Abstract
Gynodioecy is the coexistence of hermaphrodites and females in a population. It is supposed to be an intermediate stage in the evolutionary pathway from hermaphroditism to dioecy in angiosperm. Hermaphrodites gain fitness through both seed and pollen production whereas females gain fitness only through seed production. As females spread in a gynodioecious population, sexual selection prompts hermaphrodites to invest in male function and male-biased hermaphrodites prevail. In the gynodioecious shrub Daphne jezoensis (Thymelaeaceae), female frequency is stably around 50% in most populations, and fruit-set rate of hermaphrodites is commonly low. Therefore, D. jezoensis is likely at a later stage in the evolutionary pathway. Female function of hermaphrodites (fruit-set rate, selfing rate, seed size, and germination rate) was assessed in three populations under natural conditions. In order to evaluate the potential seed fertility and inbreeding depression by selfing in hermaphrodites, hand pollination treatments were also performed. Over a 2-year period under natural conditions, 18–29% of hermaphrodites and 69–81% of females set fruit. Across all three populations, the mean fruit-set rate ranged 9.5–49.2% in females and only 3.9–10.2% in hermaphrodites. Even with artificial outcross-pollination, 59–91% of hermaphrodites failed to set any fruit. When self-pollination was performed in hermaphrodites, both of fruit-set and germination rates were decreased, indicating early-acting inbreeding depression. In addition, more than half of the hermaphrodite seeds were produced by selfing under natural pollination, but pollinator service was still required. Totally, hermaphrodites performed poorly as seed producers because of the intrinsically-low fruiting ability and a combination of autogamous selfing and strong inbreeding depression, indicating the absence of reproductive assurance. These results indicate that the mating system of D. jezoensis is functionally close to dioecy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The separation of sexes in flowering plants (dioecy) may have evolved from hermaphroditic ancestors (Renner and Ricklefs 1995). One of the postulated evolutionary pathways is through gynodioecy, called as the gynodioecy–dioecy pathway (Charlesworth and Charlesworth 1978; Spigler and Ashman 2012). In gynodioecy, hermaphrodites and females coexist within a population. It occurs in approximately 2–7% of angiosperm species (Dufay et al. 2014; Richards 1997). Hermaphrodites gain fitness through both seed and pollen production whereas females gain it through seed production alone. At the first stage of the gynodioecy–dioecy pathway, female (male-sterile) plants invade a hermaphroditic population. There, they must gain seed fertility (quantitatively or qualitatively superior seed production) advantage over the hermaphrodites (Bailey et al. 2003; Charlesworth and Charlesworth 1978). As females spread within a population, selection pressure drives hermaphrodites to invest more in male function until male-biased hermaphrodites prevail (Charlesworth 1989; Lloyd 1976; McCauley and Brock 1998). Frequency-dependent selection may act on male function causing male (female-sterile) plants to appear and become part of a gynodioecious population (a stage of subdioecy in which hermaphrodites, females, and males coexist). The transition from subdioecy to dioecy is accomplished by replacing hermaphrodites with males. At a later stage of the gynodioecy–dioecy pathway, either males or male-biased hermaphrodites with low seed fertility are expected. There is much evidence to support the existence of the earlier stage of the gynodioecy–dioecy pathway (greater female than hermaphrodite seed fertility) (Shykoff et al. 2003). Nevertheless, only a few studies have addressed the evolutionary significance of the later stage (Delph and Wolf 2005; Ehlers and Bataillon 2007; Spigler and Ashman 2012). For a clarification of the gynodioecy–dioecy pathway, more studies on the mating system of male-biased hermaphrodites are needed in gynodioecious populations and species.
