Abstract
It is known that rice roots take up cadmium (Cd) via the symplastic route mediated by membrane-bound mineral transporters. Here we provide evidence that apoplastic bypass flow is another Cd uptake route in rice. High concentrations of Cd rendered apoplastic bypass flow rate increased in rice seedlings. These concentrations of Cd compromised membrane integrity in the root meristem and transition zone. Polyethleneglycol and proline inhibited the Cd-induced apoplastic bypass flow and Cd transfer to the shoots. Loss-of-function mutant of the Cd uptake transporter, nramp5, showed Cd transport to the shoot comparable to the wild type. At a low Cd concentration, increased apoplastic bypass flow rate by NaCl stress resulted in an elevation of Cd transport to shoots both in the wildtype and nramp5. These observations indicate that apoplastic bypass flow in roots carries Cd transport leading to xylem loading of Cd in addition to the symplastic pathway mediated by mineral transporters under stressed conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a toxic heavy metal to most organisms. Cd inhibits growth and transpiration of plants and disturbs mineral homeostasis (DalCorso et al. 2008; Greger and Johansson 1992; Prasad 1995). In humans, Cd exposure is associated with cancer genesis, kidney failure, osteoporosis and osteomalacia (Bertin and Averbeck 2006; Nawrot et al. 2006). The level of Cd toxicity between plants and humans are quite different. The Cd level in edible parts of plants giving no symptom can threaten human health through continually eating plant-derived food, such as wheat grain, rice grain and potato tuber (Clemens et al. 2013). Therefore, the understanding of Cd intake mechanisms of plants is associated with food security, in addition to physiological effects to plants.
Uptake of non-essential or non-beneficial minerals to plant bodies are an intriguing question in plant biology. Cd is not an essential element for plants; therefore, it is generally assumed that rice has not developed a specific transporter for Cd uptake during its evolution. Currently, it is postulated that Cd is casually incorporated in plants via the symplastic route in a non-specific manner by membrane-bound transporters for other essential metals, such as iron, zinc and manganese (Nakanishi et al. 2006; Pedas et al. 2008; Sasaki et al. 2012; for review see Clemens et al., 2013). The rice iron transporter, OsIRT1, was suggested to localize in the epidermis and hypodermis of roots and to operate to take up Cd into the cortex under iron-deficient conditions (Ishimaru et al. 2006; Nakanishi et al. 2006). The manganese uptake transporter, OsNramp5, was shown to function in the hypodermis and endodermis of rice roots in an orientation-dependent manner to intake Cd into the cortex and stele (Sasaki et al. 2012). The P-type ATPase, OsHMA3, a manganese-transporting pump, was suggested to localize in tonoplasts of root cortex cells and to sequestrate Cd in the vacuole to limit Cd transfer to the shoot (Ueno et al. 2010).
Salinity stress increases the proportion of water uptake through the apoplast in rice roots. It was proposed that radial transport of Na+ to stele is routed through the apoplastic pathway and the increase of apoplastic bypass flow is critical for Na+ uptake in rice under salinity stress (Faiyue et al. 2010a, b; Munns and Tester 2008; Ranathunge et al. 2005; Yan et al. 2021; Yeo et al. 1987). On the other hand, the relation between apoplastic bypass flow of water and uptake of heavy metals has not been well understood. A study on a rice population with different Cd-accumulating characteristics suggested that degree of Cd transport to shoots is correlated to difference in development of Casparian strip and suberin lamellae in rice roots (Qi et al. 2020).
In this study, we tested the hypothesis that Cd is incorporated into rice shoot through apoplastic bypass flow from three standpoints: (i) whether Cd increases apoplastic bypass flow rate, (ii) whether compounds that inhibit apoplastic bypass flow inhibit Cd uptake to shoots and (iii) whether Na+-induced apoplastic bypass flow enhances Cd uptake to shoots under a low Cd condition.
Materials and methods
Plant materials
Rice plants (Oryza sativa L. cultivars. ‘Nipponbare’ and ‘Zhonghua 11’, and nramp5 mutant in the ‘Zhonghua 11’ genetic background) were grown as previously reported (Sobahan et al. 2009). In brief, seeds were germinated in a water-filled Petri dish under a photo-temperature period of 12 h light at 30 °C/12 h dark at 25 °C. Illumination was provided with a white fluorescent lamp at a photon flux rate of 250 µmol m− 2 s− 1 in an environment-controlled plant growth cabinet for 7 days. Seedlings were transplanted in a pot filled with Kimura B nutrient solution (Ueno et al. 2009) and hydroponically cultured in the same chamber. Relative humidity in the chamber was maintained 55 ± 2% during the light period and 60 ± 2% during the dark period. The hydroponic solution was replaced every 2 days. Seeds of ‘Zhonghua 11’ and nramp5 were provided by Dr. Jian Feng Ma (Sasaki et al. 2012) with the permission from Rice Mutant Database (Zhang et al. 2006).
