geoperceptive layer) starch sheath layer between Bryophyllum and Nymphaea. A study conducted by Verbelen et al. (1985) on bean seedlings had also highlighted a variation in the concentration pattern of the amyloplasts among the top, middle and basal stem portions. Another striking phenomenon detected in the present study is that there exists a probable correlation between the specific curvature point at segment C (Fig. 1b) of the stem and the microscopic localization of a continuous starch sheath layer at the same segment C region (Figs. 4, 5). The study by Verbelen et al (1985) on bean seedlings demonstrated similar findings that the maximum concentration and pressure of amyloplasts was found in the stem hook region (curvature region), indicating a significant role of stem hook in graviception. The basis of such a correlation is probably due to the physiological changes induced by the continuity of the single starch sheath layer in A. philoxeroides. Bose (1920) had demonstrated through his experiments on the plant Eclipta that in the tissue cross sections following inclination induced geocurvature, there was a relative contraction of the cortex in the upper side of the stem while on the lower side there was a relative expansion. He further stated that the single starch sheath layer was noted to abut against the upper and the lower vascular bundles (phloem, in particular). The starch granules in the upper statocyte were found to press against the inner tangential wall of the cells nearest the phloem. On the other hand, in the lower side of the stem the starch grains were found to press the outer tangential wall furthest from the phloem. In the present study, the specific point of curvature of the stem and the continuous starch sheath layer, microscopically localized in the same region, i.e., segment C, probably initiated a simultaneous contraction of the upper side of the stem and expansion of the lower side of the stem, thereby resulting in the geocurvature response, following inclination.
In the present study, it was found that when the plants were mounted in the vertical orientation the starch granules were aligned in the short arm of the cell wall of the statocytes, as evident from the microscopic longitudinal sections (Fig. 4). However, in the case of the plants that were mounted in the horizontal orientation, the starch granules were aligned in the long arm of the cell wall of the statocytes (Fig. 5). This re-orientation of the amyloplasts containing starch granules within the statocytes following inclination of the whole plant from the vertical to horizontal plane was owing to the direction of the acting gravitational force. Similar experimental studies over the past few years have also demonstrated that the sedimentation of the dynamic starch granules depend on the direction of the gravitational force inside the statocytes (Hashiguchi 2013; Leitz 2009; Morita et al. 2010). Recent studies conducted on statolith dynamics of wheat coleoptiles in response to plant inclination had also reported that the starch granules move collectively to the new physical bottom of the cell wall of the statocytes upon gravitational stimulus within the presentation time (Berutet al. 2018). Bose (1920) in his research works on investigation of the mechanistic basis of geocurvature in plants has highlighted that as the starch grains become piled up at the base of the cell, they exert not only a vertical but also a lateral pressure. In the vertical orientation, the excitability of the cortex at the two sides are same; hence the two antagonistic reactions balance each other. However, when the orientation is inclined at an angle, say 35° below horizon, the upper cortex becomes directly and intensely stimulated whereas the lower cortex undergoes indirect and feeble stimulation. This ultimately results in contraction of the upper and an expansion of the lower side, thereby causing the upward or negative geocurvature (Bose 1920).
The densities of the starch granules present within the sedimentable amyloplastss, i.e., the statoliths are 1.5 times higher than that of the surrounding cytoplasm (Strohm et al. 2013). In the present study it has been found that the total time required for these starch granules to sediment to the new physical bottom of the cell wall following inclination was around 5 min (Table 1, Fig. 6). A similar kind of study based on statolith sedimentation kinetics of root columella cells of Arabidopsis plant (Leitz et al 2009) showed that the initial realignment of the statoliths to reach to the new lower cell wall surface after reorientation commenced at around 210 s (3.5 min). The study also reported that the statoliths reached the new equilibrium state (configured at the new lower cell wall) in approximately 10 min. Another study by Driss-Ecole et al. (2000) on sedimentation kinetics of lentil root statoliths in microgravity have reported that after 122 min in microgravity the bulk of amyloplastss had almost reached the new proximal pole of the cell wall which were originally (before exposure to microgravity) situated in the distal cell wall.
In the present study as per the microscopical image analysis it may thus be concluded that in the model plant A. philoxeroides, the starch granules are located in the endodermal starch sheath layer surrounding the vasculature and this starch sheath layer also follows a characteristic pattern of distribution which coincides with the points of curvature of the stem. Moreover, the realignment of the amyloplasts filled starch granules within the statocytes following a change in orientation of the plant, from the vertical to horizontal position highlights the dynamic nature of the starch granules. Understanding of the distribution of the starch sheath layer along the length of the plant and the rheology of the starch granules, thus, provided a connecting link between the functional arrangement and dynamics of the mobile starch granules present within the statocytes of the starch sheath layer at the microscopic level and the macroscopic gravisensing response of the model plant A. philoxeroides.
Data availability
The data that support the findings of the present study are available from the first/corresponding author upon reasonable request.
