Abstract
The Arabidopsis thaliana T-DNA insertion mutant glucose hypersensitive (ghs) 40-1 exhibited hypersensitivity to glucose (Glc) and abscisic acid (ABA). The ghs40-1 mutant displayed severely impaired cotyledon greening and expansion and showed enhanced reduction in hypocotyl elongation of dark-grown seedlings when grown in Glc concentrations higher than 3 %. The Glc-hypersensitivity of ghs40-1 was correlated with the hyposensitive phenotype of 35S::AtGHS40 seedlings. The phenotypes of ghs40-1 were recovered by complementation with 35S::AtGHS40. The AtGHS40 (At5g11240) gene encodes a WD40 protein localized primarily in the nucleus and nucleolus using transient expression of AtGHS40-mRFP in onion cells and of AtGHS40-EGFP and EGFP-AtGHS40 in Arabidopsis protoplasts. The ABA biosynthesis inhibitor fluridone extensively rescued Glc-mediated growth arrest. Quantitative real time-PCR analysis showed that AtGHS40 was involved in the control of Glc-responsive genes. AtGHS40 acts downstream of HXK1 and is activated by ABI4 while ABI4 expression is negatively modulated by AtGHS40 in the Glc signaling network. However, AtGHS40 may not affect ABI1 and SnRK2.6 gene expression. Given that AtGHS40 inhibited ABA degrading and signaling gene expression levels under high Glc conditions, a new circuit of fine-tuning modulation by which ABA and ABA signaling gene expression are modulated in balance, occurred in plants. Thus, AtGHS40 may play a role in ABA-mediated Glc signaling during early seedling development. The biochemical function of AtGHS40 is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
WD repeats (WD40) are conserved domains of approximately 40–60 amino acids, starting with a conserved Gly-His (GH) and typically ending with a conserved Try-Asp (WD) (Li and Roberts 2001). Most WD40 proteins have a cluster of 4 or more copies of the WD motifs to form a functional structure such as aβ-propeller (Chothia et al. 1997). The repeated WD40 motifs serve as platforms where a protein interacts with other diverse proteins, peptides or nucleic acids using multiple surfaces or modes of interaction (Ramsay and Glover 2005; Smith et al. 1999; Stirnimann et al. 2010). As of this writing, many genes that encode WD40 proteins have been identified in Arabidopsis (van Nocker and Ludwig 2003). Most Arabidopsis WD40 proteins are strongly conserved across eukaryotes. Members of the WD40 superfamily are known as key regulators of plant-specific events, which are involved in chromatin modification, RNA synthesis/processing, cytoskