Abstract
Chromosome number and genome size are important cytological characters that significantly influence various organismal traits. We investigated chromosome number and genome size variation in 73 accessions belonging to four Colocasia species from China. Five different chromosome counts (2n = 26, 28, 38, 42, and 56) were found, the largest one representing a new record in Colocasia. The basic chromosome numbers are x = 13, 14, and 19, corresponding to 2x, 3x, and 4x cytotypes. Yunnan Province, China is considered the center of Colocasia polyploid origin. The 2C values in our accessions ranged from 3.29 pg in C. gigantea to 12.51 pg in C. esculenta. All species exhibit inter- and intraspecific chromosomal variation. Differences in DNA content among the Colocasia species seem to have occurred by chromosomal gain under similar habitats. Polyploidization also obviously contributes to 2C value variation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Colocasia Schott (Araceae) contains taxa of edible, medicinal, ornamental, and cultural importance, of which taro (Colocasia esculenta (L.) Schott) is probably the best known (Ahmed 2014; Das and Das 2014; Matthews 2006). It comprises about 20 species, mainly distributed in tropical and subtropical Asia and Oceania (Li and Boyce 2010), with six species endemic to Yunnan Province, China. Yunnan appears to be the main diversity center for the genus (Cao and Long 2004; Li and Boyce 2010).
The relevance of karyological study to the knowledge of the systematics and evolution of Colocasia has long been noted. Previous cytological investigation has reported the basic chromosome number to be x = 14, and to be constant throughout the genus (Yang et al. 2003). Other studies have claimed that chromosomal variation corresponds to different ploidy levels plus several aneuploids (Cao and Long 2004; Chakraborty and Bhattacharya 1984; Huang et al. 2012; Kuruvilla and Singh 1981; Petersen 1989; Sreekumari and Mathew 1991a, b). Furthermore, some authors claim that, in addition to constancy of basic chromosome number, Colocasia species also display a morphological uniformity of chromosomes and a homogeneous karyotype arrangement (Kuruvilla and Singh 1981). However, others have found enough interspecific karyotype differences to allow species characterization (Coates et al. 1988; Yang et al. 2003). Such discrepancies have also been observed at the intraspecific level, mainly in the widely studied C. esculenta (L.) Schott. (Das and Das 2014; Nakayama et al. 2008; Parvin et al. 2008; Sreekumari and Mathew 1991a, b). Previous karyological studies have predominantly concentrated on Colocasia members from regions outside of China, and have been limited to chromosome number; thus there is a paucity of data for Chinese species so far.
In addition to chromosome number and ploidy level, genome size (nuclear DNA content) also provides useful information in many fields of plant biology, including systematics, evolution, and conservation (Bennett and Leitch 2005). Nuclear DNA content has previously been reported for only two Colocasia species. Bennett MD and Smith JB (unpublished data 1987) determined 2C = 6.65 pg in C. antiquorum, and Baum A, Kenton AY and Bennett MD (pers. commun. 1989) found 2C = 8.15 pg in C. esculenta (L.) Schott. Colocasia esculenta was also investigated by Das and Das (2014), who observed 4C values ranging from 7.24 to 18.24 pg in 10 cultivars. In our present study, four species of Colocasia from China were examined in greater detail than so in prior reports. We report the variation in ploidy level, chromosome number and morphology, and DNA content among different populations.
Materials and methods
Plant material
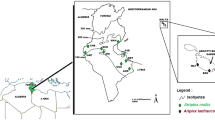
In total, 73 individual plants belonging to four Colocasia species from China were included in our study (Table 1; Fig. 1). The number of individuals per species varied from five to 44. Living plants were cultivated in a greenhouse at the Kunming Institute of Botany. Voucher specimens were deposited in Herbarium, Kunming Institute of Botany, Chinese Academy of Sciences.
