Abstract
Reproductive success of plants may be affected by interactions with co-flowering species either negatively, through competition for pollinators, or positively, by means of a magnet species effect and floral mimicry. In this study, potential interactions between Iris tuberosa, a rewarding species, and Ophrys fusca, a sexually deceptive orchid, were explored in four populations in southern Italy. In each population plots showing different ratios of the examined species were arranged in the field, and in each plot the number of pollinators and fruit set were assessed. In addition, flower size and floral hydrocarbons produced by the two species were analysed. Morphological and scent data pointed out that flower size and aliphatic compounds did not differ significantly between the two species. Interestingly, both species shared tricosane and 11-nonacosene, electrophysiologically active compounds in the shared dominant pollinator Adrena. We have found that fruit production and number of pollinators in I. tuberosa varied significantly among plots, while percentage of capsules and number of pollinators of O. fusca captured showed no significant differences across plots. These results suggested, that the presence of O. fusca contributes differentially to pollinator attraction, and thus, to total reproductive success of I. tuberosa, according to a different ratio of aggregation. These findings suggest that I. tuberosa profits from the greater abundance of insects attracted by the presence of orchid specimens, and that a sexually deceptive orchid may be a magnet species in pollination strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most flowering plants reward their pollinators with pollen or nectar as an energetic return, while some of them do not offer food rewards and therefore show a reproductive strategy called ‘deceptive pollination’, which is particularly frequent in the Orchidaceae family (Dodson and Frymire 1961; Jersáková et al. 2006). Indeed, one-third of the orchid species are able to manipulate food-foraging activities, sexual stimuli, or brood site selections of potential pollinators by means of their amazing variety of flower signals, such as colour, shape, size, and fragrance (Jersáková et al. 2006; van der Cingel 1995).
Sexual deception of male bees and wasps is one of the most remarkable mechanisms of pollination, formerly considered exclusive to the Orchidaceae (Tremblay et al. 2005).
Classical examples are those of the sexually deceptive Ophrys genus, which shows close morphological and olfactory resemblance of both mimic and model species (Ayasse et al. 2003; Schiestl 2005). If a conspecific male is deceived successfully, it will try to mate with the flower’s labellum, a behaviour termed pseudocopulation. Orchid flowers that show pseudo-copulation by male insects attract pollinators not only with floral scent, alkanes and alkenes (Schiestl et al. 1999), pyrazine (Bohman et al. 2014) or other unusual chemicals (Ayasse et al. 2003), but also with green receptor-specific contrast between the perianth and the background (Spaethe et al. 2010). The specific attraction of only one or very few bee species is thought to guarantee efficient intra specific pollen transfer (Gaskett 2010) and, in this way, pre-pollination reproductive isolation between taxa in sympatric populations (Scopece et al. 2007) mediated by differences in floral odour (Whitehead and Peakall 2014), although there are well-documented occurrences of hybrids in hybrid zones (Bellusci et al. 2010; Cortis et al. 2009; Pellegrino et al. 2005).
Recently, sexual deception through pseudocopulation has been reported outside the Ophrys genus in other orchids, such as Serapias lingua (Vereecken et al. 2012), and in other angiosperms, including both Gorteria diffusa, a daisy species from South Africa that attracts male flies (Ellis and Johnson 2010), and Iris paradoxa (Vereecken et al. 2012), suggesting that this reproductive strategy might be more widespread in flowering plants than previously thought.
Field observations pointed out that Ophrys individuals often growing sympatrically with other species (most of them belong to Iridaceae) show an apparent flower colour resemblance to those species. The majority of species of Iridaceae are pollinated by Hymenoptera (mostly bees) (Goldblatt and Manning 2006). Floral signals are primarily perianth pigmentation, complemented by a range of floral odours in many species, but flower shape and tepal orientation, in particular functional floral symmetry, may be equally important for some pollinators. The reward to visitors in the majority of Iris species is nectar, but in others it is pollen; one species offers non-volatile oil (Sapir et al. 2002).