Measurement of apoplastic bypass flow rate
Transpiration rate was measured gravimetrically. At the end of Cd exposure, plants were transplanted to a new cylinder-shape pot (100 mL) and sealed with a sheet of Parafilm to prevent non-transpirational evaporation. A pinhole was made in the Parafilm sheet by a needle to avoid negative pressure in the headspace. Water loss by evaporation from the pinhole was negligible at least for 4 h. Amount of water loss due to transpiration was determined by weight decrease after a 4-h incubation under illumination in the plant growth chamber.
Apoplastic water uptake was estimated using 8-hydroxy-1,3,6-pyrenetrisulphonic acid trisodium salt (PTS) as previously reported (Sobahan et al. 2009). In brief, rice seedlings were transferred to a fresh hydroponic solution supplemented with 300 µM PTS and incubated for 4 h under light (250 µmol m− 2 s− 1, white fluorescent tube). Tissue sap was extracted from the whole shoot. Concentration of PTS in the sap was quantified from the intensity of fluorescence at 490 nm of excitation and 525 nm of emission with a fluorescent spectrophotometer (RF-5300PC, Shimadzu, Kyoto).
Rate of apoplastic bypass flow was calculated from the rates of transpiration and apoplastic water uptake according to the equation described by Yeo et al. (1987).
Determination of leaf and root length
After the exposure, the shoot height and the length of the longest root were measured visually with a caliper.
In vivo Calcofluor staining
Staining was carried out essentially according to Ochiai and Matoh (2002). After Cd exposure, 21-day-old plants were transferred to a fresh hydroponic solution containing 0.005% of Calcofluor (Fluorescent brightener 28, MP biomedicals LLC, Solon, OH) and incubated for 24 h in a plant growth chamber. Roots were dissected, embedded in a 4% agarose block and cross-sectioned (100 μm thickness) with a vibrating microslicer (DTK-1000, Dosaka EM Co. Ltd., Tokyo). The section was observed with a fluorescent microscope (Axioscope, Carl-Zeiss) with a filter combination of band pass filter for excitation 365/12 nm, splitter 395 nm and long pass filter for emission 397nm.
Berberine-aniline blue staining
The Casparian strip was stained with berberine-aniline blue staining according to Brundrett et al. (1988) with a slight modification. The roots were fixed with 2% glutaraldehyde for 2 h at room temperature and rinsed twice with deionized water and embedded in a 4% agarose block. Section (100 μm thickness) were prepared with a vibrating microslicer. The sections were stained with 0.1% berberine hemisulfate for 1 h and rinsed three times with deionized water, and successively post-stained with 0.5% aniline blue for 30 min and rinsed three times with deionized water. The fluorescence was observed with an epifluorescence microscope as mentioned for Calcofluor.
Evans blue staining
Dead cells in roots were visualized with Evans blue staining (Li et al. 2006). Cd-exposed roots were detached from the shoot and immersed in 0.5% Evans blue (Fluka-Sigma-Aldrich, St. Louis, MO) solution for 1 h and rinsed three times with deionized water. Stained roots were observed with a stereoscope. To observe cross sections, the root was embedded in a 4% agarose block, sectioned with a vibrating microslicer and observed with a light microscope.
Determination of Cd content
The contents of Cd in shoots and roots were determined by inductivity couple plasma mass spectrometry (Agilent 7500cx, Agilent Technologies, Santa Clara, CA) essentially according to Hirayama et al. (2018). Approximately 0.1 g (fresh weight) of tissues were dried at 100 °C for 1 h and then 80 °C for 9 h, followed by weighing dry weight. Dried tissues were wet-ashed in 8 mL of 60% nitric acid (EL grade, Kanto Chemical Co. Inc., Tokyo) at 200 °C for 45 min with a microwave oven (model Start D, Milestone General KK, Kawasaki, Japan). 111Cd in the sample was quantified using external calibration standards. 89Yttrium was added to the sample as the internal standard.
Results
Cadmium increased apoplastic bypass flow rate
The effect of a wide range of Cd concentrations (0.01–100 µM) on apoplastic bypass flow of rice was examined Supplementation of Cd (as CdCl2) to hydroponic medium at 50 and 100 µM for 7 days significantly increased the uptake of the fluorescent apoplastic water transport tracer, trisodium 8-hydroxy-1,3,6-pyrenetrisulphonic acid (PTS) in rice shoot (Fig. 1). However, an exposure to lower concentrations (≤ 10 µM) did not affect the PTS uptake (Fig. 1, P > 0.05; data for 0.01 and 0.1 µM are not shown).