Chromosome number
Root tips were collected from each individual and pretreated with a solution of 0.002 mol/L 8-hydroxyquinoline at 20–21 °C for 4–5 h. After fixation for 50 min by Carnoy solution (3:1 ethanol:acetic acid) at 4 °C, the root tips were dissociated in a mixture of 1 N HCl and 45% acetic acid (1:1) at 60 °C for 30 s, stained with 1% acetic orcein for 2–3 h and squashed on a glass slide (Wang et al. 2013).
Chromosome numbers were determined for each accession from at least 50 cells of at least two seedlings for mitotic observations. Mitotic interphase nuclei and prophase chromosomes preparations followed Tanaka (1971, 1977, 1987), and the designation of the centromeric position follows Levan et al. (1964). Karyotype asymmetry was classified according to Stebbins (1971).
Determining 2C values
About 0.5 cm2 of leaf material was finely diced using a new razor blade in a Petri dish containing 1500–2000 µL of WPB nuclear solution buffer (0.2 mol L−1 Tris·HCl, 4 mmol L−1 MgCl·6H2O, 2 mmol L−1 EDTA Na2·2H2O, 86 mmol L−1 NaCl, 10 mmol L−1 Na2S2O5, 1% PVP-10, 1% (v/v) Triton X-100, pH 7.5) (Tian et al. 2011). The nuclear suspension was then filtered through disposable filters (30 μm) to remove cell debris, and then stained with 150 μL propidium iodide (PI) [50 μg mL−1; including RNAse (500 μg mL−1)] for 10 min. Samples were analyzed on a CyFlow Space (Partec, Münster, Germany) flow cytometer equipped with a blue laser operating at 488 nm. At least 5000 nuclei were measured for all samples. FlowMax ver. 2.82 was used to analyze the resulting histograms. Z. mays (B73) (2C = 5.43 pg) was chosen as an internal standard. The 2C value of a sample was calculated as follows: (mean of sample peak/mean of standard peak) × 2C value (pg) of the standard species (Table 1).
Results
Chromosome number and ploidy level
Chromosome number and ploidy level for all samples are listed in Table 1. All accessions were karyologically analyzed (supplementary figure). Five different chromosome numbers were identified (2n = 26, 28, 38, 42, and 56). Most of these are x = 14, which we regard to be the genuine basic chromosome number. Plants with 26 and 38 somatic chromosomes have previously been found in three Colocasia species (Onwueme 1978; Chaudhuri and Sharma 1979); C. gigantea and C. esculenta have 2n = 26, and C. antiquorum has 2n = 38. It appears that two other basic numbers (x = 13 and 19) have also occurred in the genus.
Genome size variation
Genome size was analyzed in 73 accessions of the genus Colocasia (Table 1). The 2C DNA content ranged from 3.29 pg for the diploid population of C. gigantea to 12.51 pg for the triploid sample of C. esculenta. The 1Cx value, which indicates DNA content per genome, ranged from 1.55 pg in C. antiquorum to 5.72 pg in C. antiquorum. Diploid species showed 2C values between 3.29 and 11.43 pg. Triploid species ranged from 4.65 to 12.51 pg, and the only tetraploid taxon (C. esculenta) had a 2C value of 6.56 pg. The dispersion diagram in Fig. 2 shows the diploid species to have a larger variation, which also occurs in triploids.
Discussion
General karyotype characteristics
The study reveals a detailed picture of chromosome features and patterns of karyotype variation in Colocasia. Our karyological analyses reveal five different chromosome counts, 2n = 26, 28, 38, 42, and 56. The somatic numbers 28 and 42 predominate; other counts were encountered in one or two taxa only (2n = 26 in C. gigantea and C. esculenta, 2n = 38 in C. antiquorum, and 2n = 56 in C. esculenta). The 2n value of 56 represents a new, and the largest yet found, chromosome number for Colocasia. Inter- and intraspecific variation in chromosome number occurred in all studied accessions.
Karyotype formula have a great uniformity among accessions of each species. At inter- and intraspecific levels, the most variable characters are type and number. The Colocasia species karyotypes we analyzed included exclusively M, m, and sm chromosomes, with st only being present in three accessions, which is inconsistent with previous studies (Cao and Long 2004; Yang et al. 2003).