Iris (subfamily Iridoideae), with more than 260 species, the largest and most widespread genus of the family, has received very little empirical attention. Early studies in Iris pollination showed that species were visited by large and small Apoids, in particular honeybees, bumblebees, and solitary bees (Imbert et al. 2014). Each outer tepal and its opposed petaloid style crest functions as a bilabiate pollination unit, and thus it appears to a bee as a single gullet flower. Bumblebees and perhaps other bees large enough to force apart the tepal from the opposed style branch then climb down the ‘gullet’ to reach nectar held below the bases of the tepals.
While reproductive fitness of food-deceptive orchids (Anacamptis, Dactylorhiza and Orchis) may be affected also by different types of interactions with co-flowering rewarding species (Pellegrino et al. 2008; Peter and Johnson 2008), we have no evidence that reproductive success of sexually deceptive orchids (Ophrys) is affected by the presence of co-flowering plants that may elicit positive or negative reactions of pollinator behaviour. Pollinators represent an important intermediary by which different plant species can influence each other’s reproductive fitness. Co-flowering species could facilitate pollination of proximate or intermingled sexually deceptive orchids, which could benefit from the greater abundance of insects attracted by the rewarding ‘magnet species’ (Pellegrino et al. 2008; Thompson 1978). At the same time, many studies have shown that some rewardless orchids increase reproductive fitness when their flower colour, shape, and odour resemble those of a co-blooming rewarding plant (Gumbert and Kunze 2001; Internicola et al. 2007; Johnson et al. 2003; Pellegrino et al. 2008), a phenomenon called ‘non-model mimicry’ (Dafni 1984).
The aim of the present study is to explore potential interactions occurring in a natural community between O. fusca and the abundant co-flowering species I. tuberosa. For field experiments, we arranged within the populations experimental plots containing different ratios of the two species. Our goals were to verify the following questions: (1) does aggregation between a rewardless orchid and a rewarding species increase the reproductive success of orchid? and/or (2) increase the reproductive success of I. tuberosa? (3) Is reproductive success associated with differences/similarities in floral morphology or floral scent of the examined taxa? (4) Is it possible to distinguish between potential facilitative and/or competitive interactions? (5) Are these interactions linked to the ratios of Ophrys–Iris within natural populations?
Materials and methods
Study species
Ophrys fusca link is a perennial herb that forms a basal rosette in late autumn and a single few-flowered inflorescence that usually bears 2–6 flowers in late winter until early summer. It is a widespread rewardless orchid, occurring mainly in the western Mediterranean region and in northern Africa (Delforge 2005).
The O. fusca labellum (brownish) has one central lobe flanked by two lateral lobes (Fig. 1a). The central lobe can be divided along its length into three main regions: basal (near the stigmatic cavity), median, and apical. Many species of the O. fusca group are pollinated by male Andrena bees (i.e. A. nigroaenea and A. flavipes) (Stökl et al. 2005).
Iris tuberosa L. is an iridacean species native to Mediterranean regions (southern Europe, the Balkans, and Northern Africa) (Mathew 1987). In Italy, it mainly occurs in central and in southern regions where it grows in dry, usually rocky places, in olive groves, and amongst hedges (Pignatti 1982). Flowers of I. tuberosa are hermaphroditic and trimerous, thus consisting of two whorls of petal-like members (an outer and an inner series of tepals), with three stamens inserted opposite the outer tepals, and an inferior ovary of three united carpels sharing a common style (Fig. 1b). Information regarding its sexual reproduction is rather scarce. The plant needs an appropriate pollinator visiting the flower to gather the nectar located at the base of the tepals, as observed by Arcangeli (1895), who identified the hymenopteran Xylocopa violacea as a pollinator of I. tuberosa flowers, since self-pollination is unlikely to occur due to the location of the anthers below the stigma lobes. Previous studies in Italy showed that I. tuberosa was pollinated exclusively by hymenopteran species of five genera: Andrena, Anthophora, Colletes, Lasioglossum, and Xylocopa (Pellegrino 2014).
Study site and experimental design
Experiments were conducted during the spring of 2013 at four sites in Pollino National Park (Calabria, southern Italy), where populations of O. fusca and I. tuberosa grow sympatrically. The O. fusca plants were, on average, numerically twice those of I. tuberosa plants. The area covers ~700 ha and consists of calcareous, dry grasslands (Festuco-Brometalia); Spartium junceum L., Cytisus sessilifolius L., and Cistus incanus L. are the frequent shrubs and Festuca circummediterranea Patzke, Bromus erectus Huds. and Dactylis glomerata L. are the dominant herbs. Ophrys fusca and I. tuberosa grow over the entire area, forming populations of a few to thousands of individuals. We selected four populations, each of which is separated from a neighbouring population by at least 1 km.