Increase of water uptake through apoplastic pathway by CdCl2. Uptake of water via apoplastic route in 21-day-old rice was examined with trisodium-8-hydroxy-1,3,6-pyrenetrisulphonic acid (PTS) after a 7-day exposure to indicated concentrations of CdCl2 in hydroponic medium. Concentration of PTS in tissue sap of shoot relative to the concentration in hydroponic medium ([PTS]shoot/[PTS]medium) is shown in Y-axis as PTS uptake. Error bars indicate standard deviation (n = 5). Asterisk indicates significant difference of the mean from the control (0 µM CdCl2) assessed with Dunnett’s test at α = 0.05
Transpiration rate and PTS uptake rate of Cd-exposed rice were determined concurrently to estimate the apoplastic bypass flow rate (Yeo et al. 1987). Rice plants were exposed to 50 µM Cd for several exposure periods as shown in Fig. 2a. PTS uptake increased gradually from day 1 to 7 of exposure (Fig. 2b). The right y-axis of Fig. 2b represents apoplastic water uptake rate calculated from PTS uptake rate. A significant decrease of total water uptake rate was observed from 5-day exposure (Fig. 2c). Apoplastic bypass flow rate was estimated as 1.3% in the absence of Cd exposure. It increased to 3.3, 7.7 and 15.3% by 3-day, 5-day and 7-day exposure to 50 µM Cd, respectively (Fig. 2d).
Increase of apoplastic bypass flow by Cd exposure. a Illustrated presentation of CdCl2 exposure protocol. Dark gray bars indicate germination in a water-filled petridish. White bars indicate hydroponic culture in Kimura B solution. Black bars indicate hydroponic culture in Kimura B solution supplemented with 50 µM CdCl2. Pale gray bars at 21 days indicate PTS uptake experiment for 4-h. b Apoplatic water flow rate examined from PTS uptake. Right Y-axis shows water flow rate calculated from PTS uptake rate, represented by the left Y-axis. c Total water uptake. d Apoplastic bypass flow rate. Error bars indicate standard deviation (n = 5). Asterisk indicates significant difference from the control (0 day) assessed with Dunnett test at α = 0.05
Calcofluor is another apoplastic water flow tracer that binds to celllulose allowing histochemical pursuit of the apoplastic water flow pathway (Ochiai and Matoh 2002). A subtle autofluorescence was observed in the cross section of roots (0 µM Cd) (Fig. S1). In comparison, a slight increase of fluorescence due to binding of Calcofluor was observed in roots with 0 µM-Cd treatment, indicating that apoplastic water uptake modestly occurred without Cd stress (Fig. S1a, d). Intense fluorescence was observed in 100- and 200-µM Cd-exposed roots (Fig. S1e, f), which is essentially in agreement with the results of PTS uptake (Fig. 2). This further supports the notion that Cd increases apoplastic bypass flow in rice roots.
In the control condition, the Calcofluor fluorescence was only modestly enhanced in the hypodermis (Fig. S1d). On the other hand, a very strong fluorescence of Calcofluor was seen at hypodermis layers in the exposed roots (Fig. S1e, f). Increase of stainability of root hairs was also observed in Cd-exposed roots (Fig. S1e, f). We anticipated that this was due to the disruption of the Casparian strip at hypodermis (exodermis) or the disruption of the membrane integrity of the exodermal and/or epidermal cell layer by the exposure to high concentrations of Cd.
Casparian strip was not critical in the increase of apoplastic bypass flow by Cd
We tested a hypothesis that the increase of apoplastic bypass flow rate in Cd-exposed plants is due to disruption of the Casparian strip in hypodermis. Intactness of the Casparian strip in Cd-treated rice roots was assessed by berberine-aniline blue staining. Autofluorescence was observed at sclerenchyma and stele (Fig. S2). Berberine staining indicating formation of Casparian strip was observed in endodermis and stele at 45 and 100 mm from the tip. On the other hand, almost no fluorescence was observed at the hypodermis from 4 to 100 mm from the tip. This result clearly showed that the Casparian strip was only subtly, or not developed in the hypodermis at this stage in this experiment. Therefore, we inferred that the increased apoplastic flow by Cd exposure is not attributed to the disruption of the Casparian strip in the hypodermis.
