Abstract
The suppression of apical growth and radial trunk growth in trees under shade is a key factor in the competition mechanism among individuals in natural and artificial forests. However, the timing of apical and radial growth suppression after shading and the physiological processes involved have not been evaluated precisely. Twenty-one Abies sachalinensis seedlings of 5-years-old were shaded artificially under a relative light intensity of 5% for 70 days from August 1, and the histological changes of the terminal bud and terminally lateral bud of terminal leader and the cambial zone of the trunk base were analyzed periodically. In shade-grown trees, cell death of the leaf primordia in a terminal bud of terminal leader was observed in one of the three samples after 56 and 70 days of shading, whereas the leaf primordia in a terminal bud of terminal leader in all open-grown trees survived until the end of the experiment. In addition, the leaf primordia of the terminally lateral buds of terminal leader retained their cell nuclei until the end of the experiment. No histological changes were observed in the cambial cells after shading, but the shade-grown trees had less cambial activity than the open-grown trees through the experiment. Strong shading appeared to inhibit the formation and survival of cells in the terminal bud of terminal leader rather than the terminally lateral buds of terminal leader and the cambium. The suppression of the terminal bud growth and elongation of the surviving lateral buds would result in an umbrella-shaped crown under shade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light is an indispensable resource for growth in plants, and a reduction of light intensity suppresses plant growth (Bormann 1965; Chazdon 1988). In trees, light restriction by shading causes the suppression of both apical growth and radial trunk growth (Beaudet and Messier 1998; Dong et al. 2015; Duchesneau et al. 2001; Schoonmaker et al. 2010). Shading affects not only individual tree growth, but also inter- and intra-specific competition in natural forests (Franklin 2002) and the efficiency of wood production in plantations (Albaugh et al. 2014).
In shaded conditions, trees decrease the apical growth and radial trunk growth depending on the relative light intensity (Denne 1974; Klinka et al. 1992; Shirley 1943; Yasuda et al. 2018). Under weak shading with a relative light intensity of 15–20% or more, such as that experienced by understory trees in a deciduous forest from 16 weeks to one growth season, apical and radial growth are suppressed (Denne 1974; Duchesneau et al. 2001; Klinka et al. 1992). When weak shading continues for several years under evergreen canopy trees, the apical and radial growth of shade-grown trees decreases greatly, but the individual trees are able to survive (Shirley 1943; Tucker et al. 1987; Zhao et al. 2015).
In contrast, strong shading with a relative light intensity of 5–15% for several years causes not only the suppression of apical and radial growth (Klinka et al. 1992; Parent et al. 2002), but also the death of individuals (Franklin et al. 1987; Kawanabe and Shidei 1968; Pedersen 1998; Peet and Christensen 1987; Shirley 1943; Wright et al. 2000). The mortality rate of shaded trees increases with the decline of light intensity (Canham et al. 1994; Kawanabe and Shidei 1968; Klinka et al. 1992; Shirley 1943; Wright et al. 2000). In extreme shading with a relative light intensity of 5% or less, the growth rates of trees further decrease and the shading leads to individual death in a shorter time (Canham et al. 1994; Shirley 1943; Wright et al. 2000).
As mentioned above, many studies have reported the suppression of apical and radial growth under shade at the individual tree level (Bormann 1965; Givnish 1988; Klinka et al. 1992; O’Connell and Kelty 1994; Parent et al. 2002). The suppression of apical and radial growth form a monolayer umbrella-shaped crown (Horn 1971; Kohyama 1980; O’Connell and Kelty 1994; Tucker et al. 1987), which enhances the light-supplementing efficiency under the shaded environment compared to a multilayer crown (Niinemets 2010) especially in late successional species such as the genera Abies and Picea (O’Connell and Kelty 1994).