The quantity and quality of seeds produced by hermaphrodites relative to females indicate the functional significance of hermaphrodites in gynodioecious populations. Some ecological factors like pollen limitation and gender-specific herbivorous attack help maintain hermaphrodites even when frequency-dependent selection acts on male function (Ashman 2006; Spigler and Ashman 2012). The reproductive success of animal-pollinated plants depends on pollinator availability. Limited pollinator service can decrease pollination success under natural conditions (Knight et al. 2005; Larson and Barrett 2000), and result in lower than expected seed production in both females and hermaphrodites (Ramsey and Vaughton 2002; Wang et al. 2014). Unlike monosexual plants (females or males), hermaphrodites may retain seed production by autonomous self-pollination (reproductive assurance) (Busch and Delph 2012; Del Castillo and Argueta 2009). In this case, pollen limitation may help maintain hermaphrodites within a gynodioecious population because relative seed production by hermaphrodites increases with pollen limitation (Ehlers and Bataillon 2007; Wolfe and Shmida 1997). Nevertheless, selfed progeny often have lower fitness than outcrossed progeny due to inbreeding depression (Charlesworth and Willis 2009; Sakai et al. 1997). Fruit production generally requires more resources than flower production (Leigh et al. 2006; Vaughton and Ramsey 2011). Therefore, the cost of seed production by selfing may exceed the fitness gain from female function of hermaphrodites if inbreeding depression is very severe. To determine whether hermaphrodite seed production functions as reproductive assurance, it is necessary to evaluate autogamous selfing in hermaphrodites and inbreeding depression under natural conditions (Wolfe and Shmida 1997).
If reproductive assurance is absent in hermaphrodites and/or high inbreeding depression exists in selfed progeny, then the production of selfed seeds decreases hermaphrodite fitness. In many gynodioecious species, hermaphrodites often produce seeds that are quantitatively or qualitatively inferior to those from females (Dufay and Billard 2012; Shykoff et al. 2003; Wang et al. 2014; Wolfe and Shmida 1997). Sexual selection may reallocate reproductive function toward the male (more flower and pollen production and less fruit production) (Spigler and Ashman 2012). When male-biased allocation is accelerated, seed fertility of hermaphrodites may decrease or a female-sterile (functional male) phenotype may occur within a population (shift to subdioecy). These transitions are predicted for the later stage of the gynodioecy–dioecy pathway but supporting empirical data is limited (e.g., Spigler and Ashman 2012).
In this study, the objective was to assess the female function of hermaphrodites in the gynodioecious shrub Daphne jezoensis Maxim. (Thymelaeaceae). The sex ratio is close to 1:1 in many D. jezoensis populations. Under natural conditions, hermaphrodites consistently produce more flowers and less fruits than females (Shibata and Kudo 2017; Sinclair et al. 2016). Therefore, D. jezoensis may be at the later stage of the gynodioecy–dioecy pathway. If the hermaphrodite fitness gain from female function is negligible in natural populations, then phenotypic hermaphrodites should act as males and there may be functional dioecy in this species. To test this prediction, we evaluated the degree of female function of hermaphrodites based on seed fertility, selfing ability, and seed quality (an index of early-acting inbreeding depression) by hand pollination treatments in three populations. The effectiveness of hermaphrodite reproductive assurance was evaluated using variations in fruit-set and selfing rates correlated to pollen limitation under natural conditions. Specifically, we ask following questions: (1) How much do hermaphrodites gain fitness through seed production under natural conditions? (2) What potential fruiting and selfing abilities do hermaphrodites have? (3) Do hermaphrodites have reproductive assurance (stable seed production even under pollen limitation)?
Methods
Study species
Daphne jezoensis (Thymelaeaceae) is a short (<30 cm) summer-deciduous shrub inhabiting the understory of cool-temperate forests in northern Japan (Fig. 1a). Flowering occurs in early spring, soon after snowmelt. Flowers persist until mid- to late May. The fruit contain a single seed and mature in July. The tubular flowers produce small amounts of nectar and are infrequently visited by moths, skippers, and bumble bees. Small beetles and thysanopterans are also observed on the flowers for pollen foraging (personal observation). Hermaphroditic flowers have developed anthers with bright yellow color (Fig. 1b), while anthers of female flowers are highly reduced and contain no pollen (Fig. 1c). Morphological character of ovary is similar between hermaphroditic and female flowers, and stigma locates at the lower position of floral tube. There is a low proportion of sexually labile plants (annual swings between hermaphrodite and female) (Sinclair et al. 2016), but these were excluded from the analyses because of low frequencies across populations in this study. The creeping stems and frequent branching of this species sometimes make visual identification of individual plants difficult. In this study, the separate stems at ground level are referred to as ramets, and a whole plant composed of genetically identical ramets is called a genet. Hermaphroditic genets are identified simply as hermaphrodites and female genets as females. In our previous studies, there was no sexual difference in ramet size (Shibata and Kudo 2017; Sinclair et al. 2016).
