Abstract
In-vitro studies of the ontogeny and mating system of the gametophytes of Lepisorus nudus were carried out through multispore and isolate cultures lasting 23 weeks. Spore germination begins early, on day 5–6. Spore germination pattern was Vittaria type and the germination percentage reached 82.69% (± 3.20%). Filamentous gametophyte did not branch and never produce separate prothalli. Occasionally the branching and separate prothalli were produced from mature and cordate gametophytes. Prothallial development was Drynaria type (cordate gametophytes with notched apex) contrary to other known species of Lepisorus, where gametophyte development was Kaulinia type (strap gametophytes without apical notch). Gametophyte production in multispore cultures reached up-to 75.6% (± 18.85%). All isolates initially produced archegonia and antheridia only after a prolonged cessation of production in archegonia. In contrast, only 37.2% (±12.63%) of individuals in multispore culture exhibited the same pattern with 29.8% (±7.56%) developing as males that did not produce archegonia by the end of the study. Only 37.2% (±12.63%) of archegoniate gametophytes developed antheridia by the end of the study and only once archegonia had degenerated; i.e., a temporal gap existed in expression of female and male gametangia. In multispore culture, only 26.21% (±5.70%) sporophytes developed on 160th day by fusion of female and male gametes that were derived from matings between sib gametophytes. In contrast, isolated gametophytes did not produce sporophytes. In isolate gametophytes, mature archegonia could not take delivery of male gametangia because antheridia were produced sequentially. This study suggests that the sequential expression of gametangia and absence of the intragametophytic selfing may also be a possible cause of reproductive barriers. Lepisorus nudus promotes inter-gametophytic selfing as an adaptive mechanism for reproductive success in multispore culture. This study presents a detailed account on reproductive biology of the taxa whose population is decreasing at distressing rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lepisorus nudus (Hook.) Ching is a fern belonging to the family Polypodiaceae. The genus comprises about 95 species globally (Hassler and Swale 2001). The genus is cosmopolitan but most of the species inhabit subtropical to tropical rain forest regions of the old world (Mitsuta 1981; Wang et al. 2010, 2011) with, 40 species in Asia (Hennipman et al. 1990; Liu et al. 2008; Qi and Zhang 2009; Zink 1993) and 18 species in India (Bir and Trikha 1974; Chandra 2000; Dixit 1984; Singh et al. 2009).Unfortunately, no attempts were made to emphasise the reproductive barriers and cause of population shrinkage for the species of genus Lepisorus. Most species of Lepisorus are epiphytic but some of them are terricolous to saxicolous. In spite of their adaptability to grow in varied habitats such as epiphytic, terricolous, saxicolous (Nampy and Madhusoodanan 1998; Negi et al. 2009) and being opportunistic colonizer (Personal observation; Schneider et al. 2004; Smith 1972), some species of Lepisorus are becoming rare and localized in their distribution (Chandra et al. 2008). Lepisorus nudus, an epiphytic (occasionally terricolous to saxicolous) fern has a cosmopolitan distribution in Bhutan, China, Central South Africa, Japan, Sumatra, Sri Lanka, North Thailand and Tibet (Chandra 2000; Tagawa and Iwatsuki 1972); however, it is scarcely distributed in the tropics of the Indian Himalayas and mountainous regions of central India with a narrow and receding population due to unknown reasons (Chandra 2000; Personal observations). Little is known about the cause of reproductive failure in ferns necessitating in-vitro investigations (Behera et al.2011; Flinn 2006; Haig and Westoby 1988; Nayar and Kaur 1971; Ranker et al. 2000; Ranker and Houston 2002). Some reproductive barriers become operative during the gametophyte development, which results inappropriate establishment of sporophytes. These barriers including other external factors could tend various adaptive mechanism of reproductive behaviour such as intra-gametophytic selfing, inter-gametophytic selfing, inter-gametophytic crossing. Amongst them the intra-gametophytic selfing had been the predominant reproductive behaviour in homosporous ferns (Crist and Farrar 1983; Klekowski 1979; Soltis and Soltis 1986) and a normal trend in polyploids (Chiou et al. 2002). In polyploid species with intra-gametophytic selfing, it is known that the duplicated loci mitigate the problems of recessive deleterious allele expression associated with selfing (Chiou et al. 2002; Klekowski and Baker 1966; Masuyama and Watano 1990), but also reflects a genetic bottleneck associated with the origin of polyploid species (Chiou et al. 2002). The inbreeding depression representing from recessive lethal genes could also be the primary factor preventing intra-gametophytic selfing (Haufler et al. 1990). In this way the inter-gametophytic fertilization could be an adaptive strategy to avoid genetic homozygosity, but it does not assure the integrity of a set gene pool in any pre-established species population over the years. It must bring some variability in the genetic pool through inter-gametophytic fertilization and change in the reproductive behaviours of the parent population. Such genetic variation gradually takes place in subsequent generations, which in the long term results in a number of genetic changes involved in speciation and new species evolution. Failure to attain bisexuality and asynchronous maturation of antheridia and archegonia also promotes out-crossing in potentially bisexual gametophytes (Chiou et al. 2002; Klekowski 1968). Such reproductive barriers might be playing a vital role in reducing the population of L. nudus and switching them towards rarity. To understand these barriers and ecology of this opportunistic colonizer, in-vitro studies on the biology of L. nudus have been conducted. Study of the reproductive biology of L. nudus inform about the development of gametophytes, reproductive behaviour, ecology of this species to contribute a large body of inquiry regarding ferns, particularly opportunistic (epiphyte, terricolous and saxicolous) fern colonization. Present study deals with spore germination, gametophyte development and sexual behaviour to understand the development of gametophytes, reproductive behaviour, ecology of this species and to interpolate that the inter-gametophytic selfing and asynchronous maturation of gametangia have been possible reproductive bottlenecks in Lepisorus nudus in addition to other external factors.