In stem of Abies species, “terminal” represents the uppermost portion of the central, vertical axis of the tree, and is not merely strong terminal shoots of the lateral branch system (Parke 1959). The branches expand their own branch system mainly by the terminal type of branching as in the case of the trunk (Kidombo and Dean 2018; Kohyama 1980; Schulte 2012; Watanabe et al. 2015). The elongation pattern of the terminal leader (current-year trunk) derived from the terminal bud and the lateral branches derived from lateral buds including terminally lateral buds determines the tree shape in the long term. When the growth of the terminal leader is suppressed by shading, the growth of the lateral branches is known to be prioritized in many tree species including the genus Abies (Klinka et al. 1992; Kohyama 1980; Tucker et al. 1987). In Abies sachalinensis Mast., long-term suppression under canopy trees causes the cessation of apical growth of terminal leader for more than several years (Yasuda et al. 2018). These studies suggest the suppression of apical growth depending on the light environment determines the plasticity of the tree shape. However, to our knowledge, no studies of the terminal bud and terminally lateral bud of the terminal leader in a shaded environment have been made using anatomical and physiological approaches (Klinka et al. 1992). To fully understand the shoot development strategy under shade in tree individuals, a study of the physiological changes in the terminal bud and the terminally lateral bud of terminal leader would be required.
On the contrary, some anatomical and physiological changes in radial growth under shade have been studied. The tree ring width becomes narrower (Rathgeber et al. 2011; Wright et al. 2000), and the radial cell number of cambial zone in the trunk is reduced (Rathgeber et al. 2011) in shade-grown trees. Yasuda et al. (2018) suggested the dysfunction of cambium progressed gradually from the trunk base to the top before individual death of A. sachalinensis under long-term shading.
Apical and radial growth in trees are considered to occur synchronously (Bormann 1965; Kienholz 1934; Schulte 2012), but Yasuda et al. (2018) suggested that apical and radial growth were independently suppressed under shading. In this study, we aimed to clarify the histological changes in the terminal bud and the terminally lateral bud of terminal leader and cambial activity in the trunk base, and their correspondence in A. sachalinensis under shading. Planted seedlings were shaded artificially in the middle of the growth period, and the time-dependent changes in the terminal bud and the terminally lateral bud of terminal leader and cambial activity in the trunk base were analyzed histologically.
Materials and methods
Study site and sample trees
Forty-two Abies sachalinensis Mast. seedlings of 5-years-old were planted on May 21, 2015 at the experimental nursery of Ashoro Research Forest, Kyushu University, Japan [43°17′N, 143°34′E, 120 m above sea level (a. s. l.)]. The average temperature, average solar radiation, total solar radiation, average rainfall, and total rainfall from May 21 to October 10, 2015 were 14.1 °C, 0.18 kW m−2, 4.8 MW m−2, 0.02 mm and 449.5 mm, respectively, at the Ashoro Research Forest weather station (369 m a. s. l.), which was 5.0 km from the experimental nursery.
Shading treatment
Twenty-one seedlings were shaded under a 5% relative light intensity, which caused wilting of the coniferous tree individuals (Canham 1988; Shirley 1943; Wright et al. 2000), for 70 days from August 1, 2015 using a black polyvinyl acetate shading sheet (Kuremona #600, Teijin, Japan) to elucidate the effect of shading (Groninger et al. 1996; Walker and Vitousek 2011) from the middle of the growth period. The other 21 seedlings were grown under open conditions with a 100% relative light intensity until the end of the experiment. The amount of photosynthetically active radiation (PAR) of 400–700 nm wavelength on the apical shoots of the shade-grown and open-grown trees was measured with a PAR sensor (S-LIA-M003, Onset Computer Corporation, EU) to control the 5% relative light intensity on the shade-grown samples.
Apical growth and radial growth measurement
On July 31, the height and trunk base diameter at 1 cm above ground level of all sampled trees were measured before the onset of shading on August 1. The mean height at the onset of shading was 325 mm ± 8 mm SE in the open-grown trees and 337 mm ± 8 mm SE in the shade-grown trees. The mean basal diameter was 8.9 ± 0.3 mm SE in the open-grown trees and 9.6 ± 0.4 mm SE in the shade-grown trees. After that, tree height and trunk base diameter were measured every 2 weeks until October 10. The number of measured open-grown and shade-grown trees decreased from 21 to 18, 15, 12, and 9 with the progression of experiment, because three individuals were sampled from both open-grown and shade-grown conditions for histological analysis of the trunk at each measurement time. In this study, the difference between the tree height at each sampling day and the height at the onset of shading was defined as apical growth, and the difference between the trunk basal diameter at each sampling day and the diameter at the onset of shading was defined as radial growth, respectively.
Anatomical analysis of apical growth and radial growth
Three seedlings each of the open-grown and shade-grown trees were sampled at 14-day intervals as mentioned in the previous section. The terminal leader and basal disks of the trunk with a 5-cm length were obtained from each individual and immediately fixed with Karnovsky fixative, which was 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer adjusted to pH 7.0 (Kuo 2014). In total, the anatomical analysis was performed on 30 terminal leaders and 30 trunk basal disks.