Basic chromosome number and ploidy level variation
The basic Colocasia chromosome number has been a matter of continuous dispute since karyological surveys began in the genus. Several different chromosome numbers have been estimated for Colocasia, including 2n counts of 26, 28, 30, 36, 38, 42, 44, 46, 52, 58, 60, 65, 72, 84, and 116 (Cao and Long 2004; Ramachandran 1978; Subramanian 1979; Yang et al. 2003), and x values of 7 and 12, while the basic chromosome number 14 has been repeatedly suggested (Coates et al. 1988; Darington and Wylie 1955; Krishnan and Magoon 1977). Kawahara (1978) suggests that plants observed with a basic chromosome number of 12 have either been misidentified as Colocasia species, or that chromosome counts have been inaccurate. In our study Colocasia esculenta is the only Colocasia species with various intraspecific 2n chromosome and basic chromosome numbers. Populations with a chromosome number of 2n = 42 are triploid with a basic chromosome number of x = 14, but are not hexaploid with a basic chromosome number of x = 7 (Yang et al. 2003). Combining our results with previous studies, x = 14 should still be considered the primary basic number. We also observed different chromosome basic numbers in another two species, C. gigantea and C. esculenta (2n = 26), and C. antiquorum (2n = 38), with x = 13 and 19. These two numbers are probably the result of secondary increases or reductions (Leong-Škorničková et al. 2007). This indicates that Colocasia is undergoing drastic differentiation. Following this logic, species investigated here correspond to 2x, 3x, and 4x cytotypes.
Chromosomes and evolution
The chromosome numbers observed in Colocasia species suggest that numerical changes may have been important in the evolution of the genus. Data from chromosome counts and 2C values confirm that ploidy/2C value levels have obvious differences in different species and in different accessions within a particular species. Our analyses show that about 51% accessions of Colocasia species are polyploidy in Yunnan Province. Therefore, our study corroborates Yunnan as the center of polyploid origin for Colocasia.
Chromosome size is also a feature subject to evolutionary change (Lavia et al. 2009). Chromosome evolution can either be directed toward an increase (Brandham and Doherty 1998) or a decrease in size (Martel et al. 2004). Furthermore, symmetrical karyotypes are widely accepted to be more primitive than asymmetrical ones (Stebbins 1971). Consider the chromosome length variation we observed, with a maximum L/S value of 3.27 in C. antiquorum, and a minimum value of 1.62 in C. esculenta, in relation to the AI values seen, ranging from 1.26 to 4.31: the type 2B C. antiquorum with AI = 4.31 has the highest asymmetric tendency in our study, and type 1A C. esculenta with AI = 1.26 has the smallest degree of asymmetry. Therefore, a possible evolutionary trend in Colocasia may be that species with smaller chromosomes and a more symmetric karyotype are more ancestral, while species with longer chromosome and more asymmetry are more derived. And, obviously, increases in chromosome arm length are accompanied by increases in karyotype asymmetry.
Difference in karyotype formula and interchromosomal asymmetries among species indicated that structure changes may lead to the diversity of the genus. Karyotype of Colocasia may be symmetrical and consisted almost exclusively of M, m, and sm chromosomes. These indicated that chromosome evolution in Colosacia may be constrained to nonrandom changes with particular restrictions for the occurrence or fixed of structural rearrangements (Seijo and Fernández 2003). The karyotype stability of Colocasia may be explained to orthoselection, which considers the occurrence of random chromosome mutation, but with the fixation of a restricted type of rearrangement (White 1978).
