Abstract
The evolution of mycoheterotrophy has been accompanied by extreme reductions in plant leaf size and photosynthetic capacity. Partially mycoheterotrophic plants, which obtain carbon from both photosynthesis and their mycorrhizal fungi, include species with leaves of normal size and others that are tiny-leaved. Thus, plant species may lose their leaves in a gradual process of size reduction rather than through a single step mutation. Little is known about how the degree of mycoheterotrophy changes during reductions in leaf size. We compared the degree of mycoheterotrophy among five Japanese Cephalanthera species, four with leaves of normal size (Cephalanthera falcata, Cephalanthera erecta, Cephalanthera longibracteata and Cephalanthera longifolia), one with tiny leaves (Cephalanthera subaphylla), and one albino form of C. falcata (as reference specimens for fully mycoheterotrophic plants). The levels of mycoheterotrophy were determined by stable isotope natural abundance analysis. All Cephalanthera species were relatively enriched in 13C and 15N in comparison with surrounding autotrophic plants. Cephalanthera subaphylla was strongly enriched in 13C and 15N to levels similar to the albinos. Species with leaves of normal size were significantly less enriched in 13C than C. subaphylla and the albinos. Thus, C. subaphylla was strongly mycoheterotrophic, obtaining most of its carbon from mycorrhizal fungi even though it has tiny leaves; species with leaves of normal size were partially mycoheterotrophic. Hence, during the evolutionary pathway to full mycoheterotrophy, some plant species appear to have gained strong mycoheterotrophic abilities before completely losing foliage leaves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 880 species of vascular and non-vascular plants are mycoheterotrophic; they obtain carbon (C) from their associated mycorrhizal fungi (Merckx et al. 2013a). The evolution of mycoheterotrophy, which is a transition phase away from photoautotrophic nutrition, is accompanied by dramatic changes in plant morphology, especially an extreme reduction in leaf size (Merckx et al. 2013b). Leaves of mycoheterotrophs are generally reduced to very small achlorophyllous scales on the inflorescence axes (Merckx et al. 2013b). Such leafless and achlorophyllous species are referred to as fully mycoheterotrophic plants, while plant species that obtain C from both photosynthesis and mycorrhizal fungi are partially mycoheterotrophic plants (Gebauer and Meyer 2003; Merckx 2013). Partial mycoheterotroph lineages include species with well-developed foliage leaves and others with strongly reduced leaves, such as Epipactis microphylla (Selosse et al. 2004) and Pyrola aphylla, which are typically leafless but often produce small leaves (Hynson et al. 2009). The existence of mycoheterotrophs with small leaves suggests that leaf loss during the evolutionary process did not result from a single step mutation, but rather a gradual process of size reduction. Little is known regarding the extent to which the mycoheterotrophic mechanism has been affected by reductions in leaf size.
The genus Cephalanthera includes both fully and partially mycoheterotrophic species and an ideal model to trace the character evolution in accordance with the evolution of mycoheterotrophy (Roy et al. 2009; Taylor and Bruns 1997). Five Cephalanthera species are distributed in Japan. Cephalanthera falcata (Thunb.) Blume, C. erecta (Thunb.) Blume, C. longibracteata Blume and C. longifolia (L.) Fritsch have foliage leaves with normal dimensions, while C. subaphylla Miyabe et Kudô has extremely reduced leaves (Fig. 1). Cephalanthera subaphylla is sister to the normal-leaved C. erecta by molecular phylogenetic analysis (Y. Sakamoto, unpublished data). In our study, we compared the extent of mycoheterotrophy among four species with normal leaves and the small-leaved species C. subaphylla using stable isotope natural abundance analyses.
Isotopic analyses can be used to quantify fungal-derived organic C and nitrogen (N), thereby revealing the level of mycoheterotrophy in plant species (Gebauer and Meyer 2003). Previous studies have reported mycoheterotrophic levels in diverse Cephalanthera species (Bidartondo et al. 2004; Gebauer and Mayer 2003; Liebel et al. 2010). We evaluated the natural abundances of 13C and 15N stable isotopes in five Japanese Cephalanthera species. Since albino individuals of green Cephalanthera species are able to function as full mycoheterotrophs (Julou et al. 2005; Roy et al. 2013), we included the albinos of normal-leaved C. falcata as a reference with full mycoheterotrophy.