To verify the effects of different combinations of the two examined species on reproductive success and pollinator attraction, we created two experimental designs. In the first experiment, we selected nine circles (plots) of 5 m diameter in each population. In each plot the selected plants of O. fusca and/or I. tuberosa were excavated and temporarily potted. Removed plants were returned to their original positions at the end of the experiments. Each circle contained a total of 60 plants of different O. fusca/I. tuberosa ratios, one circle with only one species, and other circles with different ratio (1:1, 1:2, 1:5, and 1:9) of examined species (Fig. 2). In the second experiment, because population size of each species also may be important, as shown in numerous plant species (Phillips et al. 2014), we selected nine other plots where we kept the number of plants of one species the same (60 plants) and then altered the number of individuals of the other to obtain different ratios (1:1, 1:2, 1:5, and 1:10) of the examined species (Fig. 2). Hereafter, the ratios 1:9 and 1:10 are both treated as 1:10. To avoid plants in a circle influencing pollinator attraction and fruit production of neighbouring circles, we selected plots such that each was separated from a neighbouring plot by at least 15 m. Moreover, individuals of the few other co-flowering herbaceous species (Ranunculus sp. and Bellis sylvestris) growing in the plots, which could affect pollination of the two focal species, were removed.
Scheme of the experimental design. 18 circular plots (5 m diameter) were subjected to different treatments by excavating and temporarily potting the selected plants of O. fusca and/or I. tuberosa to create different species ratios (1:1, 1:2, 1:5, and 1:10). 9 plots (black circles) contained a total of 60 plants (O. fusca + I. tuberosa) and 9 plots (grey circles) kept the number of plants of one species constant. Rows (1–5) represent the variation of O. fusca/I. tuberosa ratio
Reproductive success
For each plot, produced fruits were assessed and the percentage of flowers that set fruits determined. The effects of our treatments on the reproductive success of each species across plots and populations were evaluated by analysis of variance (ANOVA) with the SPSS software package. The significance of correlation between fruit set of O. fusca and its different aggregation with I. tuberosa, and viceversa, was tested with bivariate analysis using SAS (SAS institute, Inc., 1988).
Flower-visiting insects
Sampling of flower-visiting insects was restricted to 5–6 days for each site, leaving at least 4-day gaps between sampling days, at each site in order to minimise any negative impact on the local insect fauna and to minimise negative effects on fruit set. The sites were visited alternately between 0800 and 1800 h by four observers. The insect visitations were recorded during the peak flowering season, from 2 March to 16 April 2013, for a total of 60 h for each population and for each plot, covering the entire species phenology. Pollinators found on I. tuberosa or O. fusca flowers were captured with a handnet. The insects were then preserved in ethyl acetate for later identification. The species of each specimen were then identified using the taxonomic keys from Schmid-Egger and Scheuchl (1997).
Morphometricanalysis
Flowers of 10 individuals of O. fusca and I. tuberosa in each selected natural population were collected. Floral traits were measured to the nearest 1 mm using a digital calliper. For O. fusca flowers, we measured labellum length and median region width; for I. tuberosa flowers, we measured length and width of the outer brownish tepals. Ranges, means, and standard deviations were estimated for each trait using DataDesk 7 software (Velleman 2012). Differences in flower morphology between species were analysed by ANOVA, and pairwise contrasts were tested at α = 0.05.