Materials and methods
Survey and collection of plant materials
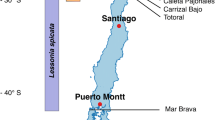
Survey and sampling of populations of Lepisorus nudus was conducted in 29 localities (comprising 4926.28 km2 area) of the forests in Pachmarhi Biosphere Reserve (PBR) of Madhya Pradesh in central India during May 2009, September 2011 and Almora of Uttarakhand in the western Himalayas during September 2010. Two populations of the species were marked in two localities viz. Brindavan, Mahadev of PBR and three populations in the forests of Almora (Kasar Devi Temple Dhanaulti).
Herbarium and specimens examined
India: Madhya Pradesh: Pachmarhi Biosphere Reserve, Brindavan, 22°28.922′N, 78°26.901′E, 3140 ft, 11.05.2009, leg. A.P. Singh & S.K. Behera 250812 (LWG); Mahadev, 22°25.053′N, 78°25.126′E, 3411ft, 07.09.2011, leg. A.P. Singh & P.B. Khare 251213 (LWG), det. A.P. Singh & P.B. Khare; Western Himalayas: Uttarakhand: Almora, Kasar Devi Temple Dhanaulti, 29°64.199′N, 79°66.085′E, 6000 ft, 08.09.2010, leg. A.P. Singh & P.B. Khare 251053 (LWG), det. A.P. Singh & P.B. Khare.Voucher specimens were deposited in the Herbarium of National Botanical Research Institute, Lucknow (LWG).
Spore sampling
One fertile frond was collected from each of the sporophytes population of L. nudus from the forests of Kasar Devi Temple Dhanaulti in Almora, Uttarakhand (western Himalayas) India. Spores were collected from single frond by drying the frond at room temperature and subsequently transferring them to desiccators.
Spore sterilization and culture media establishment
Spores collected from a single frond were surface sterilized with 2% sodium hypochlorite solution (for 2 min) and subsequently rinsed with sterilized double distilled water (three times). The culture media comprising macro (Parker’s) and micro (Thompson’s) elements were prepared in 1 L distilled water, solidified with agar and autoclaved (Klekowski 1969a, b; Klekowski and Lloyd 1968). The pH of the media was maintained at 5.6 to ensure the easy availability of required ions.
Spore sowing and observation
Spores were sown on the petridishes (8.0 cm diameter) containing culture media within 3 months from the day of frond collection. The spore density had a range of 14–26 spores per 16 mm field diameter in each of the five petridishes. The culture petridishes (5 replicates) containing spores (harvested from a single frond of an individual population) were kept in the laboratory condition at 50 µmol m−2 sec−1 light intensity with a photoperiod of 10:14 h (light and dark) and at a temperature of 25 ± 1 °C lasting 23 weeks for spore germination, gametophyte development, sex gamete expression and sporophyte production (Fig. 1 a–o). In order to test L. nudus abilities for intra-gametophytic selfing versus inter-gametophytic selfing, sequence of gender expression, spathulate gametophytes were isolated from one of the multispore cultures. These isolated spathulate gametophytes (from a multispore culture) allowed growing individually in smaller (4.5 cm diameter) petridishes (20 replicates) containing similar culture media and ambient conditions (Fig. 2a–e). The spore germination, pattern of gametophyte development, differentiation, sex organ expression, sporophyte production and development were observed for 160 days under Nikon eclipse 80i Microscope at an interval of 7-days and the photographs were taken using camera DS Fi 1.