Materials and methods
Sample collection
Plant samples were collected from six sites in Japan (Table 1). The Mt. Aoba site was in an old forest dominated by Abies firma with a dense understory; the remaining sites were in an open, mixed stand dominated by Quercus myrsinifolia, Q. serrata and/or Q. acutissima accompanied by a species-rich understory vegetation. Cephalanthera subaphylla and C. longifolia were collected from the same sites (Mt. Aoba and Yokohama, respectively) during two different years. The albino and green individuals of C. falcata grew sympatrically at Machida B and Chigasaki. Leaves of Cephalanthera species and neighboring autotrophic reference plants were collected from five or six 1 m2 plots per site following the procedures of Gebauer and Meyer (2003) (Table 2). Single 1 m2 plots included Cephalanthera species and one to six reference plant species. Two green orchids, Cymbidium goeringii and Calanthe discolor, were also included in specimens taken from Machida A. Only fresh leaves from the current year were used for the analysis. Cephalanthera subaphylla is very small and sometimes provides inadequate leaf material for samples; in these cases, we included all fresh leaves and flowers in the analysis.
Isotopic analysis
Collected samples were dried at 105 °C for 48 h, ground to a fine powder and stored in silica gel until used. Relative C and N isotopic abundances were measured using an elemental analyzer (Thermo Fisher Scientific FLASH 2000) coupled to a continuous flow isotopic ratio mass spectrometer (IRMS; Finnigan MAT Delta PLUS, Thermo Electron, Bremen, Germany). Measured abundances are reported here as δ values calculated as follows: δ13C or δ15N = (Rsample/Rstandard − 1) × 1000 ‰, where R is the ratio of heavy isotope to light isotope in the sample or the respective standard. The standards were Pee-Dee Belemnite (C) and atmospheric N2 (N).
Calculation and statistics
δ values were normalized following the procedures of Preiss and Gebauer (2008) for our comparisons of plant C and N isotopic abundances among different sites and species. Enrichment factors (ε13C and ε15N) were calculated for each site using δ values for Cephalanthera species and the reference plants as follows: ε = δs − δREF, where δs is a single δ13C or δ15N value for a Cephalanthera species, and δREF is the mean value of all reference plants from the same site.
To quantify proportional C and N gains from fungi in Cephalanthera species, we applied a linear two-source mixing model procedure to data collected at Machida B and Chigasaki, where fully mycoheterotrophic albinos grew sympatrically with pigmented individuals. The proportional C and N gains of mycoheterotrophic origin in autotrophic plants at the same site were set to 0 %, and the gains of albino plants were set to 100 %. The proportional C and N gains from mycorrhizal fungi were calculated using the following expression (Gebauer and Meyer 2003): %xdf = (δxPMH − δxREF)/εMH × 100, where %xdf is the percentage of fungal-derived C or N in a partially mycoheterotrophic plant, δxPMH is the individual ε13C or ε15N value of a particular Cephalanthera species, δxREF is the mean δ13C or δ15N value of autotrophic reference plants from the same site, and εMH is the mean enrichment factor for fully mycoheterotrophic plants (albinos of C. falcata) from the same site (εMH = δxMH − δxREF).
The data were analyzed via a generalized linear model constructed using ‘R’ v. 3.0.1 software (R Development Core Team 2013). All post hoc tests were performed using the glht-function (Hothorn et al. 2008) in the R package ‘multcomp’, which performs multiple comparisons of the means using Tukey contrasts; P values <0.01 were considered statistically significant.
Results
Intraspecific variation
To compare isotope abundances of the Cephalanthera species at different sites, we calculated enrichment factors (ε) for each location; these factors were used to quantify differences between the reference and target species (Fig. 2; Table S2). A very high 13C enrichment was measured in the albinos and C. subaphylla populations. Cephalanthera subaphylla and C. longifolia, which were sampled from the same site during two different years, were significantly different in 13C values between 2013 and 2014, but 15N did not vary between years (Fig. 2). The mean 13C enrichments in C. falcata were highly variable among populations (4.6 ± 1.8 to −0.3 ± 0.8; Table S2). At Machida A, C. falcata was significantly depleted in 13C in comparison with other populations. The δ13C values for this species at this site were not significantly different from any of the autotrophic references or two green orchids growing at the same site (Table S1); however, C. erecta and C. longibracteata were enriched in 13C. The mean 15N enrichment of C. falcata ranged from 4.8 ± 1.6 to 8.9 ± 1.2; the enrichment of this species at Machida B significantly exceeded those at Machida A and Chigasaki. 13C and 15N values of C. erecta and C. longibracteata were not significantly different among populations (Fig. 2; Table S2).