References
Bandyopadhyay S, Das S, Datta A (2020a) Comparative study and development of two contour-based image segmentation techniques for coronal hole detection in solar images. Sol Phys 295:110
Bandyopadhyay, S, Das S, Datta A (2020b) Detection of coronal holes using hough simulated parameterized online region-based active contour method. In: URSI Regional Conference on Radio Science, pp. 1–4
Bérut A, Hugo C, Valérie L, Bruno M, Pouliquen O, Yoël F (2018) Gravisensors in plant cells behave like an active granular liquid. Proc Natl Acad Sci 115:5123–5128
Bose JC (1919) Localisationofgeoperceptive layer by means of the electric probe. Trans Bose Res Inst 2:461–466
Bose JC (1920) Investigations on dia-geotropism of dorsiventral organs. Trans Bose Res Inst 3:709–727
Chauvet H, Pouliquen O, Forterre Y, Legué V, Moulia B (2016) Inclination not force is sensed by plants during shoot gravitropism. Sci Rep 6:1–8
Driss-Ecole D, Jeune B, Prouteau M, Julianus P, Perbal G (2000) Lentil root statoliths reach a stable state in microgravity. Planta 211:396–405
Edelmann HG (2018) Graviperception in maize plants: is amyloplasts sedimentation a red herring ? Protoplasma 255:1877–1881
Haberlandt G (1900) On the perception of the geotropic stimulus. Brothers Borntraeger, Germany
Halstead TW, Dutcher FR (1987) Plants in space. Annu Rev Plant Physiol 38:317–345
Hashiguchi Y, Tasaka M, Morita MT (2013) Mechanism of higher plant gravity sensing. Am J Bot 100:91–100
Jira KW, Edelmann RE, Brinckmann E, Kiss JZ (1998) The development of spaceflight experiments with Arabidopsis as a model system in gravitropism studies. J Plant Res 111:463–470
Leitz G, Kang BH, Schoenwaelder ME, Staehelin LA (2009) Statolith sedimentation kinetics and force transduction to the cortical endoplasmic reticulum in gravity-sensing Arabidopsis columella cells. Plant Cell 21:843–860
Levernier N, Pouliquen O, Forterre Y (2021) An integrative model of plant gravitropism linking statoliths position and auxin transport. Front Plant Sci 12:474
Li L, Lyu C, Huang L, Chen Q, Zhuo W, Wang X, Lu Y, Zeng F, Lu L (2019) Physiology and proteomic analysis reveals root, stem and leaf responses to potassium deficiency stress in alligator weed. Sci Rep 9:1–3
Lopez D, TocquardK VJS, Legué V, Roeckel DP (2014) Gravity sensing, a largely misunderstood trigger of plant orientated growth. Front Plant Sci 5:610
Morita MT (2010) Directional gravity sensing in gravitropism. Annu Rev Plant Biol 61:705–720
Moulia B, Der LC, Bastien R, Martin L, Rodriguez M, Gourcilleau D (2011) Integrative mechanobiology of growth and architectural development in changing mechanical environments. In: Wojtaszek P (ed) Mechanical integration of plant cells and plants, signaling and communication in plants, Springer, Heidelberg, pp 269–302
Nemec B (1900) About the way plants perceive the stimulus of gravity. Ber Dtsch Bot Ges 18:241–245
Parker ML, Ryden P, Wilde PJ, Edwards CH (2021) A simple and effective method for observing starch in whole plant cells and in raw and processed food ingredients. Stärke 73:2000056
Pouliquen O, Forterre Y, Bérut A, Chauvet H, Bizet F, Legué V, Moulia B (2017) A new scenario for gravity detection in plants: the position sensor hypothesis. Phys Biol 14:035005
Schooler SS (2012) Alternanthera philoxeroides (Martius) Grisebach (alligator weed). In: A handbook of global freshwater invasive species, Routledge, pp 43–53
Strohm A, Baldwin K, Masson PH (2013) Gravitropism in Arabidopsis thaliana. In: Maloy S, Hughes K (eds) Brenner's encyclopedia of genetics, 2ndedn. Academic Press, United States, pp 358–361
Su SH, Keith MA, Masson PH (2020) Gravity signaling in flowering plant roots. Plants 9:1290
Takahashi K, Takahashi H, Furuichi T, Toyota M, Furutani SM, Kobayashi T, Watanabe TH, Shinohara M, Numaga TT, Sakaue SA, Miyawaki A (2021) Gravity sensing in plant and animal cells. NPJ Microgravity 7:1–10
Verbelen JP, Spruyt E, De Greef JA (1985) A microscopical study of the statocyte system in stems of etiolated Phaseolus seedlings. Am J Bot 72:1054–1060
You WH, Han CM, Liu CH, Yu D (2016) Effects of clonal integration on the invasive clonal plant Alternanthera philoxeroides under heterogeneous and homogeneous water availability. Sci Rep 6:1–8
Yuheng S, Hao Y (2017) Image segmentation algorithms overview. arXiv preprint arXiv:1707.02051.
Acknowledgements
The authors remain grateful to Divisional Committee of Scientific Workers and the Technical Advisory Committee of Biological Sciences Division of Indian Statistical Institute, Kolkata for funding the project. The authors also remain thankful to the late Prof. Ratan Lal Brahmachary for the insightful discussion related to the present research study.
Funding
Indian Statistical Institute, TAC-DCSW-BSD-2020-22, Anjana Dewanji.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roy, S., Bhattacharya, B., Bandyopadhyay, S. et al. Understanding the role of starch sheath layer in graviception of Alternanthera philoxeroides: a biophysical and microscopical study. J Plant Res 136, 265–276 (2023). https://doi.org/10.1007/s10265-023-01434-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-023-01434-y