(a–o): Lepisorus nudus (Hook.) Ching sown on multispore culture. a Spore sown on P&T culture media (1st day). b Filamentous stage (3-celled) with proliferating rhizoidal initial (8th day). c Filamentous stage (4-celled) with proliferating rhizoidal initial (16th day). d Filamentous stage (5–6 celled) with dividing nucleus and discrete chloroplast (24th day). e Filamentous stage (6–7 celled) with decentralizing chlorophyllous content (32nd day). f Spathulate stage (4 celled wide) with uniseriate (2–3 celled) basal filament (40th day). g–i Spathulate stages with apical meristematic cells (48, 52 and 60th day). j–k Cordate gametophytes with apical notch and meristem cells (70 and76th day). l Gametophyte with conspicuous undulate margin (86th day). m Gametophyte bearing archegonia (98th day). n Gametophyte bearing antheridia (120th day). o Juvenile vascularised sporophyte (160th day)
a–e Lepisorus nudus (Hook.) Ching sown on isolate culture. a Spathulate gametophyte with apical small meristematic and uniseriate (3–5 celled) basal cells (48th day). b–c Semi-cordate gametophytes with slight notched apex and apical meristematic cells (56 and 60th day). d Cordate gametophytes with conspicuous margins bearing archegonia (98th day). e Gametophyte bearing numerous archegonia (98th day)
Results
Spore, spore germination, rhizoids and filamentous stage
Spores 35–50 × 55–70 µm, ellipsoid, surface rugose or undulate without perine, tuberculate or foveolate, hyaline-yellowish with rather thick walled coat and monolete (Fig. 1a). Spores germinated from the proximal end on 5–6th days in culture media. Initial germination pattern was Vittaria type. The average germination percentage of spores in petridishes (5 replicates) reached up to 82.69% (±3.20%) (Table 1). Spore content turned green with an opening of a small aperture on proximal end. The green content shifted towards aperture as a result single cell (with dense chlorophyllous content) protrudes out and germination began. The single cell divided transversely for outward appearance of two celled stage. Another division occurred in the apical cell of two celled stage resulting in a three celled filamentous stage on the 8th day. The chlorophyllous content of basal cell (enclosed in spore coat) moved towards the lateral side where rhizoid initiation began. Rhizoid initial proliferated laterally. The chlorophyllous content did not divide equally, as it was to a great extent in the apical than to the subsidiary cell (Fig. 1b). Subsequent transverse division occurred in three celled filamentous stage and as a result four celled stage was formed on the 16th day. A radical diffusion of the chlorophyllous content occurred in two basal cells so that content dispersed more homogeneously throughout the cells. In apical cell, the chlorophyllous content substantially condensed and impounded towards centre (Fig. 1c). Rapidly these contents shifted towards centre in a very definite pattern with appearance of dividing nucleus and discrete chloroplast around it. Chlorophyllous contents haulage towards apical and basal end of each cell‒more apparent in apical cells (Fig. 1d) on 24th day. Transverse division also turned the four-celled filament into six-celled stage. The basal cell (contained within spore coat) promotes rhizoidal proliferation. The contents of rhizoid condensed and became sub-hyaline. The cell next to the basal one became atypical and another rhizoidal initial proliferate out of it. Remarkable changes in the chlorophyllous content of cells were observed. These included prevalent transformations of pigment occurring in the apical cell, where the organised nucleus and discrete chloroplasts shuttle the pigment towards both regions of the cell (Fig. 1e) on 32nd day. Filamentous stage ended further divisions. Thereafter, the chlorophyllous content became thick and vertical division began as a sign of initiation of the spathulate stage. The prothallial developmental patterns are quite variable (Drynaria type) with previously known and described species (Kaulinia type).
Development of spathulate gametophyte
The chlorophyllous content of apical cell condensed with appearance of non-static nucleus and discrete chloroplast. A repetitive vertical division occurred in apical cell. Subsidiary cells divided vertically, as a result 2–4 celled wide spathulate stage was formed. Basal 2–3 cells remained uniseriate from where the rhizoids originate and proliferate. The marginal cells of the upper half of the spathulate gametophyte became smaller, thickened with dense chlorophyllous content on 40th day (Fig. 1f). During 40–48th days the upper half portions of the gametophyte (2–3 celled wide) divided vertically and horizontally to form 7–8 celled wide spathulate stage. In the basal (6–7 uniseriate cells) portion of gametophyte division occurred in upper 3–4 cells, otherwise the basal 3–4 cells remained uniseriate. Except marginal cells (in upper half portion of the gametophyte) all the cells remained homogeneous in chlorophyll content, shape and size. Comparatively, the marginal cells were smaller, thickened and densely chlorophyllous (being active meristematic cells). Rhizoids confined to the basal 3–4 uniseriate cells (Fig. 1g). In apical region the marginal cells divided continuously and shuffled the meristematic tissues into a vegetative one. This progressively widened the medial as well as apical regions of the spathulate gametophyte; as a result the spathulate gametophyte was transformed into semi-cordate stage. Except meristematic tissues, the discrete chloroplast became thick and more prevalent in other cells, particularly cells of the basal region. Numerous rhizoidal initials emerged individually or in bunches from cells. Spore coat remained with the basal most cell of the gametophyte on 52nd day (Fig. 1h). As a result of meristematic activities structural transformations occur in shape and size of the spathulate gametophyte. Apical meristematic cells divide in such a way so that apical notch became visible. Meristem cells in the proximity of the notch remain smaller and compact. These meristem cells were involved in shuttering (laterally) vegetative cells and continuously widened the spathulate gametophyte (Fig. 1i) on 60th day.