Enrichment factors (ε) for 13C (a) and 15N (b) of Cephalanthera populations. The mean ± SD was calculated for each populations. Different letters indicate significant differences among populations of each species at P < 0.01 (a generalized linear model and Tukey post hoc test). Cf C. falcata, Ce C. erecta, Clb C. longibracteata, Clf C. longifolia, Cs C. subaphylla, Cf(a) albinos of C. falcata
We calculated the proportion of C derived from mycorrhizal fungi using a mixing-model. The mean %Cdf ± SD values of C. falcata from Machida B and Chigasaki were 65 ± 26 and 19 ± 17 %; the value for C. erecta from Machida B was 42 ± 10 %. The proportional N gains from fungi in C. falcata were 85 ± 12 and 65 ± 6 % in Machida B and Chigasaki, respectively; the proportional gain of C erecta was 118 ± 8 % in Machida B.
Interspecific variation
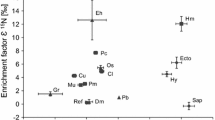
The mean 13C and 15N enrichments of the species are depicted in Fig. 3. All Cephalanthera species were relatively enriched in C (ε13C > 1.5) and N (ε15N > 5.9). The albinos and small-leaved C. subaphylla specimens were not significantly different, but both were significantly enriched in 13C in comparison with other Cephalanthera species. Cephalanthera subaphylla and the albinos were similarly highly enriched in 15N. Cephalanthera erecta had the highest level of 15N enrichment.
Discussion
All of the Cephalanthera species that we evaluated were relatively enriched in 13C and 15N (Fig. 3), indicating that they obtained C and N from their mycorrhizal fungi. Full mycoheterotrophy in the albinos was associated with strong enrichment in 13C and 15N (Figs. 2, 3). The small-leaved species C. subaphylla was also strongly enriched in 13C and 15N, the levels of which were not significantly different from those in the albinos, indicating that C. subaphylla was strongly mycoheterotrophic and obtained most of its carbon from mycorrhizal fungi and not from the small leaves borne by the plants. Single albino individual of C. subaphylla found on Mt. Aoba in 2014 was even more enriched than were green specimens of C. subaphylla collected at the same site (ε13C = 9.0, ε15N = 13.4). It is likely that green C. subaphylla plants obtain some C from their photosynthesis, but further study with larger samples is needed to confirm this fact.
The mycorrhizal fungi associated with the Cephalanthera species included in this study were identified through molecular genetic analyses by Sakamoto et al. (2015, 2016). Cephalanthera subaphylla specimens from Mt. Aoba sites dominated by ectomycorrhizal Abies firma trees were specifically associated with ectomycorrhizal basidiomycetes in the family Thelephoraceae. Mycoheterotrophic plants associated with ectomycorrhizal fungi obtain carbon from surrounding autotrophic plants via shared mycorrhizal mycelia. This association has been described as a ‘tripartite symbiosis’ (Merckx 2013). Cephalanthera subaphylla plants probably obtain C and N from thelephoracean mycorrhizal fungi that are also associated with surrounding autotrophic trees; Yagame and Yamato (2013) demonstrated such transfers in C. falcata. We showed that C. subaphylla has strong mycoheterotrophic abilities comparable to those of fully mycoheterotrophic plants. However, a more detailed analysis of samples from geographically separated populations growing in different forest types is required to fully evaluate mycoheterotrophy in C. subaphylla.