Study sites
The study was conducted on three populations in Hokkaido, northern Japan, during 2015–2016. The populations were located at Chitose (42°73′N, 141°73′E), Nopporo (43°03′N, 141°53′E), Tomakomai (42°69′N, 141°58′E), hereafter referred to as Chi, Nop, and Tom, respectively. Annual mean temperature is 7.1–7.2 °C, ranging from −6.3 °C (January) to 20.8 °C (August), and mean annual precipitation is 930–990 mm (Japan Meteorological Agency, http://www.jma.go.jp/jma/index.html). Snow usually covers the ground from mid-December to late March. Permanent plots (50 m × 50 m) at Nop and Chi were established in 2009 and 2013, respectively (Sinclair et al. 2016). A permanent 50 m × 50 m plot was set up at Tom in April 2015. All ramets with floral buds were tagged, mapped, and genotyped with genetic markers to determine genet individuality (see below). Female genet frequency ranged 42–52% in these populations during 2015–2016 (Table 1). The number of flowering ramets per genet was 1.2 ± 0.03 SE (ranging 1–9) in females and 1.4 ± 0.05 SE (ranging 1–13) in hermaphrodites throughout the populations.
Female function under natural conditions
The flower and fruit numbers of every tagged ramet in these plots were recorded in 2015 and 2016. Flowers were counted during the peak flowering period (late April to early May) and developing fruits were counted in mid-June before dispersal. The fruit-set rate (identical to the seed-set rate) was calculated as the percentage of flowers that set fruit.
Up to six mature fruits from individual ramets on every plot were harvested in mid-July 2015 and 2016. Fruit pulp was removed soon after harvest. Exposed seeds were stored at room temperature (20–25 °C) until the next procedure (i.e., size measurement, germination test, or DNA extraction). First, the dry weights of the seeds from female (F) and hermaphroditic (H) genets were measured and compared using the 2015 and 2016 seed samples. Up to four seeds per genet were oven-dried at 60 °C for at ≥3 days and individual seeds were weighed using a digital scale with an accuracy of 0.1 mg. A total of 124 seeds (F = 108, H = 16) from Chi, 75 seeds (F = 56, H = 19) from Nop, and 78 seeds (F = 65, H = 13) from Tom were weighed.
Germination rates were compared between seeds from female and hermaphroditic genets. Up to three seeds were sown per small decomposable pot (5 cm × 5 cm × 5 cm) filled with culture soil. The pots were then placed in larger planters (60 cm × 18 cm × 18 cm) and set under the deciduous forest in the private experimental garden at Higashikawa (130 km northeast of Sapporo) in August 2015 and 2016. A total of 242 seeds (F = 228, H = 14) from Chi, 254 seeds (F = 206, H = 48) from Nop, and 116 seeds (F = 98, H = 18) from Tom were used. The sample size for the germination test was strongly biased towards female genet seeds because the seed production from hermaphroditic genets was very low (see data). Germination rates were recorded in April 2016 and 2017, soon after snowmelt.
DNA extraction, genotyping, and genetic analyses
One leaf sample was collected per flowering ramet per plot over the 2013–2016 flowering seasons to genotype the mother plants. One seed per ramet per plot was selected from the seed samples harvested in 2015 and 2016. One leaf sample was collected from each seedling in the germination test of April 2016. Leaf samples were stored in a desiccator at room temperature (20–25 °C). Seed samples were stored at −60 °C until DNA extraction, which was performed using the cetyltrimethylammonium bromide (CTAB) protocol (Stewart and Via 1993). The DNA was amplified with a TaKaRa PCR Thermal Cycler Dice Gradient (Takara Bio Inc., Otsu, Shiga, Japan) using eight microsatellite markers. Three primer pairs, Dp238, Dp258, and Dp506 were adopted from Kameyama and Hirao (2014), and five primer pairs, Dp186, Dp419, Dp430, Dp499, and Dp739 were developed using genome information obtained from DNA Data Bank of Japan Sequence Read Archive: DRA001272 (Table S1). PCR was performed in a final volume of 6 μl containing 1 μl extracted DNA, 3 μl 2 × Type-it Multiplex PCR Master Mix (QIAGEN, Hilden, Germany) and 2 μl 3 × primer mix (1.26 μM for each locus) with 5 min at 95 °C, 35 cycles of 30 s at 95 °C, 90 s at 60 °C, and 30 s at 72 °C, followed by 60 °C for 30 min. The DNA fragments were analyzed using an Applied Biosystems 3730 Genetic Analyzer with GeneScan 500 LIZ Size Standard (Thermo Fisher Scientific Inc., Waltham, MA, USA) and scored genotypes were analyzed using GeneMapper ver. 4.0 (Thermo Fisher Scientific Inc.). The loci Dp186, Dp258, and Dp499 were excluded from the genetic analyses (see below) due to their high null allele frequencies.