Development of cordate gametophyte
Smaller, compact meristem cells (localized in the apical regions) of spathulate gametophyte divided transfugally and periclinally. This meristem derived vegetative cells shuttle laterally that caused widening of the cordate gametophytes with prominent notch at the apex (Fig. 1j) on 62–70th days. The chlorophyllous content thickened and turned into a dark spherical granule like structure. Numerous cells in the basal region borne rhizoidal initial on 76th day, which later turned into hyaline rhizoids (Fig. 1k). In certain gametophytes, occasionally the meristem cells behave unusual, resulting in conspicuous gametophytes with elongated lobes and undulate margins (Fig. 1l) on 86th day. Cordate gametophyte matures during 62–70 days in multispore culture. The average percentage of cordate gametophytes produced in petridishes (5 replicates) is 75.6% (±18.85%) (Table 2). Gametophytes undergo a prolonged development phase, which is yet another issue of investigation, but the potential mechanism appears to be inbreeding depression caused by a high level of genetic load.
Development of gametangia (antheridia and archegonia) in multispore culture
After reaching cordate form, certain vegetative cells in the proximity of deep notch became distinct. These cells elongate by means of successive division, as a consequence 3–4-cell-long (comprised of four vertical neck canal cell) archegonium developed. The basal cell (egg cell) embedded in the gametophyte remained more distinct. Until this distinct cell matured, the neck canal cells including apical cell become brown and started degenerating on 98th day (Fig. 1m). The average percentage of the archegoniate gametophyte development in multispore culture of petridishes (5 replicates) was 37.2% (±12.63%) (Table 2). At this stage the archegoniate gametophyte did not possess antheridial initials or antheridia. In multispore culture (at this stage) few gametophytes also posses numerous distinct cells near the apical notch. These cells having dense chlorophyllous content became more distinct and elevate out to the vegetative cells. These cells became globose-spherical containing numerous granular structures which later mature into antheridia. The average percentage of the antheridiate gametophyte development in multispore culture (5 replicates) was 29.8% (±7.56%) (Table 2). Apex of the antheridia was covered with rounded lids. Granular structures in mature antheridia were transformed into multi-flagellate antherozoids. The antherozoids swim out from the antheridia in presence of the flooded and dripping droplets of condensed water from the culture media on 120th day (Fig. 1n).
Result of mating experiments and production of sporophytes in multispore culture
In multispore culture, the eggs were fertilized by fusion of male and female gametangia derived from different gametophytes (inter-gametophytic selfing), as none of the gametophytes showed hermaphroditic conditions during observation. The fertilized eggs (embryo) prolonged into vascularised prothallium like structure which in turn developed into juvenile sporophytes on 160th day (Fig. 1o). The average percentage of the sporophytes development in petridishes (5 replicates) of multispore culture was 26.21% (±5.70%).
Development of gametangia (antheridia and archegonia) and production of sporophytes among isolates
In isolate gametophyte (Fig. 2a–c) archegonia and antheridia emerged sequentially near the apical notch (Fig. 2d). Archegonia appeared on the 98th day after spore sowing (Fig. 2e) whereas antheridia appeared on the 120th day and onwards; the latter appeared only after the former began degeneration. Archegonia neck is short, comprised of 3–4 rows of cells with 3–4 cells in a row. Amongst 20 replicates certain gametophytes posses archegonia and a few antheridia, but they never become hermaphroditic to perform intra-gametophytic selfing. The observed gap in the production of archegonia and antheridia and concomitant degradation of archegonia, among the isolates prevented production of sporophytes. Prevention of sporophytes production confirmed failure of intragametophytic selfing amongst the isolates. Sequential expression of gender appears to prevent intragametophytic selfing. Failure of intragametophytic selfing and sporophyte production among the isolates appears to be due to inbreeding depression caused by a high level of genetic load.
Discussion
Lepisorus nudus having habitat plasticity (epiphytic, terricolous to saxicolous) is known to be declining for unknown reasons in the vicinity of their previously known localities in India. The earlier known localities of L. nudus in central India (Vasudeva 1995) and western Himalayas (Khullar 1994) now harbour only small populations of this species. It appears that some reproductive barriers become operative during the spore germination and gametophyte development, which results in inappropriate establishment of sporophytes.