Large-scale analyses combining available genome size data for 3008 angiosperms have led to the proposal that genome downsizing is a widespread biological response to polyploidization leading to diploidization of polyploid genomes (Leitch and Bennett 2004). In our study, C. esculenta, the only tetraploid accession endemic to Lancang county, Pu’er City, is also in the group with no highest DNA content. Difference in DNA content between and within species could be caused by the loss or gain of entire chromosomes or by change in chromosome size (Dart et al. 2004). Chromosomes are quite homogenous among the Colocasia species. We infer that the differences in DNA content among the Colocasia species we analyzed are caused by chromosomal gain under similar habitats. Polyploidization is obviously another possible contributor to 2C value variation. In this study, 2C values in some accessions were not proportional to ploidy levels. Reduction in DNA content as ploidy levels increase may be a necessary adaptation for the establishment and stabilization of polyploidy genomes (Ozkan et al. 2003; Tuna et al. 2001).
References
Ahmed I (2014) Evolutionary dynamics in taro (Colocasia esculenta L.). Dissertation, Massey University
Bennett MD, Leitch IJ (2005) Nuclear DNA amounts in angiosperms: progress, problems, and prospects. Ann Bot 95:45–90
Brandham PE, Doherty MJ (1998) Genome size variation in the Aloaceae, an angiosperm family displaying karyotypic orthoselection. Ann Bot 82:67–73
Cao LM, Long CL (2004) Chromosome numbers of eight Colocasia taxa and karyotypes of five species occurring in China. Acta Bot Yunnan 26:310–316
Chakraborty BN, Bhattacharya GN (1984) Desynapsis as well as inversion heterozygosity in the natural population of triploid Colocasia antiquorm Schott. Cytologia 49:739–743
Chaudhuri JB, Sharma A (1979) Chromosome studies in certain members of Araceae. Genét Ibér 30–31:161–188
Coates DJ, Yen DE, Gaffey PM (1988) Chromosome variation in Taro, Colocasia esculenta: implications for origin in the Pacific. Cytologia 53:551–560
Darington CD, Wylie AP (1955) Chromosome atlas of flowering plants. George Allen and Unwin Ltd, London
Dart S, Kron P, Mable BK (2004) Characterizing polyploidy in Arabidopsis lyrata using chromosome counts and flow cytometry. Can J Bot 82:185–197
Das A, Das AB (2014) Karyotype analysis of ten draught resistant cultivars of Indian taro—Colocasia esculenta cv. antiquorom Schott. Nucleus 57:113–120
Huang XF, Ke WD, Liu YM, Ye YY, Li SM, Peng J, Liu YP, Li F (2012) Chromosomal ploidy identification of Taro (Colocasia) germplasm resources. China Veget 6:42–46
Kawahara T (1978) Chromosome number of taros in Nepal and India. Chrom Info Serv 24:4–5
Krishnan R, Magoon ML (1977) Edible aroids-new insights into phylogeny. In: Leakey CLA (ed) Proceedings of the 3rd international symposium on tropical root crops, International Institute of Tropical Agriculture, Ibadan, pp 58–60
Kuruvilla KM, Singh A (1981) Karyotypic and electrophoretic studies on taro and its origin. Euphytica 30:405–413
Lavia GI, Ortiz AM, Fernández A (2009) Karyotypic studies in wild germplasm of Arachis (Leguminosae). Genet Resour Crop Evol 56:755–764
Leitch IJ, Bennett MD (2004) Genome downsizing in polyploid plants. Biol J Linn Soc 82:651–663
Leong-Škorničková J, Šída O, Jarolímová V, Sabu M, Fér T, Trávníček P, Suda J (2007) Chromosome numbers and genome size variation in Indian species of Curcuma (Zingiberaceae). Ann Bot 100:505–526
Levan A, Fedga K, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220
Li H, Boyce PC (2010) Colocasia. In: Wu ZY, Raven PH, Hong DY (eds) Flora of China, vol 23. Science Press, St. Louis, pp 73–75
Martel E, Poncet V, Lamy F, Siljak-Yakovlev S, Lejeune B, Sarr A (2004) Chromosome evolution of Pennisetum species (Poaceae): implications of ITS phylogeny. Plant Syst Evol 249:139–149
