Abstract
Shoots of the aquatic eudicot family, Podostemaceae, exhibit unusual organogenesis with mixed leaf and stem identities. New shoots arise at the base of the older shoot with shoot apical meristem (SAM) identity but the entire SAM differentiates into a “leaf” as it develops in the Podostemoideae subfamily. The “leaves” are tightly arranged in a zigzag manner to form an apparent distichous shoot as a whole. Although previous studies have suggested that Podostemoideae shoots have evolved by modifying the ancestral sympodial branching system in the basal Tristichoideae subfamily, this evolutionary scenario requires elucidation at the molecular level. To confirm that the shoots arise as axillary shoots, in the present study, we examined gene expression patterns in plumular shoots of Zeylanidium tailichenoides using CUP-SHAPED COTYLEDON 3 (CUC3) and SHOOT MERISTEMLESS (STM) orthologs, which are involved in the determination of axils and meristem formation in model plants. Expression of the CUC3 ortholog was detected at the adaxial base of cotyledons and parental shoots where the new shoots are initiated, while STM ortholog was expressed at the initiation site and in the young shoot primordia throughout early shoot development. The results demonstrate that each Z. tailichenoides shoot arises as an axillary bud in a manner similar to axillary meristem formation in model plants involving CUC3 and STM genes. Considering that each of the two cotyledons produces an axillary bud that in turn continues to form its own axillary bud independently, the apparent distichous shoot in Z.tailichenoides is not a single shoot, but a composite of two sympodially branched shoots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vertical structure of flowering plants is an adaptation to terrestrial environments and is facilitated by indeterminate activities in the shoot apical meristem (SAM) and root apical meristem. In aquatic environments, however, plants exhibit unique morphologies that vary considerably from the structures observed in terrestrial environments (Bell 2008). An aquatic eudicot family, Podostemaceae (the river weeds), exhibit loss of conventional SAM activity, resulting in determinate shoot development (Hammond 1936; Imaichi et al. 2005; Koi et al. 2005; Rutishauser and Grubert 2000). Molecular phylogenetic studies have revealed that Podostemaceae is a sister family to Hypericaceae in Malpighiales (Wurdack and Davis 2009), suggesting that novel shoot organogenesis in Podostemaceae has evolved from ordinary SAM-mediated shoot development.
Podostemaceae are found in rocks in fast-flowing rivers in the tropical and subtropical regions of the world, where water levels change seasonally. At the beginning of the rainy seasons, the seeds germinate and the seedlings adhere to rocks. In most species, the embryonic shoot stops growing after the formation of several leaves, and adventitious roots emerge from the lateral side of the hypocotyl (Mohan Ram and Sehgal 1997; Sehgal et al. 2007; Suzuki et al. 2002). The adventitious roots grow creeping on the rock surfaces, and adventitious shoots (root-borne shoots) are formed on the dorsal or lateral sides of the roots. In the subsequent dry season, when water levels become low, the plants set flowers and fruits as they are exposed to the air and wilt.
Podostemaceae comprises three subfamilies, including Tristichoideae, Weddellinoideae, and Podostemoideae. Among the three, Tristichoideae is the most basally diverged clade, being sister to the monophyletic Weddellinoideae and Podostemoideae (Cook and Rutishauser 2007; Kita and Kato 2001; Koi et al. 2012). Although their shoots similarly exhibit determinate growth, the subfamilies exhibit different shoot development patterns. In Tristichoideae and Weddellinoideae, shoots have dome-shaped SAMs that produce scaly leaves on their flanks but the shoots soon stop growing by sympodial branching, so that new lateral shoots arise from the base of parental shoots, and the parental SAM eventually lose their organization and disappear (Fujinami and Imaichi 2009; 2015; Fujinami et al. 2013; Jäger-Zürn 1997; Kita and Kato 2005; Koi and Kato 2007). In Podostemoideae, in contrast, both embryonic and root-borne shoots lack obvious SAMs. Nevertheless, the shoots grow and form several “leaves” in a distichous fashion (Hammond 1936; Katayama et al. 2010; Mohan Ram and Sehgal 1997; Rutishauser and Grubert 2000; Sehgal et al. 2007; Suzuki et al. 2002).