Cd exposure induced cell death in root meristem and transition zone
We next hypothesized that the increase of apoplastic bypass flow is the consequence of the loss of membrane integrity of epidermal and/or hypodermal cells. Evans blue staining was conducted to test this assumption. In control roots, virtually no cells were stained with Evans blue, except root cap cells girdling the apex (Fig. 3). On the other hand, Cd exposure rendered the root cells Evans blue-positive at the region of root meristem and transition zone (Fig. 3b), indicating that membrane integrity was impaired by Cd exposure in this region. Compromised membrane integrity was not restricted in the epidermal and exodermal cell layers, but in the cortex and stele (Fig. 3d). Cd stress elicited many punctuate red autofluorescing spots in roots. Therefore, the observation of the membrane integrity impairment by propidium iodide staining was not successful. PTS does not discriminate water flow through permeabilized cells and apoplast. For the sake of expedience, the water flowed through the permeabilized cells and the apoplast were collectively referred as apoplastic bypass flow in this study. The diameter of Cd-treated roots was greater than the control (Fig. 3, S1). The thickening of roots is attributable to both cell division and swelling (Fig. S3).
Evans blue-staining of CdCl2-exposed rice roots. Stereomicroscopic evaluation of Evans blue-stained roots after control- (a) and 50 mM CdCl2-treatments (b). Cross section of roots at 3 ± 1 mm from the tip stained in vivo with Evans blue exposed to 0 µM (c) and 50 µM CdCl2 (d). Scale bar = 100 μm. Each picture is a typical picture from 15 biological replications in 3 independent experiments
Effects of polyethylene glycol and proline on apoplastic bypass flow, membrane integrity and Cd uptake
Polyethylene glycol (PEG) and proline are reported to reduce the rate of apoplastic bypass flow in NaCl-treated rice (Faiyue et al. 2010a, b; Sobahan et al. 2009). We examined the effects of these reagents on Cd-induced apoplastic bypass flow rate and Cd transport to shoots to assess whether Cd was transported through apoplastic pathway in a manner analogous to NaCl.
Cd exposure increased PTS uptake rate in the absence of PEG (Fig. 4a) in agreement with the results in Fig. 2. Addition of 10% PEG in the hydroponic solution apparently reduced the flow rate in both Cd-exposed and non-exposed rice (Fig. 4a). The effects of PEG and proline on Cd transport to shoots were examined. As expected, Cd was not detected in shoots and roots in the Cd-free hydroponic medium (Fig. 4b). Cultivation of rice in the presence of 50 µM CdCl2 for 7 days resulted in an increase of Cd content in both shoots and roots in the absence of PEG. Supplemented with 10% PEG, Cd content was dramatically reduced in the shoots compared to that with 0% PEG. On the contrary, no reduction of Cd content was observed in roots with the 10% PEG treatment but rather increased. The ratio of Cd content in shoots to that in roots is an indicator of xylem loading of Cd. The ratio of Cd content in shoots to that in roots after 7-day exposure to 50 µM CdCl2 was approximately 0.6 (Fig. 4c) in the absence of PEG. However, supplementation of 10% PEG in the hydroponic medium reduced the ratio down to 0.0086 (Fig. 4e).
Effects of polyethylene glycol and proline on PTS uptake and Cd contents in shoots and roots. a Uptake of PTS in rice shoots cultivated in hydroponic medium in the presence and absence of 50 µM CdCl2 with and without supplementation of 10% polyethylenglycol (PEG, average molecular weight: 3350, Sigma P4338) for 7 days, expressed as portion of PTS concentration in the medium. b Cd contents in shoots and roots of rice in the presence and absence of 10% PEG. c Ratio of Cd contents in shoots to roots in the presence and absence of 10% PEG. d Uptake of PTS in rice shoots cultivated in hydroponic medium in the presence and absence of 50 µM CdCl2 with and without supplementation of 5 mM proline. e Cd contents in shoots and roots of rice in the presence and absence of 5 mM proline. f Ratio of Cd contents in shoots to roots in the presence and absence of 5 mM proline. Error bars indicate standard deviation (n = 5). Significance of difference of means was assessed by Student’s t-test. * and ** indicate significance at α = 0.05 and 0.01, respectively. n.s. indicates not significant (P > 0.05)
Five mM proline did not reduce the PTS uptake rate in the absence of Cd exposure. Cd-activated PTS uptake rate was, however, substantially reduced by the addition of 5 mM proline in the solution (Fig. 4d). At first glance, the addition of 5 mM proline to the hydroponic solution did not result in significant reduction of Cd uptake to shoots, (Fig. 4e). Meanwhile, the content of Cd in roots was profoundly increased by the addition of 5 mM proline (Fig. 4d). Supplementation of 5 mM proline in the hydroponic medium reduced it as low as 0.05% (Fig. 4f). These results suggest that the xylem loading of Cd was substantially reduced by PEG and proline treatment coinciding with the apoplastic bypass flow.