Multilocus outcrossing rates (t m) were estimated and selfing rates (s = 1 − t m) were calculated for females and hermaphrodites in each population and year using MLTR ver. 3.2 (Ritland 2002). In MLTR, the maternal sex morphs were set as different groups under the same outcross pollen pool. The standard error of t m was calculated based on 1000 bootstraps. The inbreeding coefficients of all flowering adults (F is ) for each population and their significant difference from zero were estimated from five loci using GENEPOP ver. 4.2 (Rousset 2008). The actual selfing rate in each population was estimated as the proportion of the selfed seed number of the total (sum of outcrossed and selfed) seed number.
Hand pollination treatment
To evaluate seed fertility (potential fruiting ability), selfing ability, and pollen limitation under natural conditions, hand pollination was performed on all populations in April 2016. Of all plants producing floral buds, 62 genets (F = 23, H = 39) in Chi, 48 genets (F = 15, H = 33) in Nop, and 68 genets (F = 22, H = 46) in Tom were randomly selected. One inflorescence per genet at Tom and two inflorescences per genet at Chi and Nop were covered with fine-meshed bags to prevent natural pollination. Outcross- and self-pollination treatments were performed on each inflorescence per hermaphroditic genet at Chi and Nop. Either outcross- or self-pollination was performed on one inflorescence per hermaphroditic genet at Tom (outcross = 22 ramets; self = 24 ramets). For the outcross-pollination treatment, pollen was gathered from 5 to 10 hermaphroditic donors at least 10 m from the recipient genets and artificially deposited on the stigmata of bagged female and hermaphroditic inflorescences soon after opening and before hermaphrodite pollen dispersal. For the self-pollination treatment, hermaphroditic flowers received the pollen produced by their own stigmata. After pollination, the inflorescences were covered with fine-meshed bags to prevent uncontrolled pollination and herbivore damage. When the fruits matured in mid-July, they were counted and the fruit-set rate was calculated.
Mature fruits of the hand-pollinated hermaphroditic inflorescences were harvested in every plot in mid-July 2016. After removal of fruit pulp, exposed seeds were stored at room temperature (20–25 °C). In August 2016, individual seeds were sown in each of small decomposable pots, these pots were then placed in larger planters and set in the experimental garden as mentioned before. A total of 29 seeds (outcrossed 18, selfed 11), 5 seeds (outcrossed 3, selfed 2), and 25 seeds (outcrossed 19, selfed 6) were used from the harvests at Chi, Nop, and Tom, respectively. Because of small sample size, all seeds were pooled for a comparison of germination rates between the pollination treatments. Germination rates were recorded in April 2017, soon after snowmelt.
Statistical analyses
Sex differences in flower and fruit number pooled across 2 years were assessed using generalized linear mixed models (GLMM). Using the glmmADMB package, a negative binomial error distribution was postulated for flower number and a zero-inflated negative binomial error distribution was postulated for fruit number with a log-link function to reduce overdispersion (O’Hara and Kotze 2010). In the GLMM, sex morph (female and hermaphrodite) and population (Chi, Nop, and Tom) were explanatory variables and genet ID was a random factor. To evaluate the sex difference in fruit-set rate under natural conditions over the 2 years, GLMM was used postulating a zero-inflated negative binomial error distribution. In the GLMM, fruit number was a response variable, flower number was set as an offset term, sex morph, year (2015 and 2016), and population were explanatory variables, genet ID was a random factor, and the interaction effect between sex morph and year was considered. In the GLMM for fruit number and fruit-set rate, all female and fruited hermaphroditic genets setting at least one fruit in the 2 years were used. A best-fit model was selected based on Akaike’s Information Criterion (AIC).