The most diversified subfamily, Podostemoideae, is divided into three groups, paraphyletic American Podostemoideae, African Podostemoideae, and monophyletic Asian group (Koi et al. 2012). Detailed developmental examinations of Podostemoideae shoots in Asian species revealed that in the absence of SAM, new “leaf” primordia arise at the adaxial bases of preexisting “leaves” (Imaichi et al. 2005; Koi et al. 2005). In addition, gene expression analyses using orthologous genes involved in shoot development in model plants, including SHOOT MERISTEMLESS (STM), WUSCHEL (WUS), and ASYMMETRIC LEAVES1/ROUGH SHEATH1/PHANTASTICA (ARP), revealed that a new “leaf” is initiated as a SAM, but the SAM instantly differentiates into a leaf (Katayama et al. 2010). The finding revealed that each “leaf” is not a real leaf but an atypical determinate shoot with a mixture of leaf and stem identities (Katayama et al. 2010). Considering that the atypical determinate shoots initiate at the adaxial base of parental determinate shoots, it is inferred that the shoots of Podostemoideae arise as axillary shoots. Therefore, considering the phylogenetic relationships within the family, the determinate shoots in Podsotemoideae potentially evolved from the sympodial branching shoots, which are commonly found in Tristichoideae and Weddellinoideae.
To reveal the evolutionary history of the “determinate shoot”, Katayama et al. (2011, 2013) performed embryological analyses of Zeylanidium tailichenoides, an Asian member, which was previously referred to as Z. lichenoides but was renamed by Kato and Koi (2018). During embryogenesis, a morphologically unrecognizable SAM, denoted as a cryptic SAM in Katayama et al. (2013), was observed accompanying the expression of STM ortholog (ZlSTM). Following germination, ZlSTM expression is not maintained in the cryptic SAM and shifts to the adaxial base i.e., the axils of cotyledons, where the new determinate shoots arise. ZlSTM expression in shoot primordia is maintained only in the early stages of shoot development and disappears as they develop into “leaves”. Since the STM ortholog expression pattern corresponds to those in root-borne shoots of Cladopus queenslandicus and Hydrobryum japonicum, both plumular and root-borne shoots are inferred to undergo a similar developmental pathway and arise as axillary shoots (Katayama et al. 2010; Koi and Katayama 2013). The findings suggest that the shoots of Podostemoideae arise as axillary shoots.
In the present study, to elucidate the axillary origin of the Podostemoideae shoots, we investigated whether CUP-SHAPED COTYLEDON 3 (CUC3) and STM genes are involved in novel shoot development in Z. tailichenoides seedlings in a manner similar to the axillary bud formation in other eudicots. Studies on model plants have revealed the genetic basis of shoot branching and CUC genes (CUC1, CUC2, CUC3) in Arabidopsis, which encode NAC domain transcription factors, and that they are critical factors determining the boundaries between indeterminate meristem and determinate lateral organs, in addition to other regulatory genes, e.g., LATERAL SUPRESSOR (LAS) and REGULATOR OF AXILLARY MERISTEMS (RAX) genes (Aida et al. 1997; Vroemen et al. 2003). Although the molecular mechanisms remain unknown, STM and other genes involved in meristem formation are up-regulated following the determination of initiation sites of new shoots by CUC and LAS genes (Raman et al. 2008).