The effects of PEG and proline on the Cd-induced membrane integrity impairment were examined by means of Evans blue staining (Fig. S4). PEG treatment in the absence of Cd resulted in no apparent effect on the staining (Fig. S4a, c). The promotion of the staining by the Cd treatment was not apparently mitigated with 10% PEG (Fig. S4d). It is evident that the effect of 10% PEG reducing apoplastic bypass flow rate was not due to the rescue of the cells from death. The treatment with 5 mM proline resulted in a slight enhancement of the staining even in the absence of 50 µM CdCl2 compared to the control (Fig. S4g). Cd exposure rendered a strong Evans blue coloration in the meristem and transition zone, and no obvious difference was found between the absence and presence of proline (Fig. S4f, h). These results suggested that the mechanism behind the reduction of apoplastic flow by proline was not attributed to the reduction of the proportion of loss of membrane integrity or cell death in the root meristem and transition zone.
The possibility of the involvement of the Cd uptake transporter, Nramp5, in Cd transport to the shoot was assessed using the Nramp5 null mutant, nramp5 (Sasaki et al. 2012). In the absence of spiked Cd in the hydroponic solution, shoots and roots of the wild type rice cultivar, ‘Zhonghua11’, contained a subtle amount of Cd, while virtually no Cd was detected in nramp5 (Fig. 5). This result agreed with the previous report (Sasaki et al. 2012). The contents of Cd in nramp5 shoots were increased by the exposure to 50 µM Cd and achieved as comparable to that in the wild type (Fig. 5a). Increase in Cd contents in roots was significantly compromised in nramp5 (Fig. 5b). Collectively, these indicate that the uptake of Cd into root tissue at high Cd condition (50 µM) was partly attributed to the function of the Mn transporter, Nramp5, but the transport of Cd to shoots was independent or only subtly associated with Nramp5.
Cd contents in wild type and OsNramp5 null mutant. Cd contents in shoots and roots of the wild type (cv. ‘Zhonghua 11’) and the OsNramp5 null mutant (nramp5). Rice plants were exposed to 0 or 50 µM Cd in hydroponic culture for 7 days. Error bars indicate standard deviation. Significance of difference of means was assessed by Student’s t-test (n = 5). ** indicates significance at α = 0.01. n.s. indicates not significant (P > 0.05)
NaCl stress induced Cd transport to shoots at a lower Cd concentration
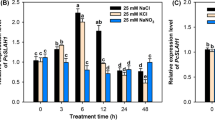
It was well established that NaCl stress increases apoplastic bypass flow rate in rice (Flowers and Yeo 1995; Ranathunge et al. 2005; Sobahan et al. 2009; Yeo et al. 1987). This was reproducible in the presence of 1 µM Cd in the hydroponic solution (Fig. S5). Here, we examined Cd contents in NaCl-stressed rice (Fig. 6) to examine whether the apoplastic bypass flow associates with Cd transport to shoots or not. Rate of Cd contents in shoots to roots was 10.2 ± 1.3% and 24.6 ± 1.5% (mean ± SD) under the presence of 1 µM CdCl2 in the hydroponic solution without NaCl treatment in ‘Nipponbare’ and ‘Zhonghua 11’, respectively. On the other hand, 50 mM NaCl treatment increased the ratio of Cd contents in shoots to roots to 35.6% ± 1.6% and 65.6 ± 6.7% (Fig. 6a, b). This suggests that the NaCl-activated apoplastic bypass flow promoted the migration of Cd to the shoot. In the nramp5 mutant, rate of Cd in the shoots was 25.8 ± 1.5% in the absence of NaCl and was increased to 59.2 ± 7.7% (Fig. 6b), while the absolute amount of Cd in the tissue was lower in this mutant than in the wildtype, ‘Zhonghua 11’ (Table S1). This suggests that Nramp5 is not a critical determinant for the Cd translocation under NaCl stress, while Cd content in root tissue was significantly affected by the lack of Nramp5. This emphasizes the potential contribution of apoplastic bypass flow in Cd uptake and xylem loading of Cd in rice in addition to symplastic and cell-to-cell Cd transport mechanisms.
