Abstract
Taxaceae s.l. comprise six genera (including Cephalotaxus) and about 35 species; The present study aims to give new insights into the evolution of this family, especially into the phylogenetic position of Cephalotaxus. Moreover, only little is known about comparative leaf anatomy of this family and this study aims to expose and interpret the diversity and evolution of leaf anatomical characters and to assess their applicability to identify taxa at the generic and species level. A detailed phylogeny was reconstructed, using both maximum likelihood and Bayesian inference, with a combined dataset of four molecular markers from the plastid and nuclear genomes. Leaf sections from 132 specimens, representing 32 species and four varieties (fresh and herbarium material) were inspected, using fluorescence microscopy. Ancestral characters were reconstructed using Mesquite. The phylogenetic analyses provided full support for Cephalotaxus as sister group to Taxaceae s.str. Within the latter, two monophyletic tribes Taxeae (comprising Austrotaxus, Pseudotaxus, and Taxus) and Torreyeae (comprising Amentotaxus and Torreya) were fully supported. Fluorescence microscopy was shown to be very useful for identifying leaf tissues and their constitution. We were able to show that particularly sclerified tissues have highest potential for the discrimination of both freshly collected samples and rehydrated herbarium specimens at the generic and species level. A correlation between the presence of different sclereid types could be shown and sclereids were hypothesized to pose a primitive trait in the evolution of Taxaceae s.l. New identification keys were generated on the basis of leaf anatomical characters. The microscopic method presented here is applicable for further studies within gymnosperms and probably in angiosperms, as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extant gymnosperms are a major group of land plants that comprises the conifers, cycads, Gnetales, and Ginkgo biloba L. (Christenhusz et al. 2011). The living conifers consist of the six families Taxaceae s.l., Cupressaceae s.l., Sciadopityaceae, Podocarpaceae s.l., Araucariaceae, and Pinaceae (Eckenwalder 2009; Farjon 2010; Knopf et al. 2012; Little et al. 2013; Schulz et al. 2005), and understanding the evolution of these groups has been assisted by both morphological, anatomical, and phylogenetic investigations (Dörken et al. 2011; Dörken and Nimsch 2014; Leslie 2012; Leslie et al. 2012; Schulz et al. 2003, 2014; Schulz and Stützel 2006; Stefanoviac et al. 1998; Stützel and Röwekamp 1999; Zou et al. 2013). Within the extant conifers, the Taxaceae s.str. (without Cephalotaxus Siebold & Zucc. ex Endl.) form a relatively small family that comprises the five genera Amentotaxus Pilg., Austrotaxus Compton, Pseudotaxus W.C.Cheng, Taxus L., and Torreya Arn. However, the relationship of Cephalotaxus to Taxaceae s.str. and to conifers in general has been a controversial discussion in the history of conifer research (Chamberlain 1935; Dörken et al. 2011; Florin 1948a, b; Hart 1987; Mundry and Mundry 2001; Pilger 1916; Sahni 1920; Stützel and Röwekamp 1999).
In modern taxonomy, Taxaceae s.str. and Cephalotaxus are both well established as a part of the conifers but they have often been treated as separate families or as a single family, which led to a wider family concept, the Taxaceae s.l., which comprises all six genera. Likewise, the number of species is inconsistent and ranges from 18 to 25 with 4 to 6 varieties in Taxaceae s.str. (Cope 1998; Eckenwalder 2009; Farjon 2010; Fu et al. 1999b) and from 5 to 10 species with 3 to 5 varieties in Cephalotaxus (Eckenwalder 2009; Farjon 2010; Fu et al. 1999a; Lang et al. 2013; Tripp 1995). In addition, two Taxus hybrids (T. × media Rehder and T. × hunnewelliana Rehder) and one unnamed Amentotaxus hybrid, derived from A. argotaenia (Hance) Pilg. and A. formosana H.L.Li (Gosling et al. 2008), are also recognized.
Molecular studies have confirmed that Taxaceae s.l. are placed within the conifers, as the sister clade to Cupressaceae s.l., which includes the former Taxodiaceae (Chaw et al. 1993; Cheng et al. 2000; Hao et al. 2008; Leslie et al. 2012; Quinn et al. 2002; Schulz et al. 2014). In addition, they also confirmed two major clades within Taxaceae s.str., the tribes Taxeae and Torreyeae that had been proposed earlier by Janchen (1949) on the basis of morphology. The tribe Taxeae includes Austrotaxus spicata Compton as the sister to Pseudotaxus chienii (W.C.Cheng) W.C.Cheng and the genus Taxus, whereas Torreyeae includes Amentotaxus and Torreya. However, the phylogenetic position of Cephalotaxus as part of the Torreyeae, and thus within Taxaceae s.str., or as the sister group to Taxaceae s.str. has remained controversial and depends on the method of reconstruction and the number of the applied molecular markers (Cheng et al. 2000; Hao et al. 2008; Leslie et al. 2012; Lu et al. 2014; Quinn et al. 2002; Schulz et al. 2014; Wang et al. 2003). Therefore, a consistent topology for Taxaceae s.l. at the generic and species level is still lacking.
Taxaceae s.l. consist of mostly dioecious (except of T. canadensis with constant monoecy), evergreen trees and shrubs that are mostly distributed in the Northern Hemisphere (Farjon and Filer 2013; Wilson et al. 1996). Following the concept by Farjon (2001, 2010), six species are recognized in North America, one in Europe, and a diversity hotspot in Asia. The only member with a restricted distribution in the Southern Hemisphere is Austrotaxus spicata, endemic to New Caledonia (Farjon and Filer 2013).
The leaves of members of Taxaceae s.l. are flattened, linear to lanceolate, and hypostomatic, with two whitish to yellowish stomatal bands of different width (Cope 1998; Eckenwalder 2009; Page 1990; Price 1990; Tripp 1995). However, species identification and delimitation is often challenging, owing to strong morphological similarities within the genera of this family, and additional traits are often required, which are only applicable within specific groups (e.g., distribution of taxa in geographically restricted areas, such as the North American species). This is only appropriate for taxa distributed in the biodiversity hotspot of Asia (Liu et al. 2011), since many Central Asian species exhibit sympatric distribution, and the occurrence of hybrids of closely related taxa was shown in situ (Poudel et al. 2012) and in cultivation in Botanical Gardens (Gosling et al. 2008). These problems especially apply to species of Taxus and have led to several classifications for the genus, ranging from the description of a single species with seven geographical subspecies (Pilger 1903) to 24 species with 55 varieties (Spjut 2007). Möller et al. (2007, 2013) have shown the benefit of the combination of a multitude of leaf morphological characters and geographical data to discriminate the closely related Asian species, referred to as Taxus wallichiana Zucc.-complex. However, most of the common identification keys additionally require a multitude of vegetative characters, including the habit of the entire shrub and individual branches, branch angle, leaf size and shape, stomatal band width and colour, and the number of stomatal rows (Eckenwalder 2009; Li 1952; Tripp 1995). Others also include reproductive characters such as the number and size of pollen cones, seed size, and aril colour (Cope 1998; Farjon 2010; Fu et al. 1999a, b; Lang et al. 2013). These keys are problematic, since reproductive structures are only available for a short time during each growing season and are generally lacking in herbarium specimens, since they do not preserve well. Growth form may also be problematic as a delimitating character, since individuals grown from seeds may differ markedly from those grown from cuttings. Furthermore, the phenotypic plasticity of leaves, stems, and habits, owing to different environmental conditions and stage of development, can even cause confusion among individuals from the same species (Collins et al. 2003).
The inherent imperfection of existing identification keys is their dependence on both, vegetative and reproductive characters, since even a single lacking or inadequate structure can deem a key inapplicable. Therefore, the use of leaf anatomy may provide additional characters for the identification of extant species, as well as for fossil leaves, requiring only less material. Extensive insight into the comparative anatomy of gymnosperm leaves has been published by Florin (1931), Napp-Zinn (1966), and Kausik and Bhattacharya (1977), with only single notes on species of Taxaceae s.l. A broader analysis of Taxaceae s.l. (10 species and 4 varieties from all six genera) was published by Ghimire et al. (2014) and represented the first attempt to produce an anatomical identification key at the generic level. Previous extensive studies on leaf anatomy in Podocarpaceae have shown that such characters are highly valuable for both diagnostics and phylogenetics and they give new insights into the evolution of this family (Knopf et al. 2007, 2012).