While CUC genes have high levels of redundancy in boundary formation, the functional differentiation of the three genes has been reported in Arabidopsis. Among the three CUC genes, CUC3 has a major function in axillary meristem formation and determination of the position of the axillary meristem (Hibara et al. 2006; Raman et al. 2008). Although CUC1, CUC2 and CUC 3 are expressed at the boundary between SAM and leaf primordia, i.e., in the axils of leaves, where the axillary meristem is initiated, a CUC3 single mutation results in stronger defects in the formation of axillary shoots than CUC1 or CUC2 mutations, suggesting that the role of CUC3 is greater in axillary meristem specification (Hibara et al. 2006; Raman et al. 2008). Although the functional differentiation of CUC genes has not been reported in eudicots other than Arabidopsis, at present, CUC3 ortholog is the most appropriate for detecting the axils of cotyledons and determinate shoots of Z. tailichenoides in the present study.
Materials and methods
Plant materials
Fruits of Z. tailichenoides were collected from Huay Kaew stream, Maethakhrai National Park, Chiang Mai, Thailand. Voucher specimens were deposited in the Herbarium of the National Museum of Nature and Science, Japan (TNS-8000188, TNS-8200207). For seedling culture, seeds were placed in 3.0% agar containing 0.05% (v/v) HYPONeX (Hyponex Japan, Tokyo, Japan) and the agar covered with 0.05% (v/v) HYPONeX liquid medium. The plants were cultured in a growth chamber at 25 °C under 14 h of light/10 h of dark conditions.
Anatomical observation
Cultured seedlings were fixed with FAA (formalin–acetic acid–50% ethanol = 1:1:18, v/v) for 24 h. Fixed materials were dehydrated in an ethanol series, embedded in Thechnovit 7100 (glycol methacrylate; Heraeus Kulzur, Wehrheim, Germany), and cut into 2-μm-thick sections on a microtome. Sections were stained in a solution of safranin, toluidine blue, and orange G (Jernstedt et al. 1992), and observed under a light microscope (Olympus 51; Olympus, Tokyo, Japan). For observation under scanning electron microscopy (SEM), the fixed material was dehydrated using a graded ethanol series, critical-point dried, and coated with platinum/palladium. The sample was observed under a Keyence VE8800 scanning electron microscope (Keyence, Osaka, Japan) at 5 kV.
Gene isolation and phylogenetic analysis of ZlCUC3
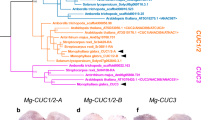
Four DNA sequences of the putative CUC3 ortholog of Z. tailichenoides were found from RNA-seq data (Accession Number: DRA008126) obtained from whole seedlings by carrying out a BLASTn search using Arabidopsis CUC1–3 sequences as the query with an E-value cutoff of e−10 and by removing short sequences ( < 900 bp) (Table S1). Because CUC3 is a member of the NO APICAL MERISTEM (NAM)/CUC3 gene family, to confirm the NAM/CUC3 homology of four obtained sequences for subsequent phylogenetic analysis, we performed BLASTP search on the National Center for Biotechnology Information (NCBI) website. Among the four sequences, two sequences showed high similarity in identity with NAM/CUC3 genes, but others showed similarity in identity with other NAC genes (Table S2). Orthology in ZlCUC3 was confirmed by phylogenetic analysis using two putative NAM/CUC3 homologs and 25 amino acid sequences, which are registered as NAM/CUC3 genes in the NCBI DNA database. The GenBank accession numbers of the sequences are listed in Table S3. The amino acid sequences of the genes and the retrieved genes were aligned using MAFFT version 7 (https://mafft.cbrc.jp/alignment/server/; Katoh and Toh 2008) and the L-INS-I method (Katoh 2005). A phylogenetic analysis was performed using RAxML version 8.0.5 (Larkin et al. 2007), based only on the conserved NAC domains of the amino acid sequences (Fig. S1) using the method of maximum likelihood. Bootstrap values were calculated using 1000 replicates.