To understand the real cause for inadequate establishment of sporophytes, reproductive barriers and ecology of the species, in-vitro studies on biology of L. nudus in multispore and isolate culture have been conducted. It is unlikely that in-vitro culture conditions reflect biology of fern gametophytes in nature. Nevertheless, there are many studies which conclude that the optimal conditions for spore germination often reflect optimal growth conditions for subsequent developmental stages in gametophytes of ferns (Nondorf et al. 2003; Paciencia and Prado 2004). In multispore culture, the germination was Vittaria type, where the rhizoidal and prothallial initial cells come forward perpendicular to each other, which was also noticed in some homosporous ferns (Nayar and Kaur 1968). In Lepisorus nudus, the germination began on 5th–6th days of spore sowing, which however is known to begin on 3–4 weeks in Lepisorus loriformis, L. thunbergianus, Weatherbya accedens and Polypodium vulgare of family Polypodiaceae (Nayar and Raza 1970). After germination, the first transverse division result two celled stage, where lower cell served as rhizoidal initial, however the upper one transformed into vegetative gametophyte as reported in a few homosporous ferns (Nayar and Kaur 1971) and Dipteris wallichii T. Moore (Behera et al. 2011). Filamentous gametophytes became six cells long, but did not branch and never produced separate prothalli as in other species of Polypodiaceous ferns (Nayar 1961, 1967; Nayar and Raza 1970). Occasionally the branching and separate prothalli were produced from mature cordate gametophytes on 86th day (Fig. 1l). Prothallial plate development began when the filamentous gametophytes were six cells long. Prothallial development in L. nudus was Drynaria type (cordate gametophyte with notched apex) in contrary to the Lepisorus loriformis (Nayar and Raza 1970), where gametophyte development was Kaulinia type (strap gametophyte without apical notch). The posterior half of the spathulate and cordate gametophytes remains 3–5 cells uniseriate like other Polypodiaceous species, except L. thunbergianus where it is one cell uniseriate. An obconical meristematic cell established in L. nudus where the prothallium became 7–10 cells broad at apex, which however is known to be very irregular, or 5 (±) cells broad in other allied species (Nayar and Raza 1970). The young cordate gametophytes in L. nudus were slightly larger than broad with notched apex and superficial rhizoids confined to lower half portion of prothallus. However, young gametophytes are narrow, elongated, ribbon-like form with cluster of marginal rhizoids and rounded apex in L. loriformis, and cordate with cluster of marginal as well as superficial rhizoids in L. thunbergianus, Weatherbya accedens, Polypodium vulgare (Nayar and Raza 1970). In L. nudus prothalli never branched except budding of some conspicuous gametophytes with elongate lobes and undulate margins (Fig. 1l) on 86th day, as was reported in Polypodium astrolepis on 4–8 months of sowing (Hooper and Haufler 1997). In contrary, the young cordate prothalli in L. loriformis, L. thunbergianus, W. accedens and P. vulgare are known to be branched, branches developing as broad ribbon-like protuberances close to the apex of thallus (Nayar and Raza 1970). Young cordate gametophytes of L. nudus exhibit organised meristem as in other species, except L. loriformis which does not exhibit organised meristem until the prothalli becomes 12–15 week old (Nayar and Raza 1970). In multispore, cordate gametophytes stage was achieved in 62–70 days. Such tendency of gametophyte production was also reported by Chiou and Farrar (1997), where they state that in general, the gametophyte begin to produce gametangia by the 60th day in Microgramma heterophylla and Polypodium pellucidum. In L. nudus gametophytes undergo a prolonged development phase, which is another issue of investigation. But the documented potential mechanism included antheridiogen activity and inbreeding depression caused by high level of genetic load (Hooper and Haufler 1997). Such mechanism may also be prevalent in L. nudus, as the gametophytes undergo a prolonged development phase.
Although, 62–70 days after sowing to reach a cordate adult pre-sexual shape in L. nudus is a very long time, it is not uncommon in Polypodiaceous ferns which are often observed to be growing slowly (Chiou and Farrar 1997). For most species the archegonia develop first and archegoniate gametophytes remained the most abundant over the 3 month (90 days) culture period (Chiou and Farrar 1997). The antheridia formation in L. nudus was also slow as it appeared on the 120 day of sowing. Such tendency of slow expression of antheridia at 75 day, even by treated with antheridiogen hormones were also known in a few Polypodiaceous ferns (Chiou and Farrar 1997).