The terminal bud and the terminally lateral bud of terminal leader, and the trunk basal disks with the cambium were excised from the fixed samples with a razor blade and embedded in PEG 1500 (Barbosa et al. 2010). Sample sections were prepared with a sliding microtome with 10 µm thickness and stained with 1% acetic acid carmine solution to visualize the cell nuclei (Nakaba et al. 2013, 2015). In addition, trunk base sections were stained with 0.5% toluidine blue solution (Abdul Khalil et al. 2010; Junghans et al. 2004) for cell structure and cytoplasm analysis. All sections were dehydrated with ethanol series from 10 to 100% aqueous solution (Barbosa et al. 2010) and embedded by Biolite (Yoshida et al. 1983).
The trunk base sections were observed using a polarized light microscope, and the radial width of cambial zone including expanding xylem and phloem cells with less birefringent cell walls (Fig. 1b, double arrowhead) was measured for quantitative evaluation of cell divisional activity (Antonova and Stasova 1993; Schweingruber 2007; Thibeault-Martel et al. 2008). Two sections were obtained from each sample and three radial rows were measured in each section to determine the radial width in each sample (Rossi et al. 2006).
Image of radial width of cambial zone including expanding xylem and phloem of Abies sachalinensis. a Light microscope image; Xy: secondary xylem, Ph: secondary phloem, C: cambial zone. b Polarized light microscope image. Double-headed arrow indicates the measurement range of the radial width of cambial zone including expanding xylem and phloem. Bar 30 µm
Statistical analysis
Comparison of the apical growth, radial growth and the width of the cambial zone including expanding xylem and phloem cells in open-grown and shade-grown trees after the onset of shading was performed using a Mann–Whitney U test.
Results
Apical growth and radial growth
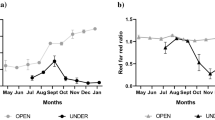
At each sampling time from 14 days after the start of shading to the end of the experiment, the apical growth (Fig. 2a) and radial growth (Fig. 2b) of the shade-grown trees were lower than those of the open-grown trees, though the difference is not significant in apical growth statistically (Mann–Whitney U test, P = 0.05). The radial growth was significantly different between shade-grown and open-grown trees after 28 days (n = 36) and 42 days (n = 30) of shading (Fig. 2b, Mann–Whitney U test, P < 0.05).
Apical growth and radial growth in the open-grown and the shade-grown trees of Abies sachalinensis after shading. a Apical growth. Y-axis represents the difference between the tree height measured on each sampling day and the tree height at the start of the shading; b Radial growth. Y-axis represents the difference between the trunk basal diameter measured on each sampling day and the trunk basal diameter at the start of the shading treatment. Values are mean ± SE. The asterisk indicates a significant difference in 95% confidence interval by Mann–Whitney U test. White circles represent the open-grown trees and black circles represent the shade-grown trees
Changes in the tissue structure of the terminal bud and terminally lateral bud of terminal leader under shading
After staining with acetic acid carmine solution, red-stained cell nuclei in the leaf primordia of the terminal bud were observed in the open-grown and shade-grown trees at 14, 28 and 42 days after the start of shading (Fig. 3a–f). Red staining of cell nuclei in the leaf primordia of the terminal bud was also observed in the open-grown trees until the end of experiment (Fig. 3g, i). However, the nuclei in the leaf primordia of the terminal bud were not observed in one sample of the shade-grown trees both at 56 days and at 70 days of shading (Fig. 3h, j). The other two samples of the shade-grown trees at 56 and 70 days of shading had cell nuclei in the leaf primordia of the terminal bud. In the leaf primordia of the terminally lateral buds, red-stained cell nuclei were observed in both the open-grown and shade-grown trees throughout the experiment (see Fig. 4a–d).