In the present study, we used a similar approach but included nearly all of the species of Taxaceae s.l., recognized by Farjon (2010). Only little is known about the diversity and evolution of leaf anatomical characters and their applicability to identify taxa at the generic but particularly at the species level of this family. Therefore, we examined the presence and constitution of several anatomical characters using fluorescence microscopy, a rarely applied method for leaf anatomy. This technique enables an unambiguous identification of tissues and their constitution without the necessity to stain sections and thus, it enables a quick investigation of a high number of taxa. We analysed the anatomical characters in an evolutionary context, and therefore reconstructed the phylogeny of Taxaceae s.l. using four molecular markers. Based upon this phylogeny, we also attempted to evaluate the phylogenetic position of Cephalotaxus within Taxaceae s.l. Since leaf anatomical characters are independent from reproductive structures, we intended to generate new keys for identification.
Materials and methods
Taxon sampling
Living material was collected from several private collections and botanical gardens (BG) (Atlanta BG, Pinetum Blijdenstein, BG Bochum, BG Bonn, Plantentuin Esveld, BG Marburg, Royal BG Edinburgh and the Jardin des Plantes Paris). Herbarium samples were obtained from collections in Leiden (Nationaal Herbarium Nederland) and Paris (Museum National d’ Historie Naturelle). Altogether, a total of 132 specimens were investigated (Table S1). For the most part, we agree with Farjon (2010) and follow his taxonomy of 23 species and four varieties for Taxaceae s.str. and an additional eight to ten species and two varieties for Cephalotaxus. However, we treated Cephalotaxus griffithii Hook.f., C. koreana Nakai, C. mannii Hook.f., C. wilsoniana Hayata, and Taxus sumatrana (Miq.) de Laub. as distinct species, as done previously by Farjon (2001) and for T. sumatrana additionally supported by molecular barcoding data (Liu et al. 2011). Thus, 32 species and four varieties (and two hybrids) of the total of 36 species and six varieties are represented in this study. For a better evaluation of anatomical differences, we investigated mature leaf material of a minimum of three specimens per taxon (Table S1). Only two specimens were available for the following taxa: Cephalotaxus fortunei Hook. var. alpina H.L. Li, C. griffithii Hook. f., C. hainanensis H.L. Li, C. harringtonia (Knight ex J. Forbes) K. Koch var. nana (Nakai) Rehder, C. koreana Nakai, C. mannii Hook. f., C. wilsoniana Hayata, Taxus cuspidata Siebold & Zucc. var. nana hort. ex Rehder, Taxus globosa Schltdl., and Taxus × hunnewelliana Rehder. For the hybrid arisen from Amentotaxus argotaenia and Amentotaxus formosana, only single reported specimen was sampled (Gosling et al. 2008).
Since most sampled individuals were shrubs or small trees, the taken living samples were preferably sun exposed leaves and character states which are dependent on sun intensity (e.g. the number of layers in the palisade parenchyma) are thus preferably applicable to sun leaves. Differences to shaded leaves, however, were not investigated in this study.
Morphological and anatomical analysis
Living material was fixed in FAA (formalin, acetic acid, and 70% [v/v] ethanol; 5:5:90 by volume) for at least 7 d and then stored in 70% ethanol. Herbarium samples were softened using the method by Ayensu (1967) and Peterson et al. (1978). Dried material was incubated for at least 72 h in a 6:1 mixture of aerosol-OT (10%, w/v) and acetone (95%, v/v) for at least 72 h and then stored in 70% ethanol. In rehydrated material, sclerified cells were of great interest since they remain in shape and size during the drying and rehydrating process, which was seen by comparing species being present by both, living and fresh material in this study. Whereas, cells of the parenchyma are compressed during drying and do not regain their shape. Thus, character states of parenchymatic cells were not used for herbarium material.
For light microscopy, cross and longitudinal sections of fixed mature leaves were obtained from the mid-leaf section using a hand microtome (Allmikro, Haga GmbH + Co KG, Nürnberg, Germany). This technique enables an immediate investigation of sections without the need of embedding material before cutting. The sections were examined using fluorescence microscopy with an Axioplan microscope (Carl Zeiss Microscopy GmbH, Göttingen, Germany; filter set 02: G 365, FT 395, LP 420). When using fluorescence microscopy, much thicker slides can be used, as for bright field microscopy, which is important for preparing sections of herbarium material, since the material tends to break when using classical microtome sectioning. Autofluorescence enables the separation and identification of tissues and cells of different constitution by their emitted colour. In this way, sclerenchymatous cells can be identified by a greenish to blueish colour, according to their chemical composition. In contrast, parenchymatous tissues (e.g. the palisade parenchyma) emit light of red to orange shades, which depends on the amount of chloroplasts, the fluorescence of cell nuclei and the storage time in ethanol. The longer samples are stored in ethanol, the less the parenchyma fluoresces. The cuticle emits bright light from yellow to white.
For the leaves of Amentotaxus formosana, hand sections were stained in order to identify tannin cells in the mesophyll. According to Gerlach (1977), slides were incubated in a 3% (w/v) ferric chloride (FeCl3) solution. The incubation time was 30 s and slides were washed in distilled water afterwards.
The slides were documented using a ColorView II camera (Olympus Soft Imaging Solutions GmbH, Münster, Germany) and the associated analysis software Cell^D v3.1. Multiple image alignment was done manually using Adobe Photoshop CS5. The aligned overview images of Fig. 1 are also provided in high resolution for each genus in the supplementary materials (Figs. S1–S6).
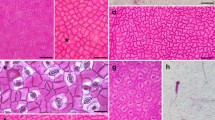
Phylogenetic reconstruction of Taxaceae s.l. (a) 50% majority rule consensus tree inferred from a Bayesian inference analysis with 50 million generations. maximum likelihood and Bayesian inference analyses exhibited identical topologies at the generic level and were almost identical at the species level. Bayesian posterior probability values are shown above branches, maximum likelihood bootstrap values are shown below branches. Top right shows tree overview. b–g Leaf cross sections of all six genera using fluorescence microscopy. b Taxus baccata. c Pseudotaxus chienii. d Austrotaxus spicata. e Amentotaxus argotaenia. f Torreya californica. g Cephalotaxus harringtonia var. harringtonia. as auxiliary sclereid, mr midrib region, st stomatal band, tt transfusion tissue. Frame colour indicates the genus in the phylogenetic tree. Scale bars 500 µm
Phylogenetic analyses
The phylogeny of Taxaceae s.l. was estimated based on the two nuclear gene regions nrITS (ITS1-5.8 S rDNA-ITS2) and PHYP and on the two chloroplast gene regions matK and rbcL. All sequences of the four phylogenetic markers were downloaded from GenBank (National Center for Biotechnology Information). Accession numbers are listed in the supplementary materials (Table S2). The outgroup contains one genus of each remaining conifer family (species of Phyllocladus were treated as part of the Podocarpaceae s.l.).
Sequences were edited using GENEIOUS v7.1.7 (http://www.geneious.com, Kearse et al. 2012), and alignments were conducted using MAFFT v7.017 (Katoh et al. 2002) in GENEIOUS. The original PHYP alignment consisted of long sequences up to 2,439 bp, as well as several shorter sequences which aligned either with the front or with the back end of the long sequences. Thus, the PHYP dataset was split into two separate parts using GENEIOUS v7.1.7, in order to treat each part as separate datasets before concatenation (for further information see Fig. S7). Phylogenetic reconstructions for all single markers were conducted using RAxML 7.2.8 (Stamatakis 2006) in GENEIOUS, with the implementation of the best fitting nucleotide substitution models which were determined using Modeltest 3.7 (Posada and Buckley 2004) in PAUP 4.0b10 (Swafford 2003). Voucher numbers were compared between the single phylogenies in order to select one accession per species and marker for the combined dataset. In the nrITS tree, the genus Cephalotaxus was sister clade to Austrotaxus + Pseudotaxus + Taxus and thus, in contrast to the topology at the generic level of the other three markers, in which Cephalotaxus is sister clade to the Taxaceae s.str. (for further information see single trees in Fig. S8). As a consequence, we tested the nrITS dataset with and without the sequences of Cephalotaxus before concatenation. As a conclusion, the topology at the generic level was the same in the reconstruction of the concatenated matrix, with Cephalotaxus as part of the nrITS dataset, as well as with Cephalotaxus discarded from the nrITS dataset. The five single datasets (matK, nrITS, rbcL, PHYP part 1, and PHYP part 2) showed no great conflicts before concatenation (see tanglegrams in Fig. S8). The final concatenated sequence alignment was conducted using Sequence-Matrix 1.7.8 (Vaidya et al. 2011) and comprised of 39 ingroup and five outgroup taxa. The matrix had a length of 6,602 sites and contained 29% of gaps or missing data, which most likely was caused by missing taxa in the single marker files (Cephalotaxus in particular).