Effect of salt stress on Cd contents in shoots and roots in the presence of 1 µM CdCl2. Ratio of Cd contents in shoots to roots in cv. ‘Nipponbare’ (a), and wild type (‘Zhonghua 11’) and nramp5 mutants (b) after a treatment with 50 mM NaCl in the hydroponic solution for 7 days. Error bars indicate standard deviation. ** indicates significance at α = 0.01 assessed by Student’s t-test (n = 5). Lower case letters indicate significance at α = 0.05 assessed by ANOVA followed by Tukey’s honestly significant difference test (n = 5)
Discussion
Apoplastic bypass flow transports water from the ambient space to the stele exclusively through the apoplast in root tissue. Although symplastic route dominates under non-stressed condition, water transport via apoplastic route becomes unignorable under salinity stress and perturbs the soil-plant-atmosphere continuum in rice (Yeo et al. 1987). The role of apoplastic bypass flow for sodium transport to the shoots has already been elucidated (Flowers and Yeo 1995; Ranathunge et al. 2005; Sobahan et al. 2009). The manganese transporter of rice, Nramp5 is known to be critical for Cd uptake in roots in a non-stressed condition (Sasaki et al. 2012). Qi et al. (2020) reported that a low Cd-accumulating rice cultivar formed a longer Casparian strip and suberin lamellae reaching near to the root tip and a high Cd-accumulating cultivar formed relatively short and did not reach to the root tip. They also demonstrated that apoplastic bypass flow rate and apparent Cd contents in the xylem sap have a positive relationship. This suggests a potential role of apoplastic pathway in Cd uptake in rice. Here, we tested a hypothesis that apoplastic bypass flow carries Cd in a manner resembling sodium transport in addition to Nramp5-associated Cd transport to rice shoots from ambient space under stressed conditions.
In this study we demonstrated that the exposure to high concentrations of Cd increased the rate of apoplastic bypass flow (Figs. 1 and 2, S1). The increase of the bypass flow rate and membrane integrity impairment in the tip region of the roots occurred concurrently and was not strongly associated with the collapse of the Casparian strips (Figs. 3, S2). Inhibition of apoplastic bypass flow by PEG and proline rendered the inhibition of Cd uptake to the shoots (Fig. 4). In low Cd concentrations, increase of apoplastic bypass flow by a NaCl-treatment increased Cd transport to shoots (Fig. 6). These results suggest that the translocation of Cd to shoots is mediated at least in part by apoplastic bypass flow, which can be promoted by NaCl and Cd stresses.
Reportedly, sodium activates apoplastic bypass flow in rice (e.g., Flowers and Yeo 1995; Ranathunge et al. 2005; Sobahan et al. 2009; Yeo et al. 1987). However, the effect of heavy metals on apoplastic bypass flow has not been elucidated well. Qi et al. (2020) discussed that 8.9 µM Cd accelerated the development of apoplastic barrier, lignin and suberin contents in rice roots, while the degree of apoplastic bypass flow enhancement was not investigated. We here showed that sodium promoted Cd uptake to shoots in a Nramp5 transporter-independent manner at a low Cd concentration (Fig. 6). This implies that non-essential metals, such as sodium and Cd, can be transported at least in part by apoplastic bypass flow, which is activated by stresses. In this study we propose a novel pathway where Cd is incorporated to the shoot via apoplastic bypass flow in addition to the conventional model with symplastic transporters (for review, see Clemens et al. 2013). Sodium is not a beneficial element for paddy rice and the main intake route of Na+ into xylem is the radial apoplastic bypass flow in roots (Yadav et al. 1996; Yeo et al. 1987). We assume that non-beneficial and non-essential elements, such as Cd, can be taken up via apoplastic bypass flow concomitantly with the bulk flow of water driven by transpiration in a manner analogous to Na+ in rice.
A study on durum wheat reported that Cd treatment did not activate apoplastic bypass flow and it is not a major route of Cd uptake (Van der Vliet et al. 2007). This discrepancy is not necessarily surprising because the activation of apoplastic bypass flow by sodium does not occur in wheat (Garcia et al. 1997). The effect of Cd on apoplastic bypass flow can be different among species as found in sodium. Beside rice, it was reported that sodium is transported by apoplastic pathway in another aquatic plant species, common reed (Fritz and Ehwald 2013). We assume that salt and Cd stress-activated apoplastic bypass flow rate might have the common feature in aquatic and semi-aquatic plant species but not in upland plants. This assumption remains to be tested.
It was shown that deposition of lignin and suberin in root, namely for reinforcement of water barrier in the Casparian strip, rendered reduction of bypass flow of Na+ (Krishnamurthy et al. 2009, 2011). Therefore, we initially hypothesized that Cd increased apoplastic bypass flow rate by disrupting the Casparian strip in the opposite manner of reduction of the bypass flow by suberin and lignin deposition. However, in the experimental condition we employed, the Casparian strip had not been substantially established in the hypodermis yet. Thus, the involvement of disruption of the Casparian strip in the increase of apoplastic bypass flow rate was excluded at least under the cultivation procedure in this study (Fig. S2). Here, we demonstrated that membrane integrity of meristem and transition zone cells by the exposure to a high concentration of Cd that increased apoplastic bypass flow rate (Fig. 3). This suggests that concomitant resistance to radial water transport decreases to increase apoplastic bypass flow rate by cell death in a particular region, although further investigation is needed on the relation between induction of cell death and activation of apoplastic bypass flow. Since the results in this study regarding the formation of Casparian strip in hypodermis was conducted only in young seedling of ‘Nipponbare’ cultivar and was not quantitative. Therefore, there is room for further investigation of the contribution of Casparian strip in Cd-induced apoplastic bypass flow. Furthermore, our study does not deny the role of Casparian strip in apoplastic bypass flow control at all.