The relationship between pollination service and hermaphrodite seed production was assessed using Pearson’s correlation coefficients between female and hermaphrodite fruit-set rates and between female fruit-set and hermaphrodite selfing rates across the populations in 2015–2016. Female fruit-set rates under natural conditions were used as an index of pollination service (or pollen limitation) in each population and year because female fruit-set success depends entirely on pollinator visits. If hermaphroditic flowers have reproductive assurance, then their fruit-set rates are expected to be highly independent of pollination service. Their selfing rates, however, may vary depending on pollination service.
To compare seed size between sex morphs, GLMM was performed using lme4 package postulating a gamma error distribution with log-link function. Seed weight was a response variable, sex morph and population were explanatory variables, and genet ID was a random factor. GLM postulating a binomial error distribution was run to compare germination rates of naturally pollinated seeds between maternal sex morphs. In the GLM, germination rate was a response variable, and sex morph and population were explanatory variables.
For the hand pollination experiment, a series of GLMM were run using the glmmADMB package postulating a negative binomial or zero-inflated negative binomial error distribution (when data included many zero values) with log-link function. Genet ID and inflorescence ID were set as random factors. First, the aim was to use GLMM to test sex differences in potential fruiting ability. Fruit-set rate by outcross-pollination was a response variable, i.e., fruit number was a response variable and flower number was set as an offset term, and sex morph and population were explanatory variables. A second objective was to use GLMM to compare hermaphrodite fruiting ability among populations. Outcrossed hermaphrodite fruit-set rate was a response variable and population was an explanatory variable. The third purpose for the GLMM was to evaluate hermaphrodite selfing ability. Hermaphrodite fruit-set rate was a response variable and pollination treatment (outcross- and self-pollination) and population were explanatory variables. The final aim of using GLMM was to evaluate pollen limitation under natural conditions. Female fruit-set rate was a response variable and pollination treatment (natural- and outcross-pollination) and population were explanatory variables. Fisher’s test was run to compare germination rates between outcrossed and selfed seeds.
Results
Fruit production and seed viability under natural conditions
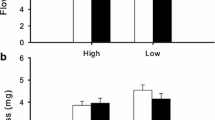
Hermaphrodites produced 1.6–2.3 times more flowers than females over 2 years under natural conditions (P < 0.001; Fig. 2a; Table S2). Only 18–29% of the hermaphroditic genets set at least one fruit (fertile hermaphrodites) during the 2 years whereas 69–81% of the female genets set fruit. Females had 2.3–4.2 times higher fruit-set rates and produced 1.5–2.7 times more fruits than fertile hermaphrodites (P < 0.001; Fig. 2b; Table 2). Although flower and fruit production varied significantly between populations, the trends in sex differences were consistent across populations. Fruit-set rate in fertile hermaphrodites (3.9–10.2%) was significantly lower than that in females (9.5–49.2%, P < 0.001), and varied between populations (P < 0.001; Table 3). A positive correlation was detected between female and fertile hermaphrodite fruit-set rates (r = 0.90, P < 0.05; Fig. 3a). Therefore, hermaphrodite fruit set also depends on pollination service.
Dry weight of naturally produced seeds varied between populations (P < 0.01), but it was similar between sex morphs (48–55 mg, P > 0.1; Tables 2, S2). Germination rates of female seeds (64.9–85.9%) were significantly higher than that of hermaphrodite seeds (35.7–88.9%, P < 0.001), and germination rates varied between populations (P < 0.001).
Selfing rate
The estimated hermaphrodite selfing rates at the seed stage ranged from 55 to 72% whereas those of female seeds were <6% across populations and years (Table 4). Therefore, more than half of the hermaphrodite seeds were produced by selfing under natural pollination. No significant correlation was found between the female fruit set and the hermaphrodite selfing rates (r = −0.42, P = 0.48; Fig. 3b). Thus, the hermaphrodite selfing rate is independent of pollination service.
The estimated selfing rates at population level were low (5–12%) because the number of hermaphrodite selfed seeds was much smaller than that of the female outcrossed seeds (dilution effect). The inbreeding coefficients of all flowering individuals (F is ) were small (0.029 for Chi, 0.068 for Nop, and 0.041 for Tom) but significantly different from zero (P < 0.05 for Chi, and P < 0.001 for Nop and Tom, respectively).