The phylogeny of the total sequence alignment was reconstructed using maximum likelihood (ML) and Bayesian Inference (BI). The ML tree was reconstructed using RAxML v8.2.4 (Stamatakis 2014). Support values were assessed from 1000 bootstrap replicates, and the dataset was partitioned by gene region. Under the Akaike Information Criterion (AIC) the general time reversible (GTR) model with gamma distribution (+G) and the additional estimation of proportion of invariable sites (+I) was selected as best fitting substitution model for the concatenated matrix by Modeltest 3.7. The BI reconstruction was performed using MrBayes 3.2.6 (Ronquist and Huelsenbeck 2003). Ten independent runs were performed with four chains each and 50 million generations with chains sampled every 1000 generations. The concatenated matrix was also partitioned by gene region in MrBayes and the number of states, as well as the estimation of proportion of invariable sites was set separately for the individual gene regions on the basis of the results by Modeltest 3.7. The analysis was finalized by discarding the first 25% of the trees as burn-in. All final trees were illustrated using FigTree (Rambaut 2012) and graphically edited using CorelDRAW Graphics Suite X6.
Character evolution
Ancestral character states were reconstructed using Mesquite v3.10 build 765 (Maddison and Maddison 2016) and traced on the maximum likelihood tree obtained using RAxML v8.2.4 (Stamatakis 2014). With the focus on leaf anatomy, only discrete characters obtained from cross and longitudinal sections were used (a list of the examined characters and character states of individual taxa is given in the Table S3). Species which were not investigated anatomically were discarded from the phylogenetic tree in Mesquite. Likewise, branches of genera and species, respectively, which were not relevant for the investigated characters and their states were also discarded from the tree and thus, not shown in the results. The ancestral character trees were exported as a pdf and graphically illustrated in CorelDRAW Graphics Suite X6.
Results
Phylogenetic analyses
Major clades within Taxaceae s.l.
The phylogenetic reconstructions indicate that Taxaceae s.l. form a monophyletic clade nested within the conifers as sister to Cupressaceae s.l. (not shown), which is highly supported in both analyses. Maximum likelihood and Bayesian inference reveal two well supported major clades within Taxaceae s.str. (bs = 90, pp = 1.00), i.e., the tribes Taxeae (bs = 100, pp = 1.00) and Torreyeae (bs = 100, pp = 1.00; Fig. 1a). In addition, both analyses of the concatenated dataset clearly indicate that Cephalotaxus forms a monophyletic sister clade to Taxaceae s.str. (bs = 100, pp = 1.00; Fig. 1a), which is in accordance to the single-marker phylogenies of matK, rbcL, and PHYP (see Fig. S8); however, Cephalotaxus is sister to Torreyeae in the single-marker analyses of nrITS (see Fig. S8).
Phylogeny of Taxaceae s.str.
The tribe Taxeae includes the monotypic Austrotaxus as sister to the clade that includes Pseudotaxus and Taxus, which is fully supported for each lineage (bs = 100, pp = 1.00; Fig. 1a). The Taxus clade comprises 14 taxa, including the hybrids T. × media and T. × hunnewelliana, and the phylogenetic reconstruction of Taxus indicates four monophyletic clades, with T. brevifolia Nutt. as the basal taxon (bs = 100, pp = 1.00). Clade 1 includes the American T. floridana Nutt. and T. globosa Schltdl. as sister species (bs = 100, pp = 1.00). Clade 2 comprises the Central Asian taxa T. chinensis Rehder, T. mairei (Lemee & H.Lév.) S.Y.Hu, T. sumatrana, and T. wallichiana (bs = 95, pp = 0.97), with T. wallichiana as sister to the closely related T. chinensis, T. mairei, and T. sumatrana (bs = 99, pp = 1.00). Clade 3 comprises of the sister species T. baccata L. and T. contorta Griff. (bs = 62, pp = 1.00). Clade 4 (bs = 63, pp = 1.00) includes T. canadensis Marshall as sister to a clade (bs = 44, pp = 1.00) comprising the two varieties of T. cuspidata and the hybrids T. × media (Taxus baccata × cuspidata) and T. × hunnewelliana (Taxus cuspidata × canadensis).
In the analyses, the genera Amentotaxus and Torreya are sister clades, and each clade is fully supported in both trees (bs = 100, pp = 1.00; Fig. 1a). In addition, both genera together form a monophyletic clade, i.e., the tribe Torreyeae (bs = 100, pp = 1.00). Our dataset contains four of the six Amentotaxus species which are divided into two sister clades. Clade 1 comprises A. formosana and A. poilanei (De Ferré & Rouane) D.K.Ferguson as sister species (bs = 85, pp = 0.63), clade 2 comprises A. yunnanensis H.L.Li and A. argotaenia with strong support (bs = 93, pp = 1.00).
The reconstruction of Torreya indicates that T. californica Torr. is sister taxon to two major clades (Fig. 1a). Clade 1 (bs = 54, pp = 0.52) comprises the Asian T. nucifera Siebold & Zucc. as sister to T. fargesii Franch var. fargesii and T. fargesii Franch. var. yunnanensis (W.C.Cheng & L.K.Fu) (bs = 100, pp = 1.00). Clade 2 comprises the North American T. taxifolia Arn. as sister to the Asian T. grandis Fortune ex Gordon, and T. jackii Chun. which both are fully supported (bs = 100, pp = 1.00).
Leaf anatomy
Midrib
The shape of the midrib is convex on the adaxial and abaxial leaf side of Amentotaxus, Cephalotaxus, Pseudotaxus, Taxus, and Torreya (Fig. 1b, c, e–g: mr), whereas the leaves of Austrotaxus show a concave (grooved) midrib on the adaxial surface and a ridge on the abaxial surface (Fig. 1d: mr). In cross sections, the midrib is approximately as wide as the distance between transfusion tissue, which is laterally adjacent to the vascular bundle in Amentotaxus, Cephalotaxus, Pseudotaxus, and Taxus (Fig. 1b–e, g: tt); whereas a well-defined midrib is absent in Torreya, and the midrib region is significantly broader and merges into the lamina at the level of the stomatal bands (Fig. 1f: st).
Vascular bundle
The structure of the vascular bundle is similar in all six genera, with the leaves exhibiting a single vascular bundle that runs centrally through the midrib (Fig. 2m–p: circles). In cross sections, the vascular bundle is tapering and flanked by a transfusion tissue (Fig. 2o, p: tt). The transfusion tissue mostly consists of sclerified cells (Fig. 2f: tt), which are easily detectable by their spiral thickening, missing in the adjacent sclereids. The transfusion tissue is unregularly interrupted by parenchymatous cells. In the leaves of Austrotaxus and Amentotaxus, cells of the transfusion tissue are in contact with the auxiliary sclereids, which are orientated perpendicular to the vascular bundle (Fig. 2m, o: as; also see Sect. “Auxiliary sclereids”). No endodermis was visible in any specimen, using fluorescence microscopy.
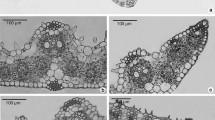
Evolution of selected characters in Taxaceae s.l. a–e Ancestral state reconstructions (ASR) for selected characters, traced on the maximum likelihood tree using Mesquite. a ASR for the presence of a papillose midrib region in Taxus. b ASR for the presence of a foliar resin canal. c ASR for the position of stomata. d ASR for the constitution of epidermal cells. e ASR for the presence of vascular sclereids. f Taxus globosa (magnified view on papillate midrib is given in the upper right corner). g Taxus canadensis (magnified view on midrib without papillae is given in the upper right corner). h Pseudotaxus chienii. i Torreya californica. j Taxus cuspidata var. cuspidata. k Torreya taxifolia. l Torreya nucifera (top-view onto adaxial leaf surface, parts of the cuticle were detached manually). m Austrotaxus spicata. n Cephalotaxus griffithii*. o Amentotaxus yunnanensis. p Torreya californica. as auxiliary sclereid, ep epidermis, pa papillae, pp palisade parenchyma, rc resin canal, st stomata, tt transfusion tissue, vs vascular sclereid. Frame colour indicates the genus in the phylogenetic tree. Rehydrated herbarium material is indicated with asterisk. Scale bars 100 µm
Vascular sclereids
Species of the Torreyeae tribe exclusively show thick-walled, fibre-shaped cells that surround the vascular bundle (Fig. 2o, p: vs) over the whole length of the leaf; such cells are missing in the taxa of the Taxeae tribe (Figs. 1b–d, 2e), as well as in Cephalotaxus (Fig. 2e, n). Due to the close proximity of these cells to the vascular bundle, they are referred to as vascular sclereids by Buchholz and Gray (1948) for leaves of Podocarpus; and this term is also applicable for the genera Amentotaxus and Torreya. Vascular sclereids are usually observed as isolated cells or cell clusters above and below the vascular bundle and partly surrounding the foliar resin canal. They almost lack a lumen and are strongly sclerified at maturity, as indicated by their intense blue colour in fluorescence microscopy (Fig. 2o, p: vs).