Scent analysis
Floral hydrocarbons produced by the two species were analysed for plants from all populations. For each population the labellum (O. fusca) and the outer tepal (I. tuberosa) of 10 un-pollinated flowers were placed in a 2 mL glass vial and rinsed in 500 μL hexane (Merck Uvasol) for 2 min and gently shaken. Before chemical analyses, the samples were concentrated to 70 μL, and 1 μg octadecane (C18) was added to each sample as internal standard. One microlitre of each sample was injected splitless at 50 °C (1 min) into a gas chromatograph (GC, HP 6890), followed by opening the split valve and programming the temperature to increase to 310 °C at a rate of 10 °C/min. The GC was equipped with a DB-5 column (30 m and 0.32 mm); helium was used as carrier gas. Relative amounts of alkanes and alkenes were calculated separately. The relative amount of each odour compound was calculated as the proportion of total alkene and alkane amounts with a chain length between 18 and 30 carbons. To reduce the number of variables, principle component analysis (PCA) was used for the analysis of interspecies floral scent variation based on scaled relative amount of hydrocarbons. The differences for each individual compound and total hydrocarbons between species and across different populations within species were analysed by ANOVA using SPSS 7.5 (SPSS 1997).
Results
Reproductive success
There were no significant differences in fruit production between plots where we kept either the number of plants of one species the same or the total number of plants (O. fusca + I. tuberosa) the same, suggesting that there are no effects of population size on reproductive success but the presence of O. fusca contributed differentially to total fruit set of I. tuberosa in respect to different ratio of aggregation.
Analysis of fitness measurements between species, considering the species-pure plots only, showed that reproductive success of sexually deceptive O. fusca was significantly higher (almost triple) than that of I. tuberosa (mean fruit set: 30.3 vs 10.5 %).
The percentage of total capsules of O. fusca detected in all 72 plots ranged from 25.7 % (O. fusca:I. tuberosa ratio of 1:2) to 30.3 % (O. fusca:I. tuberosa ratio of 1:0), with no significant differences found by ANOVA across plots (F3,67 = 0.783, P = 0.66) and across populations (F3,67 = 0.653, P = 0.54).
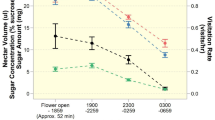
Conversely, mean values of fruit production in I. tuberosa of experimental plots varied significantly (F3,67 = 7.057, P < 0.01) from 5.7 % (O. fusca:I. tuberosa ratio of 10:1) to 19.0 % (O. fusca:I. tuberosa ratio of 1:1) (Fig. 3), while no significant differences have been found across populations (F3,67 = 0.813, P = 0.62). The highest fitness values were obtained in plots in which the O. fusca:I. tuberosa ratio were 2:1 and 1:1, respectively. An intermediate level of fruit set (12.0–13.4 %) in I. tuberosa has been observed in treated plots with O. fusca:I. tuberosa ratio corresponding to 1:2, 1:5 and 1:10. The lowest values of fruit set (5.7–7.0 %) of I. tuberosa were detected in two plots where the O. fusca:I. tuberosa ratios were 5:1 and 10:1. In plots with I. tuberosa plants only, an intermediate value of fruit set (10.5 %) was recorded (Fig. 3). Moreover, bivariate analysis showed the absence of linear regression between the percentage of fruits and the ratio of aggregation of the two species.
Flower-visiting insects
Field observations pointed out that O. fusca had an almost doubled success of pollinator attraction compared to I. tuberosa. Indeed, totals of 685 and 288 insects were observed on O. fusca and I. tuberosa flowers, respectively. All O. fusca pollinators belong to Andrena sp., while seven species were recognised as effective pollinators of I. tuberosa, all pertaining to hymenopteran insects of three genera (Andrena, Lasioglossum and Xylocopa). Among them, Andrena was the dominant genus, accounting for >87 % of I. tuberosa pollinators (Fig. 4), and 221 out of 288 insects (77 %) were male. There were no significant differences between the populations regarding the number of pollinators (F 1,3 = 1.37, P = 0.15).
The number of pollinators of O. fusca captured in all 72 plots ranged from 78 (O. fusca:I.tuberosa ratio of 1:0) to 97 (O. fusca:I.tuberosa ratio of 2:1) (Fig. 5), with no significant differences found by ANOVA across plots (F3,67 = 0.684, P = 0.68) and across populations (F3,67 = 0.783, P = 0.66). Conversely, significant differences in terms of number of pollinators of I. tuberosa were found across experimental plots. Indeed, the highest number of pollinators (118) was obtained in plots in which the O. fusca:I. tuberosa ratio was 1:1, and intermediate numbers of pollinators (49–54) were observed in treated plots where the O. fusca:I. tuberosa ratios were 1:2 and 2:1. The remaining plots showed the lowest numbers (10–15) of pollinators (Fig. 5). It is evident that the presence of O. fusca contributes differentially to pollinator behaviour of I. tuberosa according to different ratios of aggregation.