RNA in situ hybridization
Seedlings cultured in the laboratory were soaked in 3% paraformaldehyde and 0.25% glutaraldehyde (v/v) in 100 m mol L–1 sodium phosphate buffer (pH 7.2) and fixed for 14 h at 4 °C. The fixed materials were dehydrated in an ethyl and tertiary butyl alcohol series and embedded in Palaplast Plus (Oxford Labware, St. Louis, Missouri, USA). Microtome sections (10-µm thick) were put on MAS-coated glass slides (Matsunami, Osaka, Japan). For probe templates, nearly the entire coding regions of ZlCUC3 and ZlSTM were used (Table S4) and the RNA probes were synthesized using the DIG RNA Labeling Kit (Roche Diagnostics, Mannheim, Germany) according to manufacturer’s instructions. The synthesized RNA probes were partially hydrolyzed to ~ 300 bp with an alkaline solution (60 mmol L–1 Na2CO3 and 40 mmol L–1 NaHCO3, pH 10.2) at 60 °C for 80 min. RNA in situ hybridization was conducted according to Katayama et al. (2013).
Results
Plumular shoot development in Zeylanidium tailichenoides
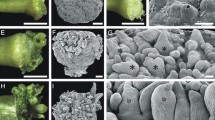
Seedlings with two cotyledons, a hypocotyl, and rhizoids were obtained approximately one week after sowing (Fig. 1a). The first and second plumular shoots developed between two cotyledons and an adventitious root began to emerge from the lateral side of the hypocotyl (Fig. 1b). Under the present culture conditions, three to four plumular shoots were produced within 30 days, while the fifth and sixth shoots were hardly observed by our rough observation under a binocular microscope (Fig. 1c). In gross morphology, the first two shoots (S1 and S2) grew at a right angle to the two cotyledons (Fig. 1b), and subsequent shoots grew three dimensionally with no apparent cladotaxis (Fig. 1c).
Plumular leaf formation in Zeylanidium tailichenoides. a–c Gross morphology of a seedling. a Seedling after germination approximately one week after sowing. b Seedling with first and second shoots between cotyledons. Adventitious root arising from the lateral side of hypocotyl. c Seedling about 30 days after sowing. Several shoots arising between cotyledons and adventitious root elongating. d Longitudinal section of seedling. Third and fourth shoots arising at the base of first and second shoots, respectively. e Cross sections of seedling. Seedling at the initiation stage of first and second shoot primordia. Cell proliferation of the primordia (surrounded by black line) was observed opposite each other at the center of the section, which corresponded to the adaxial sides of cotyledons. f SEM image of seedling with first and second shoots developing. Cotyledon removed (surrounded by white dashed line). g Seedling with third and fourth shoots arising at the adaxial side of first and second shoots, respectively. AR adventitious root, C cotyledon, H hypocotyl, S1–S4 first–fourth shoot, RH rhizoid. Scale barsa–c 100 μm d–h 50 μm
Before the gene expression analysis, we determined the initiation sites of plumular shoots in relation to the two cotyledons. Longitudinal sections of seedlings at the initiation stage of the third and fourth shoots revealed that the primordia of the third and fourth shoots are developed at the adaxial bases of the first and second shoots, respectively, and in turn, the primordia of the first and second shoots are at the adaxial bases of the two cotyledons (Fig. 1d). However, we could not obtain any median sections cut through only the middle of all the first to fourth shoot primordia, which suggests that the shoots are not arranged in two orthostichies but somewhat deviate from the orthostichies. Therefore, we carried out observations from cross sections and SEM. In the cross sections, the first and second shoots arose from the center of the adaxial side of the cotyledons (Fig. 1e). However, in the SEM observation, the growing first and second shoots were arranged at right angles to a pair of cotyledons (Fig. 1f). The observations suggest that, following their initiation, the first and second shoots grew twisting in relation to the positions of two parental cotyledons. Similar spatial changes occurred in later shoots throughout growth, as shown in Fig. 1c. The third and fourth shoots were initiated at the adaxial side of the first and second shoots, respectively (Fig. 1g). Although their initiation sites appeared to slightly deviate from the center due to twisting growth of the first and second parental shoots, the pairs of primordia of the third and fourth shoots were opposite each other (Fig. 1g).