13C was significantly less enriched in the Cephalanthera species with normal leaves (C. falcata, C. erecta, C. longibracteata and C. longifolia) than in C. subaphylla and the albinos (Fig. 3), indicating that these species with normal leaves are partially mycoheterotrophic orchids. Partial mycoheterotrophy of European C. longifolia specimens has been demonstrated by isotopic analyses (Abadie et al. 2006, Liebel et al. 2010, Johansson et al. 2015). The Cephalanthera species with normal leaves that we evaluated are associated with thelephoracean fungi that are not phylogenetically distinct from the fungi associated with C. subaphylla. Furthermore, C. falcata and C. longibracteata are additionally associated with basidiomycete ectomycorrhizal fungi in the Russulaceae and Sebacinales (Sakamoto et al. 2015, 2016). Thus, Cephalanthera species with normal leaves that grew in a habitat with abundant ectomycorrhizal Quercus trees likely obtained C and N via tripartite symbiosis via a mechanism similar to that in C. subaphylla.
Cephalanthera subaphylla and C. longifolia had significantly different 13C enrichments between the years evaluated (Fig. 2). Furthermore, 13C enrichment in C. falcata varied strongly among the populations (Fig. 2). These intraspecific variations in enrichment may be due to variations in environmental factors, particularly light flux, which is strongly correlated with the levels of mycoheterotrophy in the partial mycoheterotrophs C. damasonium and C. rubura (Preiss et al. 2010). Under low light conditions, C. damasonium and C. rubura plants increased their fungus-derived C contents to approximately 50 % of the level that occurred in fully mycoheterotrophic orchids. When irradiance was increased, the proportional contribution of heterotrophic nutrition declined markedly. Microsite differences in irradiance created by variation in the incidence of forest canopy gaps may have strong influenced the 13C enrichment variation that we detected, but other factors, such as annual variability, genetic effects, meteorological factors, soil nutrients and mycorrhizal associations should also be taken into consideration.
The highest enrichment in 15N (significantly higher than that in albinos) occurred in C. erecta, which is a sister taxon of C. subaphylla (Fig. 3). Strong 15N enrichment in C. erecta was also reported by Motomura et al. (2010). Cephalanthera erecta is mainly associated with the thelephoracean fungi closely related to members of the same family associated with C. falcata and C. longifolia (Matsuda et al. 2009; Sakamoto et al. 2016). Thus, the high 15N enrichment in C. erecta cannot be attributed to unique mycorrhizal taxa. The high enrichment levels may be related to strong mycoheterotrophic N uptake, to particular physiological processes within the tissues of C. erecta, to soil nutrient levels, or to host tree identity. The mechanism of N enrichment in C. erecta has not been identified, but it may be part of a process that paved the way to the evolution of almost complete mycoheterotorophy in C. subaphylla; importantly, these two orchids are closely related.
In conclusion, we showed that C. subaphylla had strong mycoheterotrophic carbon and nitrogen uptake despite the presence of small leaves. During the evolutionary pathway from autotrophy to full mycoheterotrophy, plant leaves have generally become extremely reduced (to achlorophyllous scales on inflorescence axes) (Merckx et al. 2013b). Our findings indicate that during the evolutionary process from partial to full mycoheterotrophy, plant species gained strong mycoheterotrophic abilities before the complete loss of foliage leaves. Strong mycoheterotrophic ability can balance the loss of photosynthetic carbon gain resulting from reductions in leaf size, which may explain why partial mycoheterotrophy leads to a reduction in leaf size that in turn triggers the evolution of full mycoheterotrophy. We found significant interspecific variation in the 13C enrichment of Cephalanthera species. Studies on C. damasonium and C. rubura (Preiss et al. 2010) indicate that the interspecific variation found in this study may to be related to the light environment in habitats occupied by the plants. Cephalanthera species may increase their dependency on fungal-derived C when growing under low light levels, which likely confers flexible adaptability to diverse environments.