Morphometric data
Morphologically, the labellum of O. fusca and the outer tepals of I. tuberosa are quite similar. Indeed, ANOVA showed no significant differences in flower morphology of the two species (F1,3 = 0.324, P = 0.33). The labellum length of O. fusca was recorded as 9–20 mm (mean 14.8 ± 0.62) and the labellum width ranged from 5 to 11 mm (mean 8.2 ± 0.33). The tepal length of I. tuberosa ranged from 12 to 20 mm (mean 16.0 ± 0.75) and the width from 7 to 13 mm (mean 8.7 ± 0.46) (Fig. 6).
Floral aliphatic compounds
In all flower extracts of selected species, we detected n-alkanes (saturated hydrocarbons) and n-alkenes (unsaturated hydrocarbons) of chain lengths of 21–29. ANOVA showed no significant differences in total amounts of aliphatic compounds between species (F1,7 = 0.425, P = 0.42) and across populations within species (F1,3 = 0.611, P = 0.53). Among the alkanes, both species showed high percentages of tricosane, pentacosane and heptacosane (Fig. 7a). In particular, tricosane (C23) represented more than 50 % of total alkanes in O. fusca and I. tuberosa while the percentages of pentacosane and heptacosane ranged from 15 to 25 %. Moreover, among the alkenes there was a high proportion (50–55 %) of 11-nonacosene in both species. The other two alkenes (11-heptacosene and 9-heptacosene) reached percentages of about 10–15 % (Fig. 7b). The remaining occurred in traces rarely exceeding 5 %. Quantitative analyses of relative amounts of alkanes and alkenes showed that aliphatic compounds did not differ significantly between the two species. The reduction of all the detected compounds with PCA produced 10 PCA factors explaining 81.6 % of the total variance. A canonical discrimination analysis, with the first two discriminant functions explaining 100 % of the variance (discriminant function 1: eigenvalue = 1.08, v2 = 103.53, P < 0.001; discriminant function 2: eigenvalue = 0.36, v2 = 30.84, P < 0.001), revealed that the floral odour bouquets did not differ significantly between the two species, showing a contiguous and overlapping group (data not shown).
Discussion
Our study reports two unusual results: O. fusca has an almost doubled success of pollinator attraction and fruit production, and a facilitating effect of sexually deceptive orchid for Iris exists. Indeed, the information presented here is sufficient to point out the possibility that the coexistence of I. tuberosa with the rewardless sexually deceptive O. fusca is advantageous for pollinator attraction and fruit production of Iris through two main interactions (i.e., the magnet species effect and the floral mimicry). These findings suggest that positive interactions among co-occurring species are more frequent than previously assumed (Johnson et al. 2003), and, for the first time, that a sexually deceptive orchid may be a magnet species in a pollination strategy. Iris tuberosa is benefiting from the greater abundance of insects attracted by the presence of O. fusca specimens, while O. fusca pollination is not affected by the presence of I. tuberosa. If a plant is a mimic of another one, it is clear that it will receive more pollinator visits in the presence of the model plant. These findings are in accordance with the observations that insects tend to visit flowers with corolla colours similar to those they have visited previously (Evans and Raine 2014; Gigord et al. 2002; Renner 2006). Indeed, morphological observations pointed out that both examined species exhibit similar flowers in terms of floral size (Fig. 6) and colour (analysis of colour reflectance, Pellegrino unpublish data). These morphological characteristics may provide a stimulus to insects approaching the flower, reinforced by the effect of the odour that acts as the primary attraction factor in orchid sexual deception (Schiestl et al. 2003, 2004). Iris tuberosa imitates O. fusca flowers in shape, colour and, most importantly, scent. In flower extracts of I. tuberosa, we found 35 hydrocarbons, which can be found also in labella extracts of O. fusca. Not only does I. tuberosa have the same alkanes and alkenes as O. fusca, but, in particular, it shares with Ophrys the electrophysiologically active compounds tricosane and 11-nonacosene (Fig. 7), which effectively play a role in pollinator attraction (Ayasse et al. 2003; Stökl et al. 2008). In this way, pollinators are attracted to the Iris flowers by olfactory signals.