Isolation of ZlCUC3
In the gene expression analysis, we isolated the orthologous gene of CUC3 in Z. tailichenoides. An alignment of the inferred amino acid sequences in ZlCUC3 with other CUC proteins revealed that ZlCUC3 encodes a NAC domain, which is shared by all NAC family proteins. Approximately 79% of amino acids in NAC domain in ZlCUC3 were identical with Arabidopsis CUC3 (Fig. S1). A phylogenetic tree inferred from NAC domain confirmed that ZlCUC3 was nested in CUC3 clade with other CUC3 orthologs with a 100% bootstrap value (Fig. 2).
Expression patterns of ZlCUC3 in comparison to ZlSTM
We examined ZlCUC3 and ZlSTM expression patterns during plumular shoot development based on RNA in situ hybridization analyses using longitudinal sections. Immediately following germination, the seedlings had no shoot primordia at the adaxial bases of the two cotyledons. In the seedlings, ZlCUC3 was expressed mainly in epidermal cells at the base of each of the two cotyledons, but its expression was more prominent in the cotyledon in which the first shoot was initiated than in the other cotyledon in which the second shoot was initiated (arrow, Fig. 3a). In a seedling at the same stage, however, ZlSTM was expressed in broad and deep regions at the bases of both cotyledons (Fig. 3b), compared to the ZlCUC3 expression region. Notably, broader expression of ZlSTM was detected in one cotyledon in which the first shoot was initiated (Fig. 3b). Soon after, one cotyledon began to form a shoot primordium i.e., the first shoot, at its adaxial base; although the second shoot primordium did not emerge in the other cotyledon at that stage (Fig. 3c, d). At that stage, distinct expression of ZlCUC3 was observed in the adaxial base of the cotyledon where the second shoot was expected to be initiated (left arrow in Fig. 3c) and ZlSTM expression was detected at the initiation site of the second shoot (Fig. 3d). Therefore, ZlCUC3 expression was observed at the initiation stages of the first and second shoots in the adaxial bases of the cotyledons.
Gene expression during plumular shoot formation in longitudinal sections of seedlings. Developing first leaves are always on the right sides of figures. a, c, e, gZlCUC3 mRNA expression. Arrows indicate putative initiation sites of new shoots with ZlCUC3 expression. b, d, f, hZlSTM mRNA expression. aZlCUC3 expression at the base of a cotyledon at the initiation stage of first plumular shoot. bZlSTM expression at the bases of both cotyledons at nearly same stage as a. cZlCUC3 expression at the base of a cotyledon and the base of the first plumular shoot. dZlSTM expression at the base of a cotyledon and protrusion of the first shoot. eZlCUC3 expression at the base of the developing first shoot. Downregulated ZlCUC3 expression was detected in the base of the second shoot primordium. fZlSTM expression in the initiation site of the second shoot but not during the protrusion of the first shoot. gZlCUC3 expression at the base of the second shoot primordium. Protrusion of the first shoot was observed on another serial section. hZlSTM expression in the protrusions of the first and second shoot primordia. C cotyledon, S1and S2 first and second shoot primordia, respectively. Scale bars a–h 50 μm
At a later stage, ZlCUC3 expression was also observed restricted to the base of the first shoot primordium, which corresponded to the initiation site of the third shoot (right arrow, Fig. 3c), while the expression of ZlSTM was expressed in the whole primordium of the first shoot (S1, Fig. 3d). At a subsequent stage where the first shoot became more developed and the second shoot primordium began to grow, ZlSTM was expressed in the first and second primordia (Fig. 3f). ZlCUC3 expression was still detected at the base of the first shoot i.e., the initiation site of the third shoot (right arrow, Fig. 3e), while ZlCUC3 expression was downregulated at the base of the second shoot primordium (left arrow, Fig. 3e). As the second shoot primordium developed further, ZlCUC3 expression was detected at the base, i.e., the initiation site of the fourth shoot (arrow, Fig. 3g), where ZlSTM expression was maintained (Fig. 3h). The expression patterns of ZlCUC3 and ZlSTM in the initiation of the third and fourth shoots are identical to those observed in the initiation of the first and second shoots. In the experiments, a signal was not detected in any case using the sense probes of ZlCUC3 and ZlSTM (Fig. S2).