Terminal bud of terminal leader in Abies sachalinensis at (a, b) 14 days, (c, d) 28 days, (e, f) 42 days, (g, h) 56 days and (i, j) 70 days after shading. (a, c, e, g, i) Open-grown trees. (b, d, f, h, j) Shade-grown trees. The sections were stained with acetic acid carmine solution. Bar 100 µm. SAM shoot apical meristem, LP leaf primordium, L leaf, WL wilted leaf
Changes in the tissue structure of the cambial zone and the neighboring secondary xylem and secondary phloem under shading
The cells in the cambial zone and adjacent secondary xylem and phloem at the trunk base of the open-grown and shade-grown trees were alive (Fig. 5a–j), and red-stained nuclei were observed in the xylem and phloem ray parenchyma adjacent to the cambial zone in the open-grown and shade-grown trees throughout the experiment (after 14–70 days, Fig. 5k–t). The radial width of cambial zone including expanding xylem and phloem cells were smaller in the shade-grown trees than in the open-grown trees on each sampling day after shading (Fig. 6), and the width was significantly different between the open-grown trees and the shade-grown trees throughout the experiment (Mann–Whitney U-test, P < 0.05).
Cambial zone including adjacent xylem and phloem of Abies sachalinensis at a, b 14 days, c, d 28 days, e, f 42 days, g, h 56 days and i, j 70 days after shading. a, c, e, g, i Open-grown trees. b, d, f, h, j Shade-grown trees. The sections were stained with (a–j) toluidine blue solution and (k–t) acetic acid carmine solution. Bar 50 µm. The white arrowhead indicates a cell nucleus in a ray parenchyma cell. Xy secondary xylem, Ph secondary phloem, C cambial zone
Radial width of cambial zone in the open-grown and the shade-grown trees of Abies sachalinensis. Y-axis represents the radial width of cambial zone including expanding xylem and phloem cells measured on each sampling day. Values are mean ± SE. The asterisk indicates a significant difference in 95% confidence interval by Mann–Whitney U test. White circles represent the open-grown trees and black circles represent the shade-grown trees
Discussion
Shading suppresses radial growth
The current-year radial growth of the shade-grown trees were lower than those of the open-grown trees after the start of shading (Fig. 2b). Previous studies reported the quick suppression of apical and radial growth after shading (Dong et al. 2015; Kawanabe and Shidei 1968). Shade-grown trees have a decreased photosynthetic rate in their crown leaves (Dong et al. 2015) and a decreased amount of newly primary and secondary xylem cells from the shoot apical meristem and cambium (Denne 1974). The reduction in cell division activity in cambial cells resulting from the limiting of the photosynthate supply would cause the suppression of radial growth during the growth season after shading.
Shading causes the death and dormancy of the terminal bud of terminal leader
At 56 and 70 days after the start of shading, the cell nuclei of the leaf primordia of the terminal bud disappeared in the one of three shade-grown trees (Fig. 3h, j). By contrast, all open-grown trees had living leaf primordia in their terminal bud through the experiment (Fig. 3a, c, e, g, i). Histological changes in the terminal bud and the terminally lateral bud of terminal leader due to shading have not been investigated thoroughly in previous studies (Duchesneau et al. 2001; Klinka et al. 1992; Wright et al. 2000). This study revealed that the terminal bud of terminal leader possibly dies when the apical growth is suppressed under shade. Insufficient light conditions reduce the supply of photosynthetic products from the leaves of the terminal bud and from the leaves in other parts of the crown. When the total amount of these photosynthetic products is below the threshold for cell maintenance in the terminal bud of terminal leader, the cells in the terminal bud would die. On the other hand, two of three shade-grown trees at 56 and 70 days after the start of shading maintained nuclei in the leaf primordia of the terminal bud. Yasuda et al. (2018) showed the heterogeneity of apical growth suppression over several years in shaded A. sachalinensis individuals with same age. In addition, Gray and Spies (1996) suggested the individual difference of mortality in Abies amabilis seedlings growing under shade condition. Shortage of photosynthetic products would cause dormancy of the bud instead of cell death in A. sachalinensis when the storage of photosynthetic products was not depleted.
Shading accelerates dormancy of cell division in cambial zone
The shading treatment did not cause cell death in the cambial zone and the adjacent ray parenchyma cells until the end of the experiment (Fig. 5l, n, p, r, t). A. sachalinensis growing under long-term shading for decades showed partial death of cambial cells in the trunk (Yasuda et al. 2018). In contrast, the sample trees were shaded only in the later part of the growth season in this experiment, although the light intensity of 5% in our study was enough to cause wilting in previous studies (Canham 1988; Shirley 1943; Wright et al. 2000). The duration and timing of the shading might have been insufficient to cause cell death in the cambium.