These findings are, to the best of our knowledge, the first conclusive reports of floral mimicry in the family Iridaceae. Data on the mechanism of mimicry are largely available for orchids, especially for food-deceptive orchids. Food-deceptive species attract pollinators by imitation of floral signals typical of rewarding plant species (Jersáková et al. 2006). Two types of mimicry are generally recognised among deceptive orchids. The first is Batesian mimicry, consisting of a ‘mimic’ that imitates signals of a ‘model’, and an ‘operator’ that responds to them (Dafni 1984). An alternative form of deception is described as ‘generalized food deception’ (Jersáková et al. 2006; Steiner 1998), where a particular model species is lacking and relies upon the perceptual exploitation of pollinators. A few examples of Batesian mimicry are well documented, such as Anacamptis israelitica (Dafni and Ivri 1981), Cephalanthera rubra (Nilsson 1983), Disa pulchra (Johnson 2000), and their respective models, in which similarities in colour between model and mimic have been reported. The I. tuberosa pollination strategy cannot be defined as Batesian mimicry or ‘generalised food deception’, because the model plant does not offer a reward to pollinators. We can define the I. tuberosa pollination strategy ‘mimicry of a sexually deceptive species’. In this case, the Iris flowers mimic O. fusca labella which mimic female insect mating signals. A situation of double deception occurs: first, the orchids deceive pollinators, then the same insects are deceived by Iris flowers, mainly by olfactory cues.
Interactions among plants that are mediated by pollinators can range from competitive to facilitative, in which plant species interfere by enhancing or diminishing one another’s ability to attract sufficient pollinators, respectively (Geber and Moeller 2006). In this respect, the maximum levels of numbers of pollinators and reproductive success of I. tuberosa were recorded in treated plots where O. fusca specimens co-occurred, suggesting a benefit that might be explained by invoking the magnet species effect (Thompson 1978) due to the higher density and greater attractiveness of the orchid. On the contrary, the lowest level of fitness of I. tuberosa in plots in which there were more O. fusca specimens than I. tuberosa (ratio of O. fusca:I. tuberosa of 10:1 or 5:1, Fig. 3) exclude the magnet species effects. In the latter case, the lowest Iris plant density could fail to attract sufficient pollinators (Moeller and Geber 2005), suggesting that pollinator saturation may be common. Moreover, an intermediate level of number of pollinators and fruit set in I. tuberosa was observed in treated plots where there were few O. fusca specimens (ratio of O. fusca:I. tuberosa of 1:5 or 1:10, Figs. 3, 5). These results point out that I. tuberosa showed a complexity of these interspecific interactions among plants. Indeed, in some cases, fitness of I. tuberosa may be affected by facilitative effects but, in other cases, by competition for attraction of pollinators.
Clearly, the direction of interactions (e.g., facilitation or competition) was ratio dependent. In this system, equal density of model and mimic species seems to be important for mediating positive interactions for pollination, while excessive differences in density of model and mimic plants seem to create competition for pollinators.
Conclusion
Our experiments showed that positive interactions among co-occurring species occur not only when an unrewarding species grows sympatrically with a rewarding species but also when a rewarding species lives in the presence of sexually deceptive species. In the first relationship, the nectariferous species has a greater attractive force of pollinators (classic example of magnet effect). In our case, for the first time, it is evident that sexual deception can outperform even reward‐based systems in terms of promotion of pollinator attraction.
We suggest that I. tuberosa adopts a complex mix of pollination strategy and magnet species effect and could represent a helpful model for studying the transition between different pollination strategies, which remains to be investigated further. Indeed, we can speculate that I. tuberosa could represent a transitional step from rewarding pollination to sexual deception (Vereecken et al. 2012). Indeed, in the case of orchid sexual deception, it has been demonstrated that pollinators were rewarded originally by food (Kullenberg and Bergström 1976) and that scent was present already before the loss of a reward (Schiestl et al. 1999).