PEG inhibited apoplastic bypass flow rate most likely by its osmotic pressure and affected similarly salt-activated apopolastic flow (Faiyue et al. 2010b). Proline is known to protect cells from heavy metal stresses (Shah and Dubey 1997; Sharma and Dietz 2006). However, the repression of apoplastic bypass flow rate was not attributed to a rescue from the Cd-induced membrane integrity impairment (Fig. S4). Although the mechanism of apoplastic flow inhibition by proline is not clear, proline also inhibits sodium-induced apoplastic flow and therefore it is deducible that the mechanisms of inhibition are different between PEG and proline. Since two different modes of action inhibiting apoplastic bypass flow reduced Cd transfer to shoot, we concluded that Cd was transferred to shoot along with apoplastic bypass flow (Fig. 4) rather than adverse effects of the reagents on Cd uptake.
The concentration of Cd used in most of this study (50–200 µM) was rather high compared to practical Cd contaminated sites in general (UNEP 2008). These experimental conditions may not reflect the real situation, and stimulation of apoplastic bypass flow by Cd might not be a major issue in paddy fields. Similar to our study, Qi et al. (2020) claimed that apoplastic bypass flow has a role in Cd uptake by comparing a variety of rice cultivars with different Cd accumulation property. As they pointed out in their article, Cd concentration used (8.9 µM) was higher than the realistic level in the paddy field. Our study showed that, even at a reasonably low Cd concentration (1 µM), an abiotic stress can increase Cd translocation to the shoot via apoplastic route (Fig. 6), and the restriction of Cd transport in the root cortex by OsHMA3 can be overwhelmed by these stresses. Here we propose that reduction of apoplastic bypass flow can be a clue for the reduction of Cd uptake in practical farming situations in addition to breeding approaches. It was reported that reduction of apoplastic bypass flow rate with silicon is effective to reduce Na+ uptake to rice shoots (Flam-Sheperd et al. 2018; Yeo et al. 1999). Application of silicon may also be effective to reduce Cd uptake through the apoplastic bypass flow in rice as an agricultural practice.
References
Bertin G, Averbeck D (2006) Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88:1549–1559
Brundrett MC, Enstone DE, Peterson CA (1988) A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 146:133–142
Clemens S, Aarts MGM, Thomie S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18:92–99
DalCorso G, Farinati S, Maistri S, Furini A (2008) How plants cope with cadmium: Staking all on metabolism and gene expression. J Integr Plant Biol 50:1268–1280
Faiyue B, Vijayalakshmi C, Nawaz S, Nagato Y, Taketa S, Ichii M, Al-Azzawi MJ, Flowers TJ (2010a) Studies on sodium bypass flow in lateral rootless mutants lrt1 and lrt2, and crown rootless mutant crl1 of rice (Oryza sativa L.). Plant Cell Environ 33:687–701
Faiyue B, Al-Azzawi MJ, Flowers TJ (2010b) The role of lateral roots in bypass flow in rice (Oryza sativa L.). Plant Cell Environ 33:702–716
Flam-Shepherd R, Huynh WQ, Coskun D, Hamam AM, Britto DT, Kronzucker HJ (2018) Membrane fluxes, bypass flows, and sodium stress in rice: the influence of silicon. J Exp Bot 69:1679–1692
Flowers TJ, Yeo AR (1995) Breeding for salinity resistance in crop plants: where next? Australian J Plant Physiol 22:875–884
Fritz M, Ehwald R (2013) Radial transport of salt and water in roots of the common reed (Phragmites australis Trin. ex Steudel). Plant Cell Environ 36:1860–1870
Garcia A, Rizzo CA, Ud-Din J, Bartos SL, Senadhira D, Flowers TJ, Yeo AR (1997) Sodium and potassium transport to the xylem are inherited independently in rice, and the mechanism of sodium: potassium selectivity differs between rice and wheat. Plant Cell Environ 20:1167–1174
Greger M, Johansson M (1992) Cadmium effects on leaf transpiration of sugar beet (Beta vulgaris). Physiol Plant 86:465–473
Hirayama T, Lei GJ, Yamaji N, Nakagawa N, Ma JF (2018) The putative peptide gene FEP1 regulates iron deficiency response in Arabidopsis. Plant Cell Physiol 59:1739–1752
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 45:335–346
Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Screiber L, Mathew MK (2009) The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planta 230:119–134