Discussion
The present study demonstrated that the CUC3 orthologous gene was expressed at the initiation sites of new shoots during plumular shoot development in Z. tailichenoides. The first and second shoots were formed at the base of each cotyledon and the third and fourth shoots at the bases of the first and second shoots, respectively. ZlCUC3 expression was detected at the initiation site but not at the protrusion of the shoot primordia, while ZlSTM was continuously expressed throughout the early stage of shoot development. Because of the spatial–temporal overlap between the initiation of new shoots and STM expression of parental cotyledons/shoots, we did not determine the time of CUC3 expression and STM expression at shoot initiation. However, we confirmed that ZlCUC3 was expressed at the shoot initiation sites at the adaxial base of parental cotyledons/shoots in the order of first to fourth shoots, corresponding to alternate cladotaxy. Therefore, ZlCUC3 is likely to be involved in the determination of the initiation sites of shoots, similar to in axillary meristem formation in Arabidopsis thaliana (Hibara et al. 2006; Raman et al. 2008; Vroemen et al. 2003). Therefore, the first and second shoots are suggested to arise as axillary buds of cotyledons and subsequent shoots occur as axillary buds of older shoots.
Detailed anatomical observations in Tristichoideae that have apparent SAM have revealed that all species investigated in five of six genera exhibit sympodial branching by extra-axillary bud formation (Fujinami and Imaichi 2009, 2015; Fujinami et al. 2013). The Tristichoideae shoots exhibit a zigzag branching pattern where new shoots arise at the base of the youngest shoot. A similar zigzag sympodial branching pattern has been reported in Weddellina squamulosa, Weddellinoideae (Koi and Kato 2007). However, here, we revealed that there is a difference in the organ composition of a “shoot” between Tristichoideae and Podostemoideae. In the plumular shoots of Z. tailichenoides, two axillary shoots of cotyledons produce each of their own axillary shoots separately, but they develop in concert, since there is no space between the cotyledons with a dome-shaped shoot apex, which should be observed in ordinary plants. The two axillary buds of cotyledons are not initiated simultaneously but one is initiated slightly earlier than the other. The maintenance of such a temporal gap through subsequent growth results in new shoots consisting of two sympodial axes that are alternately arranged. Therefore, the plumular shoot in Z. tailichenoides is considered an apparent distichous shoot, which is composed of two independent sympodially branched shoots. The finding provides new insights on the evolution of novel shoot development in Podostemaceae; an apparent plumular shoot in Podostemoideae is considered to be composed of two axillary sympodial axes, each of which is equivalent to a sympodially branched shoot of basal subfamilies, Tristichoideae and Weddellinoideae (Fujinami and Imaichi 2009, 2015; Fujinami et al. 2013; Jäger-Zürn 1997).
Regardless of monopodial or sympodial branching, axillary buds are usually formed at the adaxial bases of leaves in angiosperms (Bell 2008). This raises an interesting question regarding how adaxial-abaxial identity is established in Podostemoideae shoots where SAM is not maintained and differentiates into a leaf, since SAM activities of the major shoot in other plants determine the adaxial-abaxial character (Steeves and Sussex 1989). A potential answer is that adaxial-abaxial identity at the major axis is maintained between two rows of the apparent distichous shoot where the cryptic SAM is established. New shoots are formed at the adaxial bases of parental shoots when the region of a cryptic SAM is assumed to maintain the identity of the main axis. In addition, shoots exhibit adaxial-abaxial dorsiventrality after they are differentiated into leaves as observed in typical leaves of other angiosperms, which confirms the presence of an axis that influences the development of the apparent shoot. Therefore, the identity of an axis seems to be maintained at the center of the apparent shoot structure by an unknown regulatory mechanism. However, no molecular studies supporting the presence of an invisible axis have been carried out so far.