Foliar resin canal
The presence of a single foliar resin canal below the vascular bundle separates the tribe Torreyeae (Fig. 2o, p: rc) from the tribe Taxeae (Austrotaxus, Pseudotaxus, and Taxus; Fig. 2b), whose taxa lack this structure (Fig. 1b–d). In addition, Cephalotaxus also shows the presence of a single resin canal (Fig. 2n: rc). Resin canals are wider in the leaves of Torreya (~200 µm in diameter; Fig. 2p: rc) than in the leaves of Amentotaxus and Cephalotaxus, in which the resin canals only attain a maximum of 100 µm in diameter (Fig. 2n, o: rc).
Epidermis
Torreya differs from the other five genera by the presence of strongly sclerified epidermal cells in mature leaves (Fig. 2d). Their constitution is easily detectable using fluorescence microscopy, owing to their intense blue colour (Fig. 2i, k, l: ep). These sclerified cells almost lack a lumen when mature and surround the whole leaf, except the stomatal bands (Fig. 2k: ep). In contrast, the epidermal cells of the other genera were not sclerified in mature leaves (Fig. 2j: ep). After the cuticle was detached manually, a top-view onto the surface of Torreya leaves revealed that the sclerified epidermal cells are strongly elongated, with a length of partly up to 1000 µm (Fig. 2l: ep), in contrast to the more or less isodiametric epidermal cells found in the other five genera (Fig. 3n–q: ep).
Diversity and character evolution of sclereids in Taxaceae s.l. a–c Ancestral state reconstructions for presence and constitution of foliar sclereids, traced on the maximum likelihood tree using Mesquite. d Pseudotaxus chienii. e Austrotaxus spicata. f Torreya fargesii var. fargesii*. g Amentotaxus formosana. h Cephalotaxus koreana*. i Cephalotaxus oliveri. j Amentotaxus argotaenia × formosana. k Amentotaxus argotaenia. l Cephalotaxus harringtonia var. harringtonia. m Cephalotaxus oliveri. n Austrotaxus spicata (small window shows magnified auxiliary sclereid with a pitted cell wall). o Amentotaxus argotaenia. p Cephalotaxus koreana. q Cephalotaxus oliveri. as auxiliary sclereid, ep epidermis, hf hypodermal fibre, p pit, tt transfusion tissue. Frame colour indicates the genus in the phylogenetic tree. Rehydrated herbarium material is indicated with asterisk. Scale bars 200 µm
Palisade parenchyma
The palisade parenchyma consists of vertically elongated cells below the adaxial epidermis. In the lamina (except for the midrib region), a multi-layered palisade parenchyma is the common state within Taxaceae s.l. (Fig. 2j: pp); however, a single-layered palisade parenchyma was consistently observed in the specimens of Amentotaxus formosana (Fig. 4c, d, i: pp) and Taxus sumatrana (Fig. 4b: pp) and was also detected in the single specimen of Amentotaxus argotaenia × formosana (Fig. 4j: pp).
Characters for species identification in Taxaceae s.l. a Cephalotaxus wilsoniana*. b Taxus sumatrana. c, d Amentotaxus formosana. e Cephalotaxus fortunei var. fortunei. f Amentotaxus poilanei*. g Amentotaxus yunnanensis*. h–i Amentotaxus formosana. j Amentotaxus argotaenia × formosana. k Amentotaxus poilanei*. l Amentotaxus yunnanensis*. as auxiliary sclereid, hf hypodermal fibre, pp palisade parenchyma, tc tannin cells. Frame colour indicates the genus in the phylogenetic tree. Rehydrated herbarium material is indicated with asterisk. Scales bars a–d, i–l 100 µm; e–h 500 µm
Within Taxaceae s.l., Amentotaxus formosana is the only species to have tannin cells within the palisade parenchyma (Fig. 4c, d, h, i: tc) and spongy parenchyma (Fig. 4d: tc), identified by their black colour after staining the sections with ferric chloride solution (Fig. 4d: tc). These cells occur as different-sized clusters and are visible as yellow–brown idioblasts using bright field microscopy (Fig. 4c: tc) and as blue-grey idioblasts using fluorescence microscopy (Fig. 4i: tc). This character was consistently observed in every specimen of A. formosana and separates it from the other species of Amentotaxus.
Hypodermal fibres
Hypodermal fibres are elongated sclerified cells that run in different orientations directly below the epidermis (Fig. 3k–m: hf; Fig. 4f, g: hf), and due to their lignification, their orientation and length was visible in the external top view of the epidermis using fluorescence microscopy. Our analysis revealed that hypodermal fibres are present in only some species of Amentotaxus and Cephalotaxus but are absent in the other genera (Fig. 3b).
In Amentotaxus, only A. argotaenia (Fig. 3k: hf), A. poilanei (Fig. 4f: hf), and A. yunnanensis (Fig. 4g: hf) possess hypodermal fibres. These can either be branched (Fig. 3k: hf) or solitary (Fig. 4f: hf), but always run unordered when present in Amentotaxus (Fig. 3b). In mature leaves, species of Amentotaxus can also be distinguished by their quantity of hypodermal fibres: A. argotaenia and A. poilanei exhibit a similar pattern, with most fibres either solitary or contacting only 1–2 adjacent fibres (Fig. 3k: hf; Fig. 4f: hf); Whereas A. yunnanensis (Fig. 4g: hf) exhibits a more compact arrangement with most fibres in contact with more than 2 fibres.
In Cephalotaxus, hypodermal fibres were detected only in four investigated species (Fig. 3b). Hypodermal fibres are present in C. fortunei Hook. var. fortunei (Fig. 4e: hf), C. harringtonia (Knight ex J.Forbes) K.Koch var. harringtonia (Fig. 3l: hf), C. harringtonia (Knight ex J.Forbes) K.Koch var. nana (Nakai) Rehder, C. koreana, and C. oliveri Mast. (Fig. 3m: hf). In contrast to Amentotaxus, the hypodermal fibres were all observed to run parallel to the vascular bundle in Cephalotaxus (Fig. 3l: hf; Fig. 4e: hf), except in the leaves of C. oliveri, which possesses unordered fibres (Fig. 3m: hf). Fluorescence microscopy revealed that the hypodermal fibres differ in their maximal length between the species. In leaves of C. fortunei var. fortunei they have a maximum length of 500 µm (Fig. 4e: hf), whereas leaves of C. harringtonia and C. koreana show consistently longer fibres, both species with a similar maximum length of up to 2500 µm (Fig. 3l: hf).
Auxiliary sclereids
In the present study, auxiliary sclereids are defined as any sclerenchymatous cell within the mesophyll (except for hypodermal fibres). This term was first applied by Orr (1944) for this type of sclereids in the leaves of Podocarpus oleifolius D. Don and later adopted in Buchholz and Gray (1948) and further taxonomic revisions of the whole family of Podocarpaceae. Due to the congruence of shape and constitution, this term is adopted for species of Taxaceae s.l. in this study.
Auxiliary sclereids were found to significantly differ in shape, thickness, and orientation in Taxaceae s.l. Moreover, the occurrence of auxiliary sclereids is generally associated with the occurrence of hypodermal fibres (compare Fig. 3a, b), with the exception of Amentotaxus formosana (Fig. 4h), the single specimen of Amentotaxus argotaenia × formosana (Fig. 3j), and species having branched auxiliary sclereids (astrosclereids), i.e. Cephalotaxus wilsoniana (Fig. 4a: as) and Torreya fargesii var. fargesii (Fig. 3f: as). These four exhibit auxiliary sclereids but no hypodermal fibres.