Fruit production and seed viability under hand pollination
In the outcross-pollination treatment, hermaphrodites set fewer fruits than females; mean values of fruit-set rates ranged 1.7–21.1% in hermaphrodites and 40.7–60.4% in females across populations (P < 0.001; Fig. 4; Table S3a). Fertility of hermaphrodites (potential fruiting ability) varied significantly between populations (P < 0.01 between Chi and Nop; Table S3b). Outcrossed hermaphroditic flowers set more fruits than did selfed flowers (0.7–7.1%, P < 0.05; Table S3c). Across populations, 59–91% of hermaphrodites failed to set fruit by the outcrossing treatment, indicating potentially low fruiting ability. Outcrossed female flowers had higher fruit-set rate than naturally pollinated flowers (P < 0.001; Table S3d). Therefore, pollen limitation was common under natural conditions.
Germination rate of outcrossed seeds (72.5%) produced in hermaphrodites was significantly higher than that of selfed seeds (5.3%, P < 0.001). Inbreeding depression at the germination stage was calculated as 0.93 (=1 − 5.3/72.5).
Discussion
The hermaphrodite fruiting ability in D. jezoensis was potentially much smaller than that of the females across all populations. This finding aligns with those of previous reports (Shibata and Kudo 2017; Sinclair et al. 2016). The predominance of non-fruiting hermaphrodites (71–82% under natural pollination and 59–91% under outcross-pollination) accounts for the low overall seed productivity and fruiting ability of fruited hermaphrodites (<50% of that of females). Overall, hermaphrodites produced only 6–17% of the total seed production across populations. Because hermaphrodite seeds have low germination rates, the contribution of hermaphrodites as seed producers is negligible in natural populations.
Hermaphrodite selfing ability is an important trait affecting their sexual function (Del Castillo and Argueta 2009; Wolfe and Shmida 1997). A previous study reported that D. jezoensis might be self-incompatible, and stigma clogged with self-pollen might restrict hermaphrodite outcrossing fruit-set success (Kikuzawa 1989). Nevertheless, the pollination experiment in this study demonstrated that at least a certain proportion of hermaphrodites are physiologically self-compatible and not a few hermaphrodite seeds were produced by selfing under natural conditions. On the other hand, some of self-fertilized embryos in hermaphrodites might be aborted because selfed flowers set fewer fruits than outcrossed flowers. Therefore, autogamous self-pollination may cause negative effects not only by stigma clogging but also by abortion of selfed seeds.
Lower germination rates indicate lower viability of hermaphrodite seeds relative to female seeds. Lower germination rates of hermaphrodite seeds have been reported also for other gynodioecious species (Dalton et al. 2013; Wang et al. 2014; Wolfe and Shmida 1997). In the present study, germination rates of selfed seeds were much lower than that of outcrossed seeds, indicating a strong inbreeding depression on seed viability. On the other hand, estimating the population-level inbreeding depression using reproductive plant inbreeding coefficients and selfing rates at seed stage (Ritland 1990) was not suitable for this species because the selfing rate was too low (4–12%). Under low selfing rates, inbreeding depression estimates often become negligible (data not shown). Low population-level selfing rates may be explained by the dilution of selfed seeds by female outcrossed seeds.
Smaller flower and larger fruit production in females may indicate a strategy to allocate resources to females to maximize seed production (Delph 1990). In contrast, D. jezoensis hermaphrodites invest many resources in flower production (larger number of flowers per plant, larger corollas, more developed anthers, and greater pollen production) (Shibata and Kudo 2017; Sinclair et al. 2016). The presentation of attractive flowers to pollinators often improves success as pollen donors (Bell 1985; Eckhart 1999; Van Etten and Chang 2014). The large investment in hermaphrodite flower production may be accomplished in exchange for low fruit yield because the energetic cost of fruit production is usually higher than that of flower production (Delph 1990; Kohn 1989; Vaughton and Ramsey 2011). A trade-off between current reproductive investment (sum of flower and fruit production) and future reproductive effort (flower production) was observed for this species (Shibata and Kudo 2017). Therefore, the potentially low hermaphrodite fruiting ability may result from sexual selection to improve flower production that enhances male fitness as a pollen donor.