Prothallia of L. nudus did not develop median midrib soon after they becomes cordate, as reported in other species (Nayar and Raza 1970). Prothallia were devoid of hairs and papillae, which are usually known to occur abundantly on margin or surface of the gametophytes in many other species. Their scarce and restricted occurrence to the lower surface in the midrib region of L. loriformis was also not uncommon (Nayar and Raza 1970). Most of the gametophytes were archegoniate whereas a few the antheridiate. Antheridia were produced on vigorously growing prothalli of L. nudus, however, it is often produced by prothalli from early filamentous stage in L. loriformis, L. thunbergianus, W. accedens and P. vulgare of polypodiaceous ferns. Nevertheless, there were reports that vigorously growing prothalli also produce antheridia in a few species (Nayar and Raza 1970). Archegonia neck in L. nudus is short, comprised of 3–4 rows of cells as usually occur in other species of Lepisorus (Nayar and Raza 1970), but the neck canal cells including apical cells of the archegonia became brown and started degenerating until antheridia matured (Fig. 1m). This is possibly another sort of genetic load in L. nudus. In multispore culture the archegoniate gametophytes (having archegonia) did not borne antheridia initials or any antheridia at any stage of observation. This tendency in multispore gametophyte showed that the gametophyte was possibly dioecious or it could have been monoecious, but with an extended periodicity gap in the male gametangia expression. This became clear in the observation of isolate gametophytes, where L. nudus showed monoecious sexuality with extended periodicity gap in the male gametangia appearance.
The gametophytes with mature antheridia also had archegonia vestiges. These vestiges were degenerated archegonia, which disintegrated soon or prior to the emergence of antheridia. This event supported the hypothesis that archegonia developed prior to antheridia (Behera et al. 2011; Haig and Westoby 1988) under rich growth conditions. In multispore culture a number of sporophytes were produced by fusion of male and female gametangia from different gametophytes (inter-gametophytic selfing) of common parental origin. There was validation that the “selfing” enables individual taxon to reproduce and colonize in a new location. Ability to self fertilize extends an opportunity to reproduce at large extant in one way enhancing the plant’s capacity for colonization in another way (Flinn 2006; Lloyd 1974; Ranker et al. 2000; Singh and Roy 1977; Verma 2003). Thus, in present investigation there was an opportunity for gametangia to bring about the selfing (intra-gametophytic selfing) or crossing (inter-gametophytic selfing) between gametophytes to establish and colonize the sporophytes in substantial manner, as there were 37.2% (±12.63%) archegoniate and 29.8% (±7.56%) antheridiate gametophytes. Nevertheless, in the case of L. nudus it is only in the multispore culture where sporophytes were reasonably [26.21% (±5.70%)] produced (Table 2) by virtue of inter-gametophytic selfing. Noticeably, not a single sporophyte was produced in isolate culture by intra-gametophytic selfing (Table 3), as in case of Dipteris wallichii (Behera et al. 2011). In isolate cultures of gametophyte the archegonia appeared on 98th day, however the antheridia on 120th day. This suggested that the bisexual prothalli were never hermaphroditic (presence of female and male) during the period of observation. There are many observations about prolonged phase of archegoniate gametophytes, even more than a year (Chiou and Farrar 1997; Hooper and Haufler 1997), but in L. nudus and other Polypodiaceous ferns the archegonia began degeneration during the emergence of antheridia on archegoniate gametophytes. It becomes imperative to mention the delayed antheridia expression as one of the considerable incidence. In isolate culture, expression of both the gametangia at a definite interval (female followed by male) determined that there was a periodicity gap in the gametangia expression. Henceforth, the gametic fusion between different gametangia arising from the same gametophyte did not occur. Isolate gametophyte culture confirmed failure of gametangial fusion (between the gametangia of same gametophyte) could be one of the main causes for unsuccessful establishment of sporophytes. As a consequence, the gametophyte could not bear sporophyte, confirming failure of intra-gametophytic selfing. It was observed that until archegonia reached maturity the antheridia were either lacking or at initial stage. Eventually the archegonia also began degeneration prior to the development of antheridia. This confirmed a gap of period in the expression of two different gametangia on an isolate gametophyte. Therefore, the archegonia and antheridia hardly get a chance to demeanour sexual fusion and that could be the reason for the failure of the sporophyte establishment in isolate culture (Verma 2003). Study concluded that L. nudus has adopted inter-gametophytic selfing (fusion of gametangia from two different gametophytes of common parental origin).
In spite of possessing both the gametangia and given opportunity of substantial circumstances, fertilization did not occur in isolate gametophytes for the reason of periodicity gap in gametangial expression. Altered periodicity of gametangial expression could be the foremost reproductive barrier. In certain ferns the antheridia initiation is known to be induced by counterparts releasing antheridiogen hormones (Naf et al. 1975; Prada et al. 2008). Similarly, some sort of biochemical complex including antheridiogen activities and genetic load might be initiating the sex gamete expression and periodicity gap in L. nudus which is in need of further investigation.