Within the tribe Taxeae, no auxiliary sclereids were observed in either Pseudotaxus (Fig. 3d) or Taxus (Figs. 1b, 4b). In contrast, the leaves of Austrotaxus exhibit isodiametric to elongated and heavily pitted sclereids (Fig. 3e: as), with only a thin lignin layer when mature (Fig. 3f: small window). These sclereids show a compact pattern and were observed to run parallel to each other from the transfusion tissue to the leaf margin (Fig. 3e, n: as).
Mature auxiliary sclereids in the leaves of Amentotaxus, Cephalotaxus, and Torreya exhibit thick lignified cell walls with almost no lumens or pits (Fig. 3f-i: as). In Torreya, only T. fargesii var. fargesii possesses auxiliary sclereids, which are short and branched (astrosclereids; Fig. 3f: as) and their presence allows us to distinguish T. fargesii var. fargesii from the other species of this genus.
In Amentotaxus, auxiliary sclereids were observed in the leaves of all the investigated species (Fig. 2o: as; Fig. 3g, o: as; Fig. 4i, l: as), even though only A. argotaenia, A. poilanei, and A. yunnanensis possess hypodermal fibres (see Sect. “Hypodermal fibres”). In contrast to the arrangement of the hypodermal fibres, the auxiliary sclereids were orientated perpendicular to the vascular bundle and extend more or less parallel to each other from the midrib to the margin (Fig. 3g, o: as; 4i–l: as). Species of Amentotaxus can be distinguished also by the number of auxiliary sclereids. In longitudinal sections, leaves of A. formosana (Fig. 4i: as), A. argotaenia (Fig. 3o: as), and their common hybrid (Fig. 4j: as) possess ~10–20 sclereids per 1 mm in length of the lamina. In contrast, the investigated specimens of A. poilanei (Fig. 4k: as) and A. yunnanensis (Fig. 4l: as) possessed significantly more sclereids, with ~25–30 sclereids per 1 mm in length of the lamina.
In Cephalotaxus, C. fortunei var. fortunei, C. koreana (Fig. 3h: as), C. harringtonia var. harringtonia (Fig. 1g: as), C. harringtonia var. nana, and C. oliveri (Fig. 3i: as) possess auxiliary sclereids, that are elongated and unbranched. Like the hypodermal fibres, all auxiliary sclereids also run parallel to the vascular bundle (Fig. 1g: as; Fig. 3h, p: as), except in C. oliveri, which shows unordered sclereids in the lamina (Fig. 3i, q: as). In contrast, C. wilsoniana differs from the other Cephalotaxus species as the only found taxon with astrosclereids in its mesophyll (Fig. 4a: as).
Stomata
The stomatal complexes of Torreya differ markedly from the other genera by having deep seated stomatal complexes (Fig. 2c). Guard cells (Fig. 2i: st) and subsidiary cells are deeply sunken into the mesophyll and the external cell surface of subsidiary cells has grown out to long papillae (see "Papillae”). In the other five genera only the guard cells are slightly sunken (Fig. 2h: st), and the subsidiary cells are flush with other epidermal cells. The blue fluorescence colour of the guard cells shows that those are sclerified as reinforcement in all six genera (Fig. 2i, h: st).
Papillae
In the present study, papillae were found as outgrowths of epidermal cells of the stomatal bands in Taxus (Fig. 2g: pa) and Torreya (Fig. 2i: pa), and exclusively on the midrib of some Taxus species (Fig. 2f. pa), whereas they are absent in Pseudotaxus, Austrotaxus and Cephalotaxus. Both genera differ in the types of papillae, which are either short and isodiametric or strongly vertically elongated. Vertically elongated papillae are only present in the stomatal bands of leaves of Torreya and papillae decrease in size from the middle of the stomatal band to its edge (Fig. 2i: pa). Papillae are fully covered by cuticle and they fluoresce in bright yellow to white.
The short and isodiametric papillae (Fig. 2g: pa) were found in the stomatal bands of all species of Taxus in which the subsidiary cells and their adjacent epidermal cells bear several papillae. In the American T. brevifolia, T. floridana, and T. globosa, as well as in the Central Asian species T. wallichiana, the papillae were observed also on the abaxial surface of the midrib (Fig. 2f: pa), which is exclusive for these four species (Fig. 2a). Moreover, T. brevifolia is the only species with papillae also on its adaxial leaf surface (Fig. 2f: pa, black arrow).
Discussion
Phylogeny
Phylogeny of the American and Central Asian Taxus species
The phylogeny of the Taxus clade is ambiguous, since only a few studies used combined phylogenetic markers. In particular, the position of the three American species T. brevifolia, T. floridana, and T. globosa and of the Central Asian species T. chinensis, T. sumatrana, and T. wallichiana are uncertain. For the American species, Leslie et al. (2012) reported that the three species were paraphyletic, whereas Hao et al. (2008) reported that T. globosa and T. brevifolia were sister species and that T. floridana was the most basal lineage within Taxus. The topology presented in this study (Fig. 1a) accords with Leslie et al. (2012), that T. brevifolia was the earliest diverging lineage. However, we have full support that T. floridana and T. globosa (Fig. 1a: clade 1) are sister species and not distinct lineages, as suggested by both Hao et al. (2008) and Leslie et al. (2012).
In addition, the present study supports the Central Asian species T. chinensis, T. mairei, and T. sumatrana as closest relatives (Hao et al. 2008; Leslie et al. 2012). As reported by Leslie et al. (2012), our results show T. wallichiana as sister to the other Central Asian Taxus species (Fig. 1a: clade 2) and not as sister to a clade comprising T. cuspidata and T. canadensis, as in Hao et al. (2008). The close relationship of the four Central Asian taxa is also reflected by strong morphological similarities, and these taxa are composited as Taxus wallichiana-complex (Möller et al. 2007). This complex was not resolved in the Bayesian inference (BI) analysis of this study and the support for T. sumatrana and T. chinensis as sister species was low in the maximum likelihood (ML) analysis. However, Liu et al. (2011) have shown the value of DNA barcoding as a method to discriminate closely related species of this complex.
Phylogeny of Torreya species
Two topologies have been published, dealing with the phylogeny of Torreya species and which are based on a combined dataset (Hao et al. 2008; Leslie et al. 2012). Both accord that T. californica and T. taxifolia are sister species and that this clade is nested within the Asian species. This is contradictory to the result of this study, with highest support that T. taxifolia is closer related to Asian specie s(i.e. T. grandis and T. jackii) and that T. californica is the most basal lineage (Fig. 1a). In addition, the ML and the BI trees of this study support that T. grandis and T. fargesii which were formerly seen as varieties are treated as separate species since they are not closest relatives. However, further studies particularly for the poorly investigated taxa T. fargesii var. yunnanensis, T. jackii, and T. grandis var. jiulongshanensis are required.
Phylogenetic position of Cephalotaxus
The phylogenetic position of Cephalotaxus was a very contentious issue in gymnosperm studies, since previous studies, using only one or two molecular markers, have indicated its position to differ, either as sister to the Torreyeae (Amentotaxus and Torreya) or as sister to the Taxaceae s.str., as in Cheng et al. (2000), Quinn et al. (2002), Wang et al. (2003), and Lu et al. (2014). Extensive studies that used a broader range of markers are rare and were published by Hao et al. (2008), using five plastid markers and one nuclear marker, and by Leslie et al. (2012) and Schulz et al. (2014), each using two plastid and two nuclear markers. However, in these studies, the increased number of markers has not lead to the same result concerning the position of Cephalotaxus. This study, also dealing with two nuclear and two plastid markers, is in accordance with the topology reported by Schulz et al. (2014) and also supports an early split between the Cephalotaxus lineage and the Taxaceae s.str. lineage (Fig. 1a). Although accessions of Cephalotaxus were discarded from the nrITS matrix, the ML and the BI tree show highest support, that Cephalotaxus and Taxaceae s.str. are two distinct lineages. However, the single matrices of the nuclear markers PHYP and nrITS have shown the contradiction of the position of Cephalotaxus (see Supplementary Material, Fig. S8) which is also shown in the previously published studies dealing with this genus (Hao et al. 2008; Leslie et al. 2012; Schulz et al. 2014). In addition, sequences of Cephalotaxus are insufficiently available and further extensive molecular work is urgently required, in particular for a high support at the species level.