References
Abadie JC, Püttsepp Ü, Gebauer G, Faccio A, Bonfante P, Selosse MA (2006) Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and nonphotosynthetic individuals. Botany 84:1462–1477
Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ (2004) Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc R Soc Lond B 271:1799–1806
Development Core Team R (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Gebauer G, Meyer M (2003) 15N and 13C natural abundance of autotrophic and myco-heterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytol 160:209–223
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Hynson NA, Preiss K, Gebauer G, Bruns TD (2009) Isotopic evidence of full and partial myco-heterotrophy in the plant tribe Pyroleae (Ericaceae). New Phytol 182:719–726
Johansson VA, Mikusinska A, Ekblad A, Eriksson O (2015) Partial mycoheterotrophy in Pyroleae: nitrogen and carbon stable isotope signatures during development from seedling to adult. Oecologia 177:203–211
Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse MA (2005) Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytol 166:639–653
Liebel HT, Bidartondo MI, Preiss K, Segreto R, Stockel M, Rodda M, Gebauer G (2010) C and N stable isotope signatures reveal constraints to nutritional modes in orchids from the Mediterranean and Macaronesia. Am J Bot 97:903–912
Matsuda Y, Amiya A, Ito SI (2009) Colonization patterns of mycorrhizal fungi associated with two rare orchids, Cephalanthera falcata and C. erecta. Ecol Res 24:1023–1031
Merckx VSFT (2013) Mycoheterotrophy: an introduction. In: Merckx VSFT (ed) Mycoheterotrophy: The biology of plants living on fungi. Springer, New York, pp 1–17
Merckx VSFT, Freudenstein JV, Kissling J, Christenhusz MJM, Stotler RE, Crandall-Stotler B, Wickett N, Rudall PJ, de Kamer HM, Maas PJM (2013a) Taxonomy and classification. In: Merckx VSFT (ed) Mycoheterotrophy: The biology of plants living on fungi. Springer, New York, pp 19–101
Merckx VSFT, Mennes CB, Peay KG, Geml J (2013b) Evolution and diversification. In: Merckx VSFT (ed) Mycoheterotrophy: The biology of plants living on fungi. Springer, New York, pp 215–244
Motomura H, Selosse MA, Martos F, Kagawa A, Yukawa T (2010) Mycoheterotrophy evolved from mixotrophic ancestors: evidence in Cymbidium (Orchidaceae). Ann Bot. doi:10.1093/aob/mcq156
Preiss K, Gebauer G (2008) A methodological approach to improve estimates of nutrient gains by partially myco-heterotrophic plants. Isot Environ Health Stud 44:393–401
Preiss K, Adam IK, Gebauer G (2010) Irradiance governs exploitation of fungi: fine-tuning of carbon gain by two partially myco-heterotrophic orchids. Proc R Soc Lond B 277:1333–1336
Roy M, Watthana S, Stier A, Richard F, Vessabutr S, Selosse MA (2009) Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol 7(1):1
Roy M, Gonneau C, Rocheteau A, Berveiller D, Thomas JC, Damesin C, Selosse MA (2013) Why do mixotrophic plants stay green? A comparison between green and achlorophyllous orchid individuals in situ. Ecol Monogr 83:95–117
Sakamoto Y, Yokoyama J, Maki M (2015) Mycorrhizal diversity of the orchid Cephalanthera longibracteata in Japan. Mycoscience 56:183–189
Sakamoto Y, Yamazaki J, Yamada T, Yokoyama J, Ogura-Tsujita Y, Maki M (2016) The diversity of mycorrhizal fungi in Japanese Cephalanthera species. Plant Species Biol (in press) doi:10.1111/1442-1984.12124
Selosse MA, Faccio A, Scappaticci G, Bonfante P (2004) Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottiae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb Ecol 47:416–426
Taylor DL, Bruns TD (1997) Independent, specialized invasions of ectomycorrhizal mutualism by two nonphotosynthetic orchids. Proc Natl Acad Sci USA 94:4510–4515
Yagame T, Yamato M (2013) Mycoheterotrophic growth of Cephalanthera falcata (Orchidaceae) in tripartite symbioses with Thelephoraceae fungi and Quercus serrata (Fagaceae) in pot culture condition. J Plant Res 126:215–222
Acknowledgments
We thank T. Itagaki, T. Yamada, H. Nomura, N. Takano, Y. Waki, and T. Kimura for their advice and/or assistance in the field, and Jindai Botanical Gardens for permission to collect plants. We also thank Professor Gerhard Gebauer of the University of Bayreuth for his helpful comments on our data. This study was partly supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Sakamoto and Y. Ogura-Tsujita contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sakamoto, Y., Ogura-Tsujita, Y., Ito, K. et al. The tiny-leaved orchid Cephalanthera subaphylla obtains most of its carbon via mycoheterotrophy. J Plant Res 129, 1013–1020 (2016). https://doi.org/10.1007/s10265-016-0856-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0856-6