References
Arcangeli G (1895) Sull’ Hermodactylus tuberosus. Boll Soc Bot Ital 6:182–184
Ayasse M, Schiestl FP, Paulus HF, Ibarra F, Francke W (2003) Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. Proc R Soc B 270:517–522
Bellusci F, Pellegrino G, Palermo AM, Musacchio A (2010) Crossing barriers between the unrewarding Mediterranean orchids Serapias vomeracea and S. cordigera. Plant Species Biol 25:68–76
Bohman B, Phillips RD, Menz MHM, Berntsson BW, Flematti GR, Barrow RA, Dixon KW, Peakall R (2014) Discovery of pyrazines as pollinator sex pheromones and orchid semiochemicals: implications for the evolution of sexual deception. New Phytol 203:939–952
Cortis P, Vereecken NJ, Schiestl FP, Barone Lumanga MR, Scrugli A, Cozzolino S (2009) Pollinator convergence and the nature of species’ boundaries in sympatric Sardinian Ophrys (Orchidaceae). Ann Bot 104:497–506
Dafni A (1984) Deception, mimicry and parasitism in pollination. Ann Rev Ecol Syst 15:159–278
Dafni A, Ivri Y (1981) Floral mimicry between Orchis israelitica Baumann and Dafni (Orchidaceae) and Bellevalia felexuosa Boiss (Liliaceae). Oecologia 49:229–232
Delforge P (2005) Guide des orchidées d’Europe, d’Afrique du Nord et du Proche-Orient. Delachaux & Niestlé, Paris
Dodson CH, Frymire GP (1961) Natural pollination of orchids. Mo Bot Gard Bull 49:133–152
Ellis AG, Johnson SD (2010) Floral mimicry enhances pollen export: the evolution of pollination by sexual deceit outside of the Orchidaceae. Am Nat 176:143–151
Evans LJ, Raine NE (2014) Changes in learning and foraging behaviour within developing bumble bee (Bombus terrestris) colonies. PLoS One 9:e90556
Gaskett AC (2010) Orchid pollination by sexual deception: pollinator perspectives. Biol Rev 86:33–75
Geber MA, Moeller DA (2006) Pollinator responses to plant communities and implications for reproductive character evolution. In: Hardes LD, Barrett SCH (eds) The ecology and evolution of flowers. Oxford University Press, Oxford, pp 102–119
Gigord LDB, Macnair MR, Stritesky M, Smithson A (2002) The potential for floral mimicry in rewardless orchids: an experimental study. Proc R Soc B 269:1389–1395
Goldblatt P, Manning J (2006) Radiation of pollination systems in the Iridaceae of sub-saharan Africa. Ann Bot 97:317–344
Gumbert A, Kunze J (2001) Color similarity to rewarding model plants affects pollination in a food deceptive orchid, Orchis boryi. Biol J Linn Soc 72:419–433
Imbert E, Wang H, Conchou L, Vincent H, Talavera M, Schatz B (2014) Positive effect of the yellow morph on female reproductive success in the flower colour polymorphic Iris lutescens (Iridaceae), a deceptive species. J Evolut Biol 27:1965–1974
Internicola AI, Page PA, Bernasconi G, Gigord LDB (2007) Competition for pollinator visitation between deceptive and rewarding artificial inflorescences: an experimental test of the effects of floral color similarity and spatial mingling. Funct Ecol 21:864–872
Jersáková J, Johnson SD, Kindlmann P (2006) Mechanisms and evolution of deceptive pollination in orchids. Biol Rev 81:219–235
Johnson SD (2000) Batesian mimicry in the non-rewarding orchid Disa pulchra, and its consequences for pollinator behavior. Biol J Linn Soc 71:119–132
Johnson SD, Peter CI, Nilsson A, Ågren J (2003) Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84:2919–2927
Kullenberg B, Bergström G (1976) The pollination of Ophrys orchids. Bot Not 129:11–19
Mathew B (1987) The smaller bulbs. Batsford BT, London
Moeller DA, Geber MA (2005) Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communities, and reproductive assurance. Evolution 59:786–799
Nilsson AL (1983) Mimesis of bellflower (Campanula) by the red helleborine orchid Cephalanthera rubra. Nature 305:799–800
Pellegrino G (2014) Pollinator limitation on reproductive success in Iris tuberosa L. (Iridaceae). AoB Plants. doi:10.1093/aobpla/plu089