Leaf anatomy
Foliar sclereids as new delimitating characters
Foliar sclereids are common within the leaves of gymnosperm families (Burrows and Bullock 1999; Hu et al. 1992; Kausik and Bhattacharya 1977; Napp-Zinn 1966; Rao 1965, 1977; Rao and Bhupal 1972; Rao and Malaviya 1963), and the present study confirms that Taxaceae s.l. possess the same broad spectrum of sclereid types found in other gymnosperm families (i.e., vascular sclereids, auxiliary sclereids, and hypodermal fibres). It was previously unknown whether foliar sclereids were suitable as diagnostic characters in Taxaceae s.l. and a first attempt was made by Ghimire et al. (2014). The presence of thick walled fibre cells around the vascular bundle was mentioned for Amentotaxus and Torreya (Ghimire et al. 2014). Using fluorescence microscopy, their presence and constitution were easily detectable in this study and we propose them to be termed as vascular sclereids as well, following the definition by Buchholz and Gray (1948). The present study found that vascular sclereids are present around the vascular bundle (Fig. 2o: vs) in both Amentotaxus and Torreya, but are absent in Taxeae (Taxus, Pseudotaxus, and Austrotaxus), thus providing an anatomical character for distinguishing between the two major clades within Taxaceae s.str.
The use of foliar sclereid characters for species identification within conifers has already been demonstrated for the Podocarpaceae (Buchholz and Gray 1948; Knopf et al. 2007, 2012). However, these characters had not yet been investigated for their application to identifying the particularly morphologically similar species of Taxaceae s.l. Ferguson (1985) first mentioned the diagnostic applicability of sclereid types to distinguish Amentotaxus assamica (not available for the present study) from the other species of this genus by the absence of auxiliary sclereids in the mesophyll. In the present study, we expanded this approach for all six genera of this family and were thus able to distinguish the species of Amentotaxus, Austrotaxus, Cephalotaxus, and Torreya based on auxiliary sclereids (Fig. 3e–i: as) and hypodermal fibres (Fig. 3k–m: hf; Fig. 4f, g: hf).
Hypodermal fibres, in particular, turned out to be useful as a diagnostic character. The presence of such were reported as hypodermal region in Ghimire et al. (2014), found in the investigated species Cephalotaxus harringtonia, as well as in Amentotaxus yunnanensis, which we agree with on the basis of our results. However, in contrast to Ghimire et al. (2014) we have found hypodermal fibres in Cephalotaxus fortunei var. fortunei, but interestingly not in Cephalotaxus fortunei var. alpina. Thus, both can be delimited by the absence of such fibres. Furthermore, for both Cephalotaxus and Amentotaxus, interspecific differences in the orientation, length, and quantity of hypodermal fibres had not been reported earlier. The here presented method and the large number of specimens investigated revealed that type, orientation, and frequency of foliar sclereids are consistent and could be used as distinctive characters. Fluorescence microscopy has been shown to be useful in detecting and distinguishing different types of sclereids and hypodermal fibres are easy to visualize in the top view of the adaxial epidermis which represents a rapid method for identification at the generic and also at the species level. Likewise, this approach is useful for distinguishing between morphologically similar species (e.g., Cephalotaxus, Amentotaxus, and Torreya) including sterile herbarium material, as shown in the present study.
Differentiation of Taxus species by a papillose midrib
Previous identification keys have indicated that species identification is hardly feasible for species of Taxus without additional information about habit or reproductive structures (Cope 1998; Eckenwalder 2009; Fu et al. 1999b). In this study, we did not detect any significant anatomical differences in the mesophyll that could be used for species identification either. Thus, it was important to determine whether species differ in their papillae, which are characteristic for Taxus and which are visible also when using fluorescence microscopy. The presence of papillae on the abaxial midrib, as well as on the adaxial leaf surface of T. brevifolia, was found to be highly useful as a diagnostic character for separating T. brevifolia, T. floridana, T. globosa, and T. wallichiana from the other species (Fig. 2f: pa). However, no differences were obtained between the species having a papillose midrib nor between the species lacking this character. An adequate separation may require further investigation of their micro-morphological differences, such as cuticle characters, the width of the stomatal bands or structure of epicuticular waxes.
Character evolution
Evolution of different foliar sclereid types
We discovered a broad range of foliar sclereids in several species of Taxaceae s.l. and were able to show that vascular sclereids are present in Amentotaxus and Torreya (Torreyeae) but absent in Taxus, Pseudotaxus, and Austrotaxus (Taxeae). In addition, since Cephalotaxus, as sister to Taxaceae s.str., also lacks vascular sclereids, the feature may represent an apomorphy for the Torreyeae clade, a conclusion that is also supported by ancestral state reconstruction (ASR; Fig. 2e). However, Buchholz and Gray (1948) first defined vascular sclereids for members of Podocarpaceae, and Napp-Zinn (1966) described similar sclerified cells as sclerenchymatous caps (germ.: Sklerenchymkappen) in the leaves of various other gymnosperm species. It seems that these cells are similar to the sclereids in Amentotaxus and Torreya, but if this indicates a homology, the vascular sclereids are homologous throughout gymnosperms and were independently lost in both Taxeae and Cephalotaxus. With a potential function as reinforcement in broad-leaved gymnosperms, the loss of vascular sclereids may also be correlated with the smaller leaf size in Taxus, Pseudotaxus, and Cephalotaxus.
On the basis of this study, we accord with Ghimire et al. (2014) in the fact that a sclerenchymatous epidermis is present exclusively in Torreya. This extensive study of almost all listed species of this genus has shown, that all feature thick walled epidermal cells and the ASR supports that this is an apomorphy for the genus Torreya (Fig. 2d). Moreover, longitudinal sections could show that epidermal cells in Torreya are fusiform fibres (Fig. 2l: black arrows) and significantly longer than rectangular cells in the other five genera, as reported in Ghimire et al. (2014).
Different types of auxiliary sclereids and hypodermal fibres were found to differ characteristically between the distinct lineages of Amentotaxus, Austrotaxus, Cephalotaxus, and Torreya. Thus, the sclereids could have either evolved several times independently or evolved from a common ancestral state. However, the ASR indicated no possible ancestral state, neither for the shape nor for the orientation of both, auxiliary sclereids and hypodermal fibres (Fig. 3a-c). They likely diversified exclusively in each lineage of this family. In particular, elongated auxiliary sclereids which run parallel to the vascular bundle were detectable only for a few species of Cephalotaxus and it remains uncertain whether this features the primitive trait or evolved separately for each species (Fig. 3c). However, character reconstruction revealed that the occurrence of hypodermal fibres is strictly associated with the occurrence of auxiliary sclereids, but not vice versa (Fig. 3a, b), which indicates that hypodermal fibres were lost in A. formosana. In addition, we found that the auxiliary sclereids in both Austrotaxus and Amentotaxus resemble the pattern of the so called accessory transfusion tissue in the leaves of Podocarpaceae and Cycadaceae (Buchholz and Gray 1948; Kausik 1975; Kausik and Bhattacharya 1977; Knopf et al. 2012; Locosselli and Ceccantini 2012; Worsdell 1897), and thus, the term accessory transfusion tissue also seems appropriate for the formation of auxiliary sclereids found in Austrotaxus and Amentotaxus. The accessory transfusion tissue is thought to function as an extra hydraulic pathway in single veined leaves (Brodribb et al. 2007, 2010). Thus, the presence and constitution of auxiliary sclereids is likely influenced by climatic conditions. However, their occurrence in all gymnosperm families (Kausik and Bhattacharya 1977; Knopf et al. 2012; Napp-Zinn 1966; Rao 1965; Rao and Bhupal 1972) supports the notion that sclereids are plesiomorphic in Taxaceae s.l. Thus, sclereids were subsequently lost in the Pseudotaxus and Taxus lineage, which may also be correlated to their smaller leaf size.
Evolution of papillae in the midrib region of Taxus leaves
The occurrence of protuberances is common in the stomatal bands of all Taxus species, and protuberances were also detected on the abaxial midrib of T. brevifolia, T. floridana, T. globosa, and T. wallichiana. Since T. brevifolia is the basal lineage of the genus, a papillose midrib may be the ancestral state in Taxus, as was also indicated by ASR, and the occurrence of papillae on the abaxial midrib is likely to be the ancestral state (Fig. 2a). Florin (1958) described T. harrisii Florin as a possible ancestor of Taxus, and the species already contained papillae from the stomatal bands towards the margin. However, the midrib region was not described in detail. Thus, the conclusion cannot be supported by the state of its possible ancestor, but a papillose midrib is likely the ancestral state for the recent Taxus lineages.
Foliar resin canals as primitive state in Taxaceae s.l.