Krishnamurthy P, Ranathunge K, Nayak S, Schreiber L, Mathew MK (2011) Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J Exp Bot 62:4215–4228
Li J, Zhu S, Song X, Shen Y, Chen H, Yu J, Yi K, Liu Y, Karplus VJ, Wu P, Deng XW (2006) A rice glutamate receptor-like gene is critical for the division and survival of individual cells in the root apical meristem. Plant Cell 18:340–349
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:951–981
Nakanishi H, Ogawa I, Ishimaru U, Mori S, Nishizawa NK (2006) Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr 52:464–469
Nawrot T, Plusquin M, Hogevorst J, Roels HA, Celis H, Thijs L, Vangronsveld J, Van Hecke E, Staessen JA (2006) Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol 7:119–126
Ochiai K, Matoh T (2002) Characterization of the Na+ delivery from roots to shoots in rice under saline stress: excessive salt enhances apoplastic transport in rice plants. Soil Sci Plant Nutr 43:371–337
Pedas P, Ytting CK, Fuglsang AT, Jahn TP, Schjoerring JK, Husted S (2008) Manganese efficiency in barley: Identification and characterization of the metal ion transporter HIRT1. Plant Physiol 148:455–466
Prasad MNV (1995) Cadmium toxicity and tolerance in vascular plants. Envir Exp Bot 35:525–545
Qi X, Tam NF, WC L, Ye Z (2020) The role of root apoplastic barriers in cadmium translocation and accumulation in cultivars of rice (Oryza sativa L.) with different Cd-accumulating characteristics. Environ Pollut 254:1147736
Ranathunge K, Steudle E, Laritte R (2005) Blockage of apoplastic bypass-flow of water in rice roots by insoluble salt precipitates analogous to a Pfeffer cell. Plant Cell Environ 28:121–133
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24:2155–2167
Shah K, Dubey RS (1997) Effect of cadmium on proline accumulation and ribonuclease activity in rice seedlings: role of proline as a possible enxyme protectant. Biol Plant 40:121–130
Sharma SS, Dietz KJ (2006) The significance of amino acid and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Sobahan MA, Arias CR, Okuma E, Shimoishi Y, Nakamura Y, Hirai Y, Mori IC, Murata Y (2009) Exogenous proline and glycinebetaine suppress apoplastic flow to reduce Na+ uptake in rice seedlings. Biosci Biotechnol Biochem 73:2037–2042
Ueno D, Kono I, Yokosho K, Ando T, Yano M, Ma JF (2009) A major quantitative trait locus controlling cadmium translocation in rice (Oryza sativa). New Phytol 182:644–653
Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA 107:16500–16505
UNEP (United Nations Environmental Programme) (2008) Draft final review of scientific information on cadmium. http://www.chem.unep.ch/Pb_and_Cd/SR/Draft_final_reviews/Cd_Review/Final_UNEP_Cadmium_review_Nov_2008.pdf. Accessed 30 Sept 2018
Van der Vliet L, Peterson C, Hale B (2007) Cd accumulation in roots and shoots of durum wheat: the roles of transpiration rate and apoplastic bypass. J Exp Bot 58:2939–2947
Yadav R, Flowers TJ, Yeo AR (1996) The involvement of the transpirational bypass flow in sodium uptake by high- and low-sodium-transporting lines of rice developed through intravarietal selection. Plant Cell Environ 19:329–336
Yan GC, Fan XP, Tan L, Yin C, Li TQ, Liang YC (2021) Root silicon deposition and its resultant reduction of sodium bypass flow is modulated by OsLsi1 and OsLsi2 in rice. Plant Physiol Biochem 158:219–227
Yeo AR, Yeo ME, Flowers TJ (1987) The contribution of an apoplastic pathway to sodium uptake by rice roots in saline condition. J Exp Bot 38:1141–1153
Yeo AR, Flowers SA, Rao G, Welfare K, Senanayake N, Flowers TJ (1999) Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ 22:559–565
Zhang J, Li C, Wu C, Xiong L, Chen G, Zhang Q, Wang S (2006) RMD: a rice mutant database for functional analysis of the rice genome. Nucleic Acids Res 34:D745–D748
Acknowledgements
ICP analysis and histochemical analysis were helped by Ms. Sanae Rikiishi and Ms. Chiemi Hasegawa (Institute of Plant Science and Resource, Okayama University). The authors thank Dr. Jian Feng Ma and Rice Mutant Database for providing the mutant seeds. This study was funded by The Japan Society for the Promotion of Science KAKENHI (17078006 to Y.M. and I.C.M).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mori, I.C., Arias-Barreiro, C.R., Ooi, L. et al. Cadmium uptake via apoplastic bypass flow in Oryza sativa. J Plant Res 134, 1139–1148 (2021). https://doi.org/10.1007/s10265-021-01319-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-021-01319-y