The width of expanding cell region including phloem, cambial zone and xylem had smaller in the shade-grown trees than in the open-grown trees in all sampling time after shading (Fig. 6). The radial growth period is known to be shortened considerably in suppressed trees (Duchesneau et al. 2001; Kozlowski and Peterson 1962; Petritan et al. 2009). The shading in this experiment would suppress the cambial activity and accelerate early dormancy of cell division under the limited photosynthate supply from shaded crown leaves.
The terminally lateral buds can act as a backup for apical growth in shaded conditions
Shading led to the death of the terminal bud of terminal leader in two sample trees (Fig. 3). However, the cell nuclei of all terminally lateral bud in the terminal leader survived after the shading (Fig. 4b, d). Although the width of the cambial zone and the expanding xylem and phloem cells were smaller in the shade grown trees than in the open-grown trees (Fig. 6), the cells of ray parenchyma and cambial zone survived until the end of the shading in the shade-grown trees as in the open-grown trees (Fig. 5k–t). Coniferous trees such as fir, spruce, and pine form an umbrella-like crown in shaded environments (Duchesneau et al. 2001; Greis and Kellomaki 1981; King 1997; Kohyama 1980; O’Connell and Kelty 1994; Tucker et al. 1987). The results of this study explain the process of umbrella-like crown formation histologically. When the apical growth of the terminal leader stops for several years because of the death or the dormancy of the terminal bud over 1 year (Yasuda et al. 2018), the lateral buds including terminally lateral buds keep their activities and elongate lateral shoots. Accordingly, the tree’s crown would change from a conical shape to an umbrella shape. In contrast, if light conditions improve after the death of the terminal bud of the terminal leader, terminally lateral buds adjacent to the dead terminal bud would elongate vertically (Häsler et al. 2008) and form a conical-shaped crown again. The difference of shade tolerance between the meristems of terminal bud and terminally lateral bud would regulate the crown shape in the long-term. To elucidate these plasticity of tree form accurately, long-term detail analyses under shading would be needed in future.
References
Abdul Khalil HPS, Yusra AFI, Bhat AH, Jawaid M (2010) Cell wall ultrastructure, anatomy, lignin distribution, and chemical composition of Malaysian cultivated kenaf fiber. Ind Crops Prod 31:113–121. https://doi.org/10.1016/j.indcrop.2009.09.008
Albaugh JM, Albaugh TJ, Heiderman RR et al (2014) Evaluating changes in switchgrass physiology, biomass, and light-use efficiency under artificial shade to estimate yields if intercropped with Pinus taeda L. Agrofor Syst 88:489–503. https://doi.org/10.1007/s10457-014-9708-3
Antonova G, Stasova V (1993) Effects of environmental factors on wood formation in Scots pine stems. Trees 7:214–219. https://doi.org/10.1007/BF00202076
Barbosa ACF, Pace MR, Witovisk L, Angyalossy V (2010) A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA J 31:373–383
Beaudet M, Messier C (1998) Growth and morphological responses of yellow birch, sugar maple, and beech seedlings growing under a natural light gradient. Can J For Res 28:1007–1015. https://doi.org/10.1139/x98-077
Bormann FH (1965) Changes in the growth pattern of white pine trees undergoing suppression. Ecology 46:269–277
Canham CD (1988) Growth and canopy architecture of shade-tolerant trees: response to canopy gaps. Ecology 69:786–795. https://doi.org/10.2307/1941027
Canham CD, Finzi AC, Pacala SW, Burbank DH (1994) Causes and consequences of resource heterogeneity in forets: interspecific variation in light transmission by canopy trees. Can J For Res 24:337–349
Chazdon RL (1988) Sunflecks and their importance to forest understorey plants. Adv Ecol Res 18:1–63. https://doi.org/10.1016/S0065-2504(08)60179-8
Denne MP (1974) Effects of light intensity on tracheid dimensions in Picea sitchensis. Ann Bot 38:337–345