Within Taxaceae s.str., a single foliar resin canal is present in Torreyeae (Amentotaxus and Torreya) but absent in Taxeae (Taxus, Pseudotaxus, and Austrotaxus). Since Cephalotaxus, as sister to Taxaceae s.str., also features a single resin canal, it may have been lost in the Taxeae. Likewise, the ASR shows that the presence of resin canals is the most likely ancestral state (Fig. 2b), and foliar resin canals have also been found in several species among all conifer families (Eckenwalder 2009; Hamidipour et al. 2011; Knopf et al. 2012; Napp-Zinn 1966; Saxton 1930; Wu and Hu 1997). Thus, we assume that the occurrence of resin canals is a plesiomorphic trait in Taxaceae s.l., which was lost in the Taxeae tribe.
References
Ayensu ES (1967) Aerosol ot solution—an effective softener of herbarium specimens for anatomical study. Biotech Histochem 42:155–156. doi:10.3109/10520296709115000
Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol 144:1890–1898. doi:10.1104/pp.107.101352
Brodribb TJ, Feild TS, Sack L (2010) Viewing leaf structure and evolution from a hydraulic perspective. Funct Plant Biol 37:488. doi:10.1071/FP10010
Buchholz JT, Gray NE (1948) Taxonomic revision of Podocarpus. I. The sections of the genus and their subdivisions with special reference to leaf anatomy. J Arnold Arbor 29:49–63
Burrows GE, Bullock S (1999) Leaf anatomy of wollemi pine (Wollemia nobilis, Araucariaceae). Aust J Bot 47:795. doi:10.1071/BT98019
Chamberlain CJ (1935) Gymnosperms, structure and evolution. The University of Chicago Press, Chicago
Chaw S, Long H, Wang B, Zharkikh A, Li W (1993) The phylogenetic position of Taxaceae based on 18 S rRNA sequences. J Mol Evol 37:624–630
Cheng Y, Nicolson RG, Tripp KE, Chaw S (2000) Phylogeny of Taxaceae and Cephalotaxaceae genera inferred from chloroplast matK gene and nuclear rDNA ITS Region. Mol Phyl Evol 14:353–365. doi:10.1006/mpev.1999.0710
Christenhusz M, Reveal JL, Farjon A, Gardner MF, Mill RR, Chase MW (2011) A new classification and linear sequence of extant gymnosperms. Phytotaxa 19:55–70
Collins D, Mill RR, Möller M (2003) Species separation of Taxus baccata, T. canadensis. cuspidata (Taxaceae) and origins of their reputed hybrids inferred from RAPD and cpDNA data. Am J Bot 90:175–182. doi:10.3732/ajb.90.2.175
Cope E (1998) Taxaceae: The genera and cultivated species. Bot Rev 64:291–322
Dörken VM, Nimsch H (2014) Morpho-anatomical investigations of cones and pollen in Cathaya argyrophylla Chung & Kuang (Pinaceae, Coniferales) under systematical and evolutional aspects. Feddes Rep 125:25–38. doi:10.1002/fedr.201400035
Dörken VM, Zhang Z, Mundry I, Stützel T (2011) Morphology and anatomy of male cones of Pseudotaxus chienii (Cheng WC) Cheng WC (Taxaceae). Flora 206:444–450. doi:10.1016/j.flora.2010.08.006
Eckenwalder JE (2009) Conifers of the world: the complete reference. 1st edn. Timber Press, Portland
Farjon A (2001) World checklist and bibliography of conifers, 2nd edn. Royal Botanic Gardens, Kew
Farjon A (2010) A handbook of the World’s Conifers. BRILL, Leiden
Farjon A, Filer D (2013) An atlas of the world’s conifers: an analysis of their distribution, biogeography, diversity and conservation status. BRILL, Leiden
Ferguson DK (1985) A new species of Amentotaxus (Taxaceae) from Northeastern India. Kew Bull 40:115. doi:10.2307/4108483
Florin R (1931) Untersuchungen zur Stammesgeschichte der Coniferales und Cordaitales. In: I. Morphologie und Epidermisstruktur der Assimilationsorgane bei den rezenten Koniferen. 10, vol I. K Svenska Vetensk Akad Handl, Stockholm
Florin R (1948a) On Nothotaxus, a new species of the Taxaceae, from Eastern China. Acta Horti Bergiani 14:385–395
Florin R (1948b) On the morphology and relationships on the Taxaceae. Bot Gaz 110:31–39
Florin R (1958) On Jurassic taxads and conifers from north-western Europe and eastern Greenland. Acta Horti Bergiani 17:257–402
Fu L, Li N, Mill RR (1999a) Cephalotaxaceae Fl China 4:85–88
Fu L, Li N, Mill RR (1999b) Taxaceae Fl China 4:89–96
Gerlach D (1977) Botanische Mikrotechnik: Eine Einführung, 2., überarb. u. erw. Aufl. Thieme, Stuttgart
Ghimire B, Lee C, Heo K (2014) Leaf anatomy and its implications for phylogenetic relationships in Taxaceae s. l. J Plant Res 127:373–388. doi:10.1007/s10265-014-0625-3
Gosling PG, McCartan SA, Ives LM, Cunningham VJ, Squirrell J, Thomas P (2008) Preliminary observations on fruit handling, seed germination and chloroplast inheritance of an Amentotaxus hybrid arising at the Royal Botanic Garden Edinburgh from A. argotaenia (F) x A. formosana (M). Sibbaldia 6:101–115
Hamidipour A, Radjabian T, Charlet DA, Zarrei M (2011) Leaf anatomical investigation of Cupressaceae and Taxaceae in Iran. Wulfenia 18:95–111
Hao DC, Xiao PG, Huang B, Ge GB, Yang L (2008) Interspecific relationships and origins of Taxaceae and Cephalotaxaceae revealed by partitioned Bayesian analyses of chloroplast and nuclear DNA sequences. Plant Syst Evol 276:89–104. doi:10.1007/s00606-008-0069-0
Hart JA (1987) A cladistic-analysis of conifers - preliminary-results. J Arnold Arbor 68:269–307
Hu Y, Wang H, Wang F (1992) Leaf anatomy of Austrotaxus in relation to its systematic position. Cathaya 4:69–77
Janchen E (1949) Das System der Koniferen. Oesterr Akad Wiss Math-Naturwiss. Kl Sitzungsber Abt 1 Biol 158:155–262
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucl Acid Res 30:3059–3066. doi:10.1093/nar/gkf436
Kausik S (1975) The leaf structure in Podocarpus brevifolia (Stapf.) Foxw. Proc Ind Acad Sci B 81:197–206
Kausik S, Bhattacharya S (1977) Comparative foliar anatomy of selected gymnosperms: leaf structure in relation to leaf form in Coniferales and Taxales. Phytomorphology 27:146–160
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi:10.1093/bioinformatics/bts199
Knopf P, Nimsch H, Stützel T (2007) Dacrydium × suprinii, sp. nova – a natural hybrid of Dacrydium araucarioides × D. guillauminii. Feddes Rep 118:51–59. doi:10.1002/fedr.200711126
Knopf P, Schulz C, Little DP, Stützel T, Stevenson DW (2012) Relationships within Podocarpaceae based on DNA sequence, anatomical, morphological, and biogeographical data. Cladistics 28:271–299. doi:10.1111/j.1096-0031.2011.00381.x
Lang X, Su J, Zhang Z, Lu S (2013) A taxonomic revision of the genus Cephalotaxus (Taxaceae). Phytotaxa 84. doi:10.11646/phytotaxa.84.1.1
Leslie AB (2012) Branching habit and the allocation of reproductive resources in conifers. Ann Bot 110:915–921. doi:10.1093/aob/mcs150
Leslie AB, Beaulieu JM, Rai HS, Crane PR, Donoghue MJ, Mathews S (2012) Hemisphere-scale differences in conifer evolutionary dynamics. Proc Natl Acad Sci USA 109:16217–16221. doi:10.1073/pnas.1213621109
Li H (1952) The genus Amentotaxus. J Arnold Arbor 33:192–198
Little DP, Knopf P, Schulz C, Hajibabaei M (2013) DNA barcode identification of Podocarpaceae—the second largest conifer family. PLoS One 8:e81008. doi:10.1371/journal.pone.0081008
Liu J, Möller M, Gao LM, Zhang D, Li D (2011) DNA barcoding for the discrimination of Eurasian yews (Taxus L., Taxaceae) and the discovery of cryptic species. Mol Ecol Resour 11:89–100. doi:10.1111/j.1755-0998.2010.02907.x