Both hermaphrodite and female fruit-set success depended on pollination service (Fig. 3a). Therefore, both sex morphs need pollinators to set fruits. More than half of the hermaphrodite seeds were produced by selfing, and the rates were relatively stable under natural conditions regardless of pollen limitation severity (Fig. 3b). The tubular shape of the upward facing flowers in this species forces pollinators to touch the anthers arranged at upper part of the floral tubes in the attempt to reach the nectaries (Fig. 1b). Therefore, hermaphrodites cannot avoid autogamous self-pollination during insect visits. These results strongly suggest that hermaphrodites do not have a reproductive assurance function. Most hermaphrodites (59–91%) did not set any fruit even after artificial outcross-pollination, so functional males (female-sterile plants) prevail in a population and the mating system of D. jezoensis is subdioecious (population composed of male, female, and hermaphroditic plants).
Low seed fertility, high selfing rate, early-acting inbreeding depression, and lack of reproductive assurance function were apparent in hermaphrodites of D. jezoensis. Thus, hermaphrodites may gain little fitness through seed production. Then, why are seed-fertile hermaphrodites maintained in the subdioecious populations? If hermaphrodites gain negligibly small fitness through seed production, selective force should accelerate the shift from seed-fertile phenotype (hermaphrodite) to sterile phenotype (male). However, potentially low seed fertility and strong pollinator limitation under natural conditions may minimize the cost of fruit production for hermaphrodites. Actually, negative effects of previous fruit production on current flower production were not detected under natural conditions in our previous study (Sinclair et al. 2016). As a result, selective force acting on seed-fertile hermaphrodites may be mitigated, resulting in remaining some low-fruiting individuals within a population.
Conclusion
The present study demonstrates that the hermaphrodite fitness through female function was minimal in this species because of the intrinsically-low fruiting ability and a combination of autogamous selfing and strong inbreeding depression, i.e., absence of reproductive assurance. These results suggest that mating system in D. jezoensis functionally resembles dioecy and locates at the later stage of the gynodioecy–dioecy pathway (Wang et al. 2014). Nevertheless, the fitness of phenotypic hermaphrodites was assessed only through female function in this study. To continue evaluating the evolutionary status of this species, it is necessary to compare the frequencies of functional hermaphrodites and males among populations and the relationships between reproductive performance (flower production) and male function (success as a pollen donor).
References
Ashman T-L (2006) The evolution of separate sexes : a focus on the ecological context. In: Harder L, Barrett SCH (eds) The ecology and evolution of flowers. Oxford University Press, New York, pp 204–222
Bailey MF, Delph LF, Lively CM (2003) Modeling gynodioecy: novel scenarios for maintaining polymorphism. Am Nat 161:762–776
Bell G (1985) On the function of flowers. Proc R Soc B Biol Sci 224:223–265
Busch JW, Delph LF (2012) The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Ann Bot 109:553–562
Charlesworth D (1989) Allocation to male and female function in hermaphrodites, in sexually polymorphic populations. J Theor Biol 139:327–342
Charlesworth B, Charlesworth D (1978) A model for evolution of dioecy and gynodioecy. Am Nat 112:975–997
Charlesworth D, Willis JH (2009) The genetics of inbreeding depression. Nat Rev Genet 10:783–796
Dalton RM, Koski MH, Ashman T-L (2013) Maternal sex effects and inbreeding depression under varied environmental conditions in gynodioecious Fragaria vesca subsp. bracteata. Ann Bot 112:613–621
Del Castillo RF, Argueta ST (2009) Reproductive implications of combined and separate sexes in a trioecious population of Opuntia robusta (Cactaceae). Am J Bot 96:1148–1158
Delph LF (1990) Sex-differential resource allocation patterns in the subdioecious shrub Hebe Subalpina. Ecol Soc Am 71:1342–1351
Delph LF, Wolf DE (2005) Evolutionary consequences of gender plasticity in genetically dimorphic breeding systems. New Phytol 166:119–128
Dufay M, Billard E (2012) How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Ann Bot 109:505–519