Matthews PJ (2006) Written records of taro in the Eastern Mediterranean. In: Ertug ZF (ed) Proceedings of the IVth International Congress of Ethnobotany (ICEB 2005), Yeditepe University, Istanbul pp 419–426
Nakayama S, Uga Y, Maw-Oo T, Kawase M (2008) Chromosomes and 5S rDNA-repeats of wild taro Colocasia esculenta from Myanmar. J Agric Rural Dev Trop 52:32–35
Onwueme IC (1978) The tropical tuber crops: yam, cassava, sweet potato and cocoyam. Wiley, Chichester
Ozkan H, Tuna M, Arumuganathan K (2003) Nonadditive changes in genome size during allopolyploidization in the wheat (Aegilops-Triticum) group. J Hered 94:260–264
Parvin S, Kabir G, Ud-Deen MM, Sarker JK (2008) Karyotype analysis of seven varieties of Taro Colocasia esculenta (L.) Schott. from Bangladesh. J Bio Sci 16:15–18
Paszko B (2006) A critical review and a new proposal of karyotype asymmetry indices. Plant Syst Evol 258:39–48
Petersen G (1989) Cytology and systematics of Araceae. Nord J Bot 9:119–166
Ramachandran K (1978) Cytological studies on South Indian Araceae. Cytologia 43:289–303
Seijo JG, Fernández A (2003) Karyotype analysis and chromosome evolution in South American species of Lathyrus (Leguminosae). Amer J Bot 90:980–987
Sreekumari MT, Mathew (1991a) Karyomorphology of five morphotypes of taro (Colocasia esculenta (L.) Schott). Cytologia 56:215–218
Sreekumari MT, Mathew (1991b) Karyotypically distinct morphotypes in taro (Colocasia esculenta (L.) Schott). Cytologia 56:399–402
Stebbins GL (1971) Chromosomal evolution in higher plants. Edward Arnold, London
Subramanian D (1979) Cytological studies in Colocasia antiquorum Schott. J Cytol Gene 14:179–184
Tanaka R (1971) Types of resting nuclei in Orchidaceae. Bot Mag Tokyo 84:118–122
Tanaka R (1977) Recent karyotype studies. In: Ogawa K, Koike S, Kurosumi I Sato M (eds) Plant cytology. Asakura Publisher, Tokyo, pp 293–326
Tanaka R (1987) The karyotype theory and wide crossing as an example in Orchidaceae. In: Hong DY (ed) Plant chromosome research 1989, Proceedings of the Sino-Japanese Symposium on Plant Chromosomes, Hiroshima pp 1–10
Tian XM, Zhou XY, Gong N (2011) Applications of flow cytometry in plant research—analysis of nuclear DNA content and ploidy level in plant cells. Chin Agric Sci Bull 27:21–27
Tuna M, Vogel KP, Arumuganathan K, Gill KS (2001) DNA content and ploidy determination of bromegrass germplasm accessions by flow cytometry. Crop Sci 41:1629–1634
Wang GY, Meng Y, Yang YP (2013) Karyological analyses of 33 species of the tribe Ophiopogoneae (Liliaceae) from Southwest China. J Plant Res 126:597–604
White MJD (1978) Modes of speciation. W. H. Freeman and Company, San Francisco
Yang ZY, Yi TS, Li H, Gong X (2003) A cytological study on three species of Colocasia (Araceae) from Yunnan. Caryologia 56:323–327
Acknowledgements
We are grateful to Professor Chen Yu for providing some necessary materials, and Ms. Zhang Chunling for her important contributions in experiment. The work was financially supported by the National Natural Science Foundation of China (NSFC) (31590823, 41271058), the Basic Research Project of Ministry of Science and Technology of China (2012FY111400), and the General Project of Natural Science Research in Anhui Province (AQKJ2015B018), and the Key Project of Natural Science Research of Education Department in Anhui (KJ2017A358).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guang-Yan Wang and Xiao-Ming Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, GY., Zhang, XM., Qian, M. et al. Chromosome number and genome size variation in Colocasia (Araceae) from China. J Plant Res 130, 989–997 (2017). https://doi.org/10.1007/s10265-017-0959-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0959-8