Dong T, Li J, Zhang Y et al (2015) Partial shading of lateral branches affects growth, and foliage nitrogen-and water-use efficiencies in the conifer Cunninghamia lanceolata growing in a warm monsoon climate. Tree Physiol 35:632–643. https://doi.org/10.1093/treephys/tpv036
Duchesneau R, Lesage I, Messier C, Morin H (2001) Effects of light and intraspecific competition on growth and crown morphology of two size classes of understory balsam fir saplings. For Ecol Manage 140:215–225. https://doi.org/10.1016/S0378-1127(00)00281-4
Franklin J (2002) Disturbances and structural development of natural forest ecosystems with silvicultural implications, using douglas-fir forests as an example. For Ecol Manage 155:399–423. https://doi.org/10.1016/S0378-1127(01)00575-8
Franklin JF, Shugart HH, Harmon ME (1987) Tree death as an ecological process. Bioscience 37:550–556. https://doi.org/10.2307/1310665
Givnish TJ (1988) Adaptation to sun and shade: a whole-plant perspective. Aust J Plant Physiol 15:63–92. https://doi.org/10.1071/PP9880063
Gray AN, Spies TA (1996) Gap size, within-gap position and canopy structure effects on conifer seedling establishment. J Ecol 84:635–645
Greis I, Kellomaki S (1981) Crown structure and stem growth of norway spruce undergrowth under varying shading. Silva Fenn 15:306–322
Groninger JW, Seiler JR, Peterson JA, Richard E (1996) Growth and photosynthetic responses of four Virginia Piedmont tree species to shade. Tree Physiol 16:773–778
Häsler H, Senn J, Edwards PJ (2008) Light-dependent growth responses of young Abies alba to simulated ungulate browsing. Funct Ecol 22:48–57. https://doi.org/10.1111/j.1365-2435.2007.0
Horn HS (1971) The adaptive geometry of trees. Princeton University Press, Princeton
Junghans U, Langenfeld-Heyser R, Polle A, Teichmann T (2004) Effect of auxin transport inhibitors and ethylene on the wood anatomy of poplar. Plant Biol 6:22–29. https://doi.org/10.1055/s-2003-44712
Kawanabe S, Shidei T (1968) Ecological studies on the influence of light intensity upon the growth and development of forest trees (III): Effects of shading on the growth of some coniferous seedlings. Bull Kyoto Univ For 40:111–121 (in Japanese)
Kidombo SD, Dean TJ (2018) Growth response of branches to variation in the intra- and interbranch supply of photosynthate. Trees (Online). https://springerlink.bibliotecabuap.elogim.com/article/https://doi.org/10.1007/s00468-018-1711-2. Accessed 5 June 2018
Kienholz R (1934) Leader, needle, cambial, and root growth of certain conifers and their interrelations. Bot Gaz 96:73–92
King DA (1997) Branch growth and biomass allocation in Abies amabilis saplings in contrasting light environments. Tree Physiol 17:251–258
Klinka K, Wang Q, Kayahara GJ et al (1992) Light-growth response relationships in pacific silver fir (Abies amabilis) and sub-alpine fir (Abies lasiocarpa). Can J Bot 70:1919–1930. https://doi.org/10.1139/b92-239
Kohyama T (1980) Growth pattern of Abies mariesii saplings under conditions of open-growth and suppression. Bot Mag Tokyo 93:13–24. https://doi.org/10.1007/BF02489483
Kozlowski TT, Peterson TA (1962) Seasonal growth of dominant, intermediate, and suppressed red pine trees. Bot Gaz 124:146–154
Kuo J (ed) (2014) Electron microscopy methods and protocols, 3rd edn. Humana, New York City
Nakaba S, Funada R, Sano Y (2013) Disappearance of microtubules, nuclei and starch during cell death of ray parenchyma in Abies sachalinensis. IAWA J 34:135–146. https://doi.org/10.1163/22941932-00000012
Nakaba S, Takata N, Yoshida M, Funada R (2015) Continuous expression of genes for xylem cysteine peptidases in long-lived ray parenchyma cells in Populus. Plant Biotechnol 32:21–29. https://doi.org/10.5511/plantbiotechnology.14.1208a
Niinemets Ü (2010) A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol Res 25:693–714. https://doi.org/10.1007/s11284-010-0712-4
O’Connell BM, Kelty MJ (1994) Crown architecture of understory and open-grown white pine (Pinus strobus L.) saplings. Tree Physiol 14:89–102. https://doi.org/10.1093/treephys/14.1.89
Parke RV (1959) Growth periodicity and the shoot tip of Abies concolor. Am J Bot 46(2):110–118