Furthermore, the isolate gametophytes exhibit periodicity gap in gametangial expression (female followed by male) and early degeneration of archegonia. This phenomenon confirms that the sequence of gametangia maturation greatly limits if not precludes selfing. The temporal gap of gametangia expression in L. nudus is a symptomatic possibility of inbreeding depression caused by high levels of genetic load, in addition to many other reasons as also speculated by Hooper and Haufler (1997).
References
Behera SK, Rawat VK, Singh AP, Khare PB (2011) Studies on the spore germination, developmental pattern and sexuality of gametophyte in Dipteris wallichii (R. Br. ex Hook. et Grev.) T. Moore. Indian Fern J 28:172–178
Bir SS, Trikha CK (1974) Taxonomic revision of the Polypodiaceous genera of India-VI. Lepisorus excavatus Group. Am Fern J 64:49–63
Chandra S (2000) The ferns of India (Enumeration, Synonyms and Distribution). International Book Publisher and Distributors, Dehradun p (i–xii) 1–459
Chandra S, Fraser-Jenkins CR, Kumari A, Srivastava A (2008) A summary of the status of threatened Pteridophytes of India. Taiwania 53:170–209
Chiou WL, Farrar DR (1997) Antheridiogen production and response in Polypodiaceae species. Am J Bot 84:633–640
Chiou WL, Farrar DR, Ranker TA (2002) The mating systems of some epiphytic Polypodiaceae. Am Fern J 92:65–79
Crist KC, Farrar DR (1983) Genetic load and long distance dispersal in Asplenium platyneuron. Can J Bot 61:1809–1814
Dixit RD (1984) A census of Indian Pteridophytes. Director, Botanical Survey of India, Howrah, p (i–iii) 1–177
Flinn KM (2006) Reproductive biology of three fern species may contribute to differential colonization success in post agricultural forests. Am J Bot 93:1289–1294
Haig D, Westoby M (1988) Sex expression in homosporus ferns: an evolutionary perspective. Evol Trend Plant 2:111–119
Hassler M, Swale B (2001) Checklist of ferns and fern allies. In: Hassler M, Swale B [com.], pp 1–872. http://homepages.caverock.net.nz/~bj/fern/list.htm. Accessed 28 Mar 2016
Haufler CH, Windham MD, Ranker TA (1990) Biosystematic analysis of the Cystopteris tennesseensis complex. Ann Mo Bot Gard 77:314–329
Hennipman E, Kramer KU, Veldhoen P (1990) Polypodiaceae. In: Kubitzki K (ed) The families and genera of vascular plants. Springer, Berlin, p 203–230
Hooper EA, Haufler CH (1997) Genetic diversity and breeding system in a group of neotropical epiphytic ferns (Pleopeltis; Polypodiaceae). Am J Bot 84:1664–1674
Khullar SP (1994) An illustrated fern flora of West Himalaya (I). International Book Publisher and Distributors, Dehradun, p (i–xxxx) 1–506
Klekowski EJ Jr, Baker HG (1966) Evolutionary significance of polyploidy in the Pteridophyta. Science 153:305–307
Klekowski EJ Jr (1968) Reproductive biology and evolution in the Pteridophyta. Ph. D. dissertation. University of California, Berkeley
Klekowski EJ Jr, Lloyd RM (1968) Reproductive biology of the Pteridophyta, I. General considerations and a study of Onoclea sensibilis L. J Linn Soc (Bot) 60:315–324
Klekowski EJ Jr (1969a) Reproductive biology of the Pteridophyta II, theoretical consideration. Bot J Linn Soc 62:347–359
Klekowski EJ Jr (1969b) Reproductive biology of the Pteridophyta III, a study of Blechnaceae. Bot J Linn Soc 62:361–377
Klekowski EJ Jr (1979) The genetics and reproductive biology of ferns. In: Dyer AF (ed) The experimental biology of ferns, Academic Press, London, pp 133–170
Liu QR, Ming GH, Ge Y, Zhang XC (2008) A taxonomic revision of Lepisorus sect. Hymenophyton Polypodiaceae from China. J Syst Evol 46:906–915
Lloyd RM (1974) Mating systems and genetic load in pioneer and non-pioneer Hawaiian Pteridophyta. Bot J Linn Soc 69:23–35
Masuyama S, Watano Y (1990) Trends for inbreeding in polyploid Pteridophytes. Plant Species Biol 5:13–17
Mitsuta S (1981) Venation of Lepisorus and Pleopeltis: Polypodiaceae. Acta Phytotaxon et Geobot 32:147–164
Naf U, Nakanishi K, Endo M (1975) On the physiology and chemistry of fern antheridiogens. Bot Rev 41:315–359
Nampy S, Madhusoodanan PV (1998) Fern flora of South India - taxonomic revision of Polypodioid ferns. Daya Publishing House, New Delhi