Considerable variation in morphology is observed in the mixed leaf-shoot organ of Podostemoideae, ranging from linear shoots in Z. tailichenoides and Cladopus queenslandicus to highly dissected organ-like compound leaves e.g., Mourera fluviatilis and Marathrum rubrum (Rutishauser 1995; Rutishauser and Grubert 1999). In addition, leafy organs with two sheaths that have been referred to as a “double-sheathed leaf” or a “dithecous leaf” occur in Z. sublatum and Ledermaniella linearifolia (Imaichi et al. 2005; Moline et al. 2007). Since the Podostemoideae shoots examined in previous studies on species ranging from American, African, to Asian groups do not exhibit typical shoot development associated with SAM, SAM-less shoot development could be a synapomorphy in Podostemoideae (Hammond 1936; Imaichi et al. 2005; Koi et al. 2005; Rutishauser and Grubert 2000; Suzuki et al. 2002). In addition, since both organs have been interpreted as leaves in previous studies, further studies based on the view that they are initiated as shoots would offer novel insights for understanding the diversification process in Podostemoideae shoots. Detailed observations in early developmental stages are required to understand the variations in shoot development.
References
Aida M, Ishida T, Fukaki H et al (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9:841–857
Bell AD (2008) Plant form: an illustrated guide to flowering plant morphology. Timber Press, Portland
Cook CDK, Rutishauser R (2007) Podostemaceae. In: Kubitzki K (ed) The families and genera of vascular plants. Springer, Berlin, pp 304–344
Fujinami R, Imaichi R (2009) Developmental anatomy of Terniopsis malayana (Podostemaceae, subfamily Tristichoideae), with implications for body plan evolution. J Plant Res 122:551–558
Fujinami R, Imaichi R (2015) Developmental morphology of flattened shoots in Dalzellia ubonensis and Indodalzellia gracilis with implications for the evolution of diversified shoot morphologies in the subfamily Tristichoideae (Podostemaceae). Am J Bot 102:848–859
Fujinami R, Ghogue JP, Imaichi R (2013) Developmental morphology of the controversial ramulus organ of Tristicha trifaria (subfamily Tristichoideae, Podostemaceae): implications for evolution of a unique body plan in Podostemaceae. Int J Plant Sci 174:609–618
Hammond BL (1936) Regeneration of Podostemon ceratophyllum. Bot Gaz 97:834–845
Hibara KI, Karim MR, Takada S et al (2006) Arabidopsis CUP-SHAPED COTYLEDON3 regulates postembryonic shoot meristem and organ boundary formation. Plant Cell 18:2946–2957
Imaichi R, Hiyama Y, Kato M (2005) Leaf development in the absence of a shoot apical meristem in Zeylanidium subulatum (Podostemaceae). Ann Bot 96:51–58
Jäger-Zürn I (1997) Comparative morphology of the vegetative structures of Tristicha trifaria, Indotristicha ramosissima and Dalzellia ceylanica (Podostemaceae, Tristichoideae): a review. Aquat Bot 57:71–96
Katayama N, Koi S, Kato M (2010) Expression of shoot meristemless, wuschel, and a symmetric leaves1 homologs in the shoots of Podostemaceae: implications for the evolution of novel shoot organogenesis. Plant Cell 22:2131–2140
Katayama N, Kato M, Yamada T (2013) Origin and development of the cryptic shoot meristem in Zeylanidium lichenoides (Podostemaceae). Am J Bot 100:635–646
Kato M, Koi S (2018) Molecular phylogeny of Zeylanidium (Podostemaceae) showing a new cryptic species from Thailand. Acta Phytotax Geobot 69:1–9
Katoh K (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucl Acids Res 33:511–518
Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9:286–298