Dufay M, Champelovier P, Käfer J, Henry JP, Mousset S, Marais GAB (2014) An angiosperm-wide analysis of the gynodioecy–dioecy pathway. Ann Bot 114:539–548
Eckhart VM (1999) Sexual dimorphism in flowers and inflorescences. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 123–148
Ehlers BK, Bataillon T (2007) “Inconstant males” and the maintenance of labile sex expression in subdioecious plants. New Phytol 174:194–211
Kameyama Y, Hirao AS (2014) Development and evaluation of microsatellite markers for the gynodioecious shrub Daphne jezoensis (Thymelaeaceae). Appl Plant Sci 2:3–5
Kikuzawa K (1989) Floral biology and evolution of gynodioecism in Daphne kamtchatica var. jezoensis. Oikos 56:196–202
Knight T, Steets J, Vamosi J, Mazer S, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman T-L (2005) Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst 36:467–497
Kohn JR (1989) Sex ratio, seed production, biomass allocation, and the cost of male function in Cucurbita foetidissima HBK (Cucurbitaceae). Evolution 43:1424–1434
Larson BM, Barrett SC (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc 69:503–520
Leigh A, Cosgrove MJ, Nicotra AB (2006) Reproductive allocation in a gender dimorphic shrub: anomalous female investment in Gynatrix pulchella? J Ecol 94:1261–1271
Lloyd DG (1976) The transmission of genes via pollen and ovules in gynodioecious angiosperms. Theor Popul Biol 9:299–316
McCauley DE, Brock MT (1998) Frequency-dependent fitness in Silene vulgaris, a gynodioecious plant. Evolution 52:30–36
O’Hara RB, Kotze DJ (2010) Do not log-transform count data. Methods Ecol Evol 1:118–122
Ramsey M, Vaughton G (2002) Maintenance of gynodioecy in Wurmbea biglandulosa (Colchicaceae): gender differences in seed production and progeny success. Plant Syst Evol 232:189–200
Renner SS, Ricklefs RE (1995) Dioecy and its correlates in the flowering plants. Am J Bot 82:596–606
Richards A (1997) Plant breeding systems, 2nd edn. Chapman and Hall, London
Ritland K (1990) Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution 44:1230–1241
Ritland K (2002) Extensions of models for the estimation of mating systems using n independent loci. Heredity 88:221–228
Rousset F (2008) GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour 8:103–106
Sakai AK, Weller SG, Chen M-L, Chou S-Y, Tasanont C (1997) Evolution of gynodioecy and maintenance of females: the role of inbreeding depression, outcrossing rates, and resource allocation in Schiedea adamantis (Caryophyllaceae). Evolution 51:724–736
Shibata A, Kudo G (2017) Size-dependent sex allocation and reproductive investment in a gynodioecious shrub. AoB Plants. doi:10.1093/aobpla/plw089
Shykoff JA, Kolokotronis S-O, Collin CL, López-Villavicencio M (2003) Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia 135:1–9
Sinclair JP, Kameyama Y, Shibata A, Kudo G (2016) Male-biased hermaphrodites in a gynodioecious shrub, Daphne jezoensis. Plant Biol 18:859–867
Spigler RB, Ashman T-L (2012) Gynodioecy to dioecy: are we there yet? Ann Bot 109:531–543
Stewart CN, Via LE (1993) A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. Biotechniques 14:748–750
Van Etten ML, Chang SM (2014) Frequency-dependent pollinator discrimination acts against female plants in the gynodioecious Geranium maculatum. Ann Bot 114:1769–1778
Vaughton G, Ramsey M (2011) Reproductive allocation and costs in gynodioecious Leucopogon melaleucoides (Ericaceae): implications for the evolution of gender dimorphism. Plant Biol 13:888–895
Wang H, Matsushita M, Tomaru N, Nakagawa M (2014) Differences in female reproductive success between female and hermaphrodite individuals in the subdioecious shrub Eurya japonica (Theaceae). Plant Biol 17:194–200
Wolfe LM, Shmida A (1997) The ecology of sex expression in a gynodioecious Israeli desert shrub (Ochradenus Baccatus). Ecology 78:101–110
Acknowledgements
The authors would like to thank Y. Mizunaga, Y. Amagai, K. Onizawa, A. Wakui, S. Nakamura, and T. Kohyama for their kind assistance with fieldwork and their helpful discussions and comments. This study was partly supported by JSPS KAKENHI (15H02641).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shibata, A., Kameyama, Y. & Kudo, G. Restricted female function of hermaphrodites in a gynodioecious shrub, Daphne jezoensis (Thymelaeaceae). J Plant Res 131, 245–254 (2018). https://doi.org/10.1007/s10265-017-0978-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0978-5