Locosselli GM, Ceccantini G (2012) Plasticity of stomatal distribution pattern and stem tracheid dimensions in Podocarpus lambertii: an ecological study. Ann Bot 110:1057–1066. doi:10.1093/aob/mcs179
Lu Y, Ran J, Guo D, Yang Z, Wang X, Buerki S (2014) Phylogeny and divergence times of gymnosperms inferred from single-copy nuclear genes. PLoS One 9:e107679. doi:10.1371/journal.pone.0107679
Maddison W, Maddison D (2016) Mesquite: a modular system for evolutionary analysis. Version 3.03. https://mesquiteproject.wikispaces.com. Accessed 24 Oct 2016
Möller M, Gao LM, Mill RR, Li D, Hollingsworth ML, Gibby M (2007) Morphometric analysis of the Taxus wallichiana complex (Taxaceae) based on herbarium material. Bot J Linn Soc 155:307–335. doi:10.1111/j.1095-8339.2007.00697.x
Möller M, Gao LM, Mill RR, Liu J, Zhang D, Poudel RC, Li D (2013) A multidisciplinary approach reveals hidden taxonomic diversity in the morphologically challenging Taxus wallichiana-complex. Taxon 62:1161–1177. doi:10.12705/626.9
Mundry I, Mundry M (2001) Male cones in Taxaceae s. l.—an example of Wettstein’s pseudanthium concept. Plant Biol 3:405–416
Napp-Zinn K (1966) Anatomie des Blattes: I. Blattanatomie der Gymnospermen. Gebrüder Bornträger, Berlin-Nikolassee
Orr MY (1944) The Leaf Anatomy of Podocarpus Trans Bot Soc Edinburgh 34:1–54. doi:10.1080/13594864409441551
Page CN (1990) Taxonomic concepts in conifers and ginkgoids. In: Kramer KU, Green PS (eds) Pteridophytes and gymnosperms. Springer, Berlin Heidelberg
Peterson RL, Hersey RE, Brisson JD (1978) Embedding softened herbarium material in Spurr’s resin for histological studies. Biotech Histochem 53:1–9. doi:10.3109/10520297809111436
Pilger R (ed) (1903) Taxaceae. Das Pflanzenreich, IV.5. W. Engelmann, Leipzig and Berlin
Pilger R (1916) Die Taxales. Mitt Dtsch Dendrol Ges 25:1–30
Posada D, Buckley T (2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808. doi:10.1080/10635150490522304
Poudel RC, Möller M, Gao LM, Ahrends A, Baral SR, Liu J, Thomas P, Li D, Joly S (2012) Using morphological, molecular and climatic data to delimitate yews along the Hindu Kush-Himalaya and adjacent regions. PLoS One 7:e46873. doi:10.1371/journal.pone.0046873
Price RA (1990) The genera of Taxaceae in the southeastern United States. J Arnold Arbor 71:69–91
Quinn CJ, Price RA, Gadek PA (2002) Familial concepts and relationships in the conifer based on rbcL and matK sequence comparisons. Kew Bull 57:513. doi:10.2307/4110984
Rambaut A (2012) FigTree v1.4.0: tree figure drawing tool. http://tree.bio.ed.ac.uk/software/figtree. Accessed 26 Oct 2016
Rao A (1965) Studies on foliar sclereids in gymnosperms. Proc Ind Acad Sci B 61:196–203
Rao A (1977) Morphogenesis of sclereids in Gnetum gnemon. Ann Bot 39:973–974
Rao A, Bhupal OP (1972) Topology of sclereids. Bot Surv India 14:41–55
Rao A, Malaviya M (1963) The peculiar sclereids of Cephalotaxus drupacea Sieb. et Zucc. Proc Ind Acad Sci B 59B:228–236
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Sahni B (1920) On certain archaic features in the seed of Taxus baccata, with remarks on the antiquity of the Taxinae. Ann Bot 34:117–132
Saxton WT (1930) Notes on conifers: IV. Some points in the leaf anatomy of Fokienia hodginsii Henry and Thomas and Libocedrus macrolepis B. and H. Ann Bot 44:167–171
Schulz C, Stützel T (2006) Variability of male cones in Chamaecyparis as an example for Cupressaceae male cones. Feddes Rep 117:146–157. doi:10.1002/fedr.200511085
Schulz C, Jagel A, Stützel T (2003) Cone morphology in Juniperus in the light of cone evolution in Cupressaceae s.l. Flora 198:161–177. doi:10.1078/0367-2530-00088
Schulz C, Knopf P, Stützel T (2005) Identification key to the Cypress family (Cupressaceae). Feddes Rep 116:96–146. doi:10.1002/fedr.200411062
Schulz C, Klaus KV, Knopf P, Mundry M, Dörken VM, Stützel T (2014) Male cone evolution in conifers: not all that simple. Am J Plant Sci 5:2842–2857. doi:10.4236/ajps.2014.518300
Spjut R (2007) Taxonomy and nomenclature of Taxus (Taxaceae). J Bot Res Inst Texas 1:203–289
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi:10.1093/bioinformatics/btl446
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi:10.1093/bioinformatics/btu033
Stefanoviac S, Muriel J, Deutsch J, Broutin J, Masselot M (1998) Phylogenetic relationships of conifers inferred from Partial 28 S rRNA gene sequences. Am J Bot 85:688–697
Stützel T, Röwekamp I (1999) Female reproductive structures in Taxales. Flora 194:145–157
Swafford DL (2003) PAUP∗: Phylogenetic analysis using parsimony (∗and other methods). Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts. http://paup.sc.fsu.edu/index.html. Accessed 24 Oct 2016
Tripp KE (1995) Cephalotaxus: the plum yews. Arnoldia 55:25–39
Vaidya G, Lohman DJ, Meier R (2011) SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27:171–180. doi:10.1111/j.1096-0031.2010.00329.x
Wang T, Su Y, Zheng B, Li X, Zeng Q, Qu L, Gu H (2003) Cladistic analysis of chloroplast rbcL gene and trnL-trnF intergenic spacer sequences in Taxaceae and related taxa. In: Sharma AK, Sharma A (eds) Plant genome: biodiversity and evolution, vol 1, part A: Phanerogams. Scientific Publications, Enfield, pp 103–116
Wilson P, Buonopane M, Allison TD (1996) Reproductive biology of the monoecious clonal shrub Taxus canadensis. Bull Torrey Bot Club 123:7. doi:10.2307/2996301
Worsdell WC (1897) VIII. On transfusion-tissue: its origin and function in the leaves of Gymnospermous Plants. // VIII. On “Transfusion-tissue”. Trans Linn Soc London Bot 5:301–319. doi:10.1111/j.1095-8339.1897.tb00205.x
Wu H, Hu Z (1997) Comparative anatomy of resin ducts of the Pinaceae. Trees 11:135. doi:10.1007/s004680050069
Zou J, Sun Y, Li L, Wang G, Yue W, Lu Z, Wang Q, Liu J (2013) Population genetic evidence for speciation pattern and gene flow between Picea wilsonii, P. morrisonicola and P. neoveitchii. Ann Bot 112:1829–1844. doi:10.1093/aob/mct241
Acknowledgements
We would like to thank all botanical institutions and Botanical Gardens (BG) (Atlanta BG, Pinetum Blijdenstein, BG Bochum, BG Bonn, Plantentuin Esveld, BG Marburg, Royal BG Edinburgh, and the Jardin des Plantes Paris), as well as several private collections (Mr. Hubertus Nimsch and Mr. Albrecht Weiss) for their support in providing access to living material. Likewise, we thank the Nationaal Herbarium Nederland in Leiden and the Museum National d’ Historie Naturelle in Paris for their generous access to herbarium material.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Identifications keys
Identifications keys
Identification key at the genera level on the basis of fluorescence-microscopic characters

Identification key for Amentotaxus on the basis of leaf anatomical characters

Identification key for Cephalotaxus on the basis of leaf anatomical characters

Identification key for Taxus on the basis of leaf anatomical characters

Identification key for Torreya on the basis of leaf anatomical characters

Rights and permissions
About this article
Cite this article
Elpe, C., Knopf, P., Stützel, T. et al. Diversity and evolution of leaf anatomical characters in Taxaceae s.l.—fluorescence microscopy reveals new delimitating characters. J Plant Res 131, 125–141 (2018). https://doi.org/10.1007/s10265-017-0973-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0973-x