Pellegrino G, Musacchio A, Noce ME, Palermo AM, Widmer A (2005) Reproductive versus floral isolation among morphologically similar Serapias L. species (Orchidaceae). Jour Hered 96:15–23
Pellegrino G, Bellusci F, Musacchio A (2008) Double floral mimicry and the magnet species effect in dimorphic co-flowering species, the deceptive orchid Dactylorhiza sambucina and rewarding Viola aethnensis. Preslia 80:411–422
Peter CI, Johnson SD (2008) Mimics and magnets: the importance of color and ecological facilitation in floral deception. Ecology 89:1583–1595
Phillips RD, Peakall R, Hutchinson MF, Linde CC, Xu T, Dixon KW, Hopper SD (2014) Specialized ecological interactions and plant species rarity: the role of pollinators and mycorrhizal fungi across multiple spatial scales. Biol Conserv 169:285–295
Pignatti S (1982) Flora d’Italia. Edagricole Bologna, Italy
Renner SS (2006) Rewardless flowers in the angiosperms and the role of insect cognition in their evolution. In: Waser NM, Ollerton J (eds) Plant–pollinator interactions: from specialization to generalization. University of Chicago Press, Chicago, pp 123–144
Sapir Y, Shmida A, Fragman O, Comes HP (2002) Morphological variation of the Onocyclus irises (Iris: iridaceae) in the southern Levant. Bot J Linn Soc 139:369–382
Schiestl FP (2005) On the success of a swindle: pollination by deception in orchids. Naturwissenschaften 92:255–264
Schiestl FP, Ayasse M, Paulus HF, Löfstedt C, Hansson BS, Ibarra F, Francke W (1999) Orchid pollination by sexual swindle. Nature 399:421–422
Schiestl FP, Peakall R, Mant J, Ibarra F, Schulz C, Francke S, Francke W (2003) The chemistry of sexual deception in an orchid–wasp pollination system. Science 302:437–438
Schiestl FP, Peakall R, Mant J (2004) Chemical communication in the sexually deceptive orchid genus Cryptostylis. Bot J Linn Soc 144:199–205
Schmid-Egger C, Scheuchl E (1997) Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs, Band III, Andrenidae. Eigenverlag, Velden
Scopece G, Musacchio A, Widmer A, Cozzolino S (2007) Pattern of reproductive isolation in Mediterranean deceptive orchids. Evolution 61:2623–2642
Spaethe J, Streinzer M, Paulus HF (2010) Why sexually deceptive orchids have colored flowers. Commun Integr Biol 3:139–141
Steiner KE (1998) The evolution of beetle pollination in a South African orchid. Am J Bot 85:1180–1193
Stökl J, Paulus H, Dafni A, Schulz C, Francke W, Ayasse M (2005) Pollinator attracting odour signals in sexually deceptive orchids of the Ophrys fusca group. Plant Syst Evol 254:105–120
Stökl J, Schlüter PM, Stuessy TF, Hannes F, Paulus GA, Ayasse M (2008) Scent variation and hybridization cause the displacement of a sexually deceptive orchid species. Am J Bot 95:472–481
Thompson JD (1978) Effect of stand composition on insect visitation in two-species mixtures of Hieracium. Am Midl Nat 100:431–440
Tremblay R, Ackerman JD, Zimmerman JK, Calvo RN (2005) Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biol J Linn Soc 84:1–54
van der Cingel NA (1995) An atlas of orchid pollination. Balkema AA, Rotterdam
Velleman PF (2012) DataDesk: an interactive package for data exploration, display, model building, and data analysis. WIREs Comput Stat 4:407–414
Vereecken NJ, Wilson CA, Hötling S, Schulz S, Banketov SA, Mardulyn P (2012) Pre-adaptations and the evolution of pollination by sexual deception: cope’s rule of specialization revisited. Proc R Soc B 279:4786–4794
Whitehead MR, Peakall R (2014) Pollinator specificity drives strong prepollination reproductive isolation in sympatric sexually deceptive orchids. Evolution 68:1561–1575
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pellegrino, G., Bellusci, F. & Palermo, A.M. Who helps whom? Pollination strategy of Iris tuberosa and its relationship with a sexually deceptive orchid. J Plant Res 129, 1051–1059 (2016). https://doi.org/10.1007/s10265-016-0853-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0853-9