Parent S, Morin H, Messier C (2002) Missing growth rings at the trunk base in suppressed balsam fir saplings. Can J For Res 32:1776–1783. https://doi.org/10.1139/x02-102
Pedersen BS (1998) The role of stress in the mortality of midwestern oaks as indicated by growth prior to death. Ecology 79:79–93. 10.1890/0012-9658(1998)079[0079:TROSIT]2.0.CO;2
Peet RK, Christensen NL (1987) Competition and tree death. Bioscience 37:586–595
Petritan AM, von Lüpke B, Petritan IC (2009) Influence of light availability on growth, leaf morphology and plant architecture of beech (Fagus sylvatica L.), maple (Acer pseudoplatanus L.) and ash (Fraxinus excelsior L.) saplings. Eur J For Res 128:61–74. https://doi.org/10.1007/s10342-008-0239-1
Rathgeber CBK, Rossi S, Bontemps JD (2011) Cambial activity related to tree size in a mature silver-fir plantation. Ann Bot 108:429–438. https://doi.org/10.1093/aob/mcr168
Rossi S, Deslauriers A, Anfodillo T (2006) Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the alpine timberline. IAWA J 27:383–394. https://doi.org/10.1163/22941932-90000161
Schoonmaker AL, Hacke UG, LandhäUsser SM et al (2010) Hydraulic acclimation to shading in boreal conifers of varying shade tolerance. Plant Cell Environ 33:382–393. https://doi.org/10.1111/j.1365-3040.2009.02088.x
Schulte PJ (2012) Vertical and radial profiles in tracheid characteristics along the trunk of douglas-fir trees with implications for water transport. Trees 26:421–433. https://doi.org/10.1007/s00468-011-0603-5
Schweingruber FH (2007) Wood structure and environment. Springer, Berlin
Shirley HL (1943) Is torelance the capacity to endure shade? J For 41:339–345
Thibeault-Martel M, Krause C, Morin H, Rossi S (2008) Cambial activity and intra-annual xylem formation in roots and stems of Abies balsamea and Picea mariana. Ann Bot 102:667–674. https://doi.org/10.1093/aob/mcn146
Tucker GF, Hinckley TM, Leverenz J, Jiang S (1987) Adjustments of foliar morphology in the acclimation of understory pacific silver fir following clearcutting. For Ecol Manage 21:249–268. https://doi.org/10.1016/0378-1127(87)90047-8
Walker LR, Vitousek PM (2011) An invader alters germination and growth of native dominant tree in Hawai’i. Ecology 72:1449–1455
Watanabe Y, Ichikawa S, Kubota M et al (2015) Morphological defects in native Japanese fir trees around the Fukushima Daiichi Nuclear Power Plant. Sci Rep 5:1–7. https://doi.org/10.1038/srep13232
Wright EF, Canham CD, Coates KD (2000) Effects of suppression and release on sapling growth for 11 tree species of northern, interior British Columbia. Can J For Res 30:1571–1580. https://doi.org/10.1139/x00-089
Yasuda Y, Utsumi Y, Tashiro N, Koga S, Fukuda K (2018) Cessation of annual apical growth and partial death of cambium in stem of Abies sachalinensis under intensive shading. J Plant Res 131:261–269. https://doi.org/10.1007/s10265-017-0984-7
Yoshida Y, Nakamura K, Hiura A (1983) Contraction of chromosomes and depression RNA synthesis by Isopropyl N- (3-Chlorophenyl) Carbamate (CIPC) in Vicia faba root tip cells. Cytologia (Tokyo) 48:707–717
Zhao Q, Pang X, Bao W, He Q (2015) Effects of gap-model thinning intensity on the radial growth of gap-edge trees with distinct crown classes in a spruce plantation. Trees Struct Funct 29:1861–1870. https://doi.org/10.1007/s00468-015-1267-3
Acknowledgements
The authors thank Mr. Yan Xiang and the staff of Ashoro Research Forest, Kyushu University for supporting nursery experiment. The authors thank the members of the Laboratory of Forest Production Control, Kyushu University, for valuable comments on our manuscript. This study was supported by JSPS KAKENHI Grant numbers 26450233 and 15H0245.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yasuda, Y., Utsumi, Y., Tan, X. et al. Suppression of growth and death of meristematic tissues in Abies sachalinensis under strong shading: comparisons between the terminal bud, the terminally lateral bud and the stem cambium. J Plant Res 131, 817–825 (2018). https://doi.org/10.1007/s10265-018-1051-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-018-1051-8