Kita Y, Kato M (2001) Infrafamilial phylogeny of the aquatic angiosperm Podostemaceae inferred from the nucleotide sequences of the matK gene. Plant Biol (Stuttg) 3:156–163
Kita Y, Kato M (2005) Seedling developmental anatomy of an undescribed Malaccotristicha species (Podostemaceae, subfamily Tristichoideae) with implications for body plan evolution. Plant Syst Evol 254:221–232
Koi S, Katayama N (2013) Gene expression analysis of aquatic angiosperms podostemaceae to gain insight into the evolution of their enigmatic morphology. Methods Mol Biol 959:83–95
Koi S, Kato M (2007) Developmental Morphology of the Shoot in Weddellina squamulosa and implications for shoot evolution in the Podostemaceae. Ann Bot 99:1121–1130
Koi S, Imaichi R, Kato M (2005) Endogenous leaf initiation in the apical-meristemless shoot of Cladopus queenslandicus (Podostemaceae) and implications for evolution of shoot morphology. Int J Plant Sci 166:199–206
Koi S, Kita Y, Hirayama Y et al (2012) Molecular phylogenetic analysis of Podostemaceae: implications for taxonomy of major groups. Bot J Linn Soc 169:461–492
Larkin MA, Blackshields G, Brown NP et al (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Mohan Ram H, Sehgal A (1997) In vitro studies on developmental morphology of Indian Podostemaceae. Aquat Bot 57:97–132
Moline P, Thiv M, Ameka G et al (2007) Comparative morphology and molecular systematics of African Podostemaceae-Podostemoideae, with emphasis on Dicraeanthus and Ledermanniella from Cameroon. Int J Plant Sci 168:159–180
Raman S, Greb T, Peaucelle A et al (2008) Interplay of miR164, CUP-SHAPED COTYLEDON genes and LATERAL SUPPRESSOR controls axillary meristem formation in Arabidopsis thaliana. Plant J 55:65–76
Rutishauser R (1995) Developmental patterns of leaves in podostemaceae compared with more typical flowering plants—saltational evolution and fuzzy morphology. Can J Bot 73:1305–1317
Rutishauser R, Grubert M (1999) The architecture of Mourera fluviatilis (Podostemaceae): developmental morphology of inflorescences, flowers, and seedlings. Am J Bot 86:907–922
Rutishauser R, Grubert M (2000) Developmental morphology of Apinagia multibranchiata (Podostemaceae) from the Venezuelan Guyanas. Bot J Linn Soc 132:299–323
Sehgal A, Khurana JP, Sethi M et al (2007) Organ identity of the thalloid plant body of Griffithella hookeriana and Polypleurum stylosum—Podostemoideae (Podostemaceae). Plant Syst Evol 267:93–104
Steeves TA, Sussex IM (1989) Patterns in plant development. Cambridge University Press, New York
Suzuki K, Kita Y, Kato M (2002) Comparative developmental anatomy of seedlings in nine species of podostemaceae (subfamily Podostemoideae). Ann Bot 89:755–765
Vroemen CW, Mordhorst AP, Albrecht C, Kwaaitaal MACJ. de Vries SC (2003) The CUP-SHAPED COTYLEDON3 gene is required for boundary and shoot meristem formation in Arabidopsis. Plant Cell 15:1563–1577
Wurdack KJ, Davis CC (2009) Malpighiales phylogenetics: gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. Am J Bot 96:1551–1570
Acknowledgements
The authors would like to thank T. Wongprasert, M. Kato and S. Koi for their help during the collection trips in Thailand. We thank Y. Hirayama for providing a part of anatomical data. This study was supported by a Research Fellowship to N.K. and a Grant-in-Aid for Scientific Research to M.K. from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Katayama, N., Tanaka, R., Fujinami, R. et al. Expression pattern of CUC3 ortholog in Zeylanidium tailichenoides (Podostemaceae) infers organization of a unique distichous shoot in Podostemoideae. J Plant Res 132, 521–529 (2019). https://doi.org/10.1007/s10265-019-01113-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-019-01113-x