Nayar BK (1961) Studies in Polypodiaceae. VII. Morphology of the gametophyte of Lepisorus excavatus (Bory) Ching. Sci Cult 27:345–347
Nayar BK (1967) Morphology of the spores and prothallus of Christiopteris tricuspis. Am Fern J 57:15–27
Nayar BK, Kaur S (1968) Spore germination in homosporus ferns. J Palynol 4:1–14
Nayar BK, Kaur S (1971) Gametophytes of homosporus ferns. Bot Rev 37:295–396
Nayar BK, Raza F (1970) The prothalli of some Polypodiaceae–II. Lepisorus loriformis, L. thunbergianus, Polypodium vulgare and Weatherbya accedens. J Indian Bot Soc 49:81–86
Negi S, Tewari LM, Pangtey YPS, Kumar S, Martolia A, Jalal J, Upreti K (2009) Taxonomic studies on the family Polypodiaceae (Pteridophyta) of Nainital Uttarakhand. N Y Sci J 2:47–83
Nondorf LS, Dooley AM, Palmieri M, Swatzell J (2003) The effects of pH, temperature, light intensity, light quality, and moisture levels on spore germination in Cheilanthes feei of Southeast Missouri. Am Fern J 93:56–69
Paciencia MLB, Prado J (2004) Efeitos de borda sobre a comunidade de pteridófitas na Mata Atlântica da região de Uma, sul da Bahia, Brasil. Rev Bras de Bot 27:641–653
Prada C, Moreno V, Gabriel JM, Galan Y (2008) Gametophyte development, sex expression and antheridiogen system in Pteris incompleta Cav. (Pteridaceae). Am Fern J 98:14–25
Qi XP, Zhang XC (2009) Taxonomic revision of Lepisorus (J. Sm.) Ching sect. Lepisorus (Polypodiaceae) from China. J Syst Evol 47:581–598
Ranker TA, Houston HA (2002) Is gametophyte sexuality in the laboratory a good predictor of sexuality in nature? Am Fern J 92:112–118
Ranker TA, Gemill CEC, Trapp PG (2000) Microevolutionary pattern and processes of the native Hawaiian colonizing fern Odontosoria chinensis (Lindsaeaceae). Evol Int J Org Evol 54:828–839
Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Magallon S, Lupia R (2004) Ferns diversified in the shadow of angiosperms. Nature 428:553–557
Singh VP, Roy SK (1977) Mating systems and distribution in some tropical ferns. Ann Bot (Lond) 41:1055–1060
Singh AP, Mishra S, Gupta S, Behera SK, Khare PB (2009) Studies on the Genus Ophioglossum L. in Pachmarhi biosphere reserve, Madhya Pradesh-India. Taiwania 54:353–364
Smith AR (1972) Comparison of fern and flowering plant distributions with some evolutionary interpretations for ferns. Biotropica 4:4–9
Soltis DE, Soltis PS (1986) Electrophoretic evidence for inbreeding in the fern Botrychium virginianum (Ophioglossaceae). Am J Bot 74:504–509
Tagawa M, Iwatsuki K (1972) Families and genera of the pteridophytes known from Thailand. Mem Fac Sci Kyoto Univ Ser Biol 5:67–88
Vasudeva SM (1995) Peculiarities of pteridophytic flora of Pachmarhi, Satpura Hills (central India). Indian Fern J 12:29–42
Verma SC (2003) Some aspects of reproductive biology of the gametophyte generation of homosporus ferns. In: Chandra S, Srivastava M (eds) Pteridology in the New Millennium. Kluwer Academic Publishers, Netherland, p 455–484
Wang L, Qi XP, Xiang QP, Heinrichs J, Schneider H, Zhang XC (2010) Phylogeny of the paleotropical fern genus Lepisorus (Polypodiaceae, Polypodiopsida) inferred from four chloroplast DNA regions. Mol Phylogenet Evol 54:211–225
Wang L, Wu ZQ, Bystriakova N, Ansell SW, Xiang QP (2011) Phylogeography of the Sino-Himalayan fern Lepisorus clathratus on ‘‘the roof of the world’’. PLoS One 6:e25896. doi:10.1371/journal.pone.0025896
Zink MJ (1993) Systematics of the fern genus Lepisorus (J. Smith) Ching (Polypodiacea-Lepisoreae). Ph. D. Dissertation Zurich University, Zurich, p 3–30, 55–119
Acknowledgements
Authors are thankful to Director, CSIR-National Botanical Research Institute, Lucknow for providing infrastructural facilities. Thanks are also due to the Council of Scientific & Industrial Research, New Delhi for financial assistance under SIP-005 to support this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.P., Johari, D. & Khare, P.B. Studies on ontogeny and reproductive behaviour of Lepisorus nudus (Hook.) Ching (Polypodiaceae). J Plant Res 130, 281–290 (2017). https://doi.org/10.1007/s10265-016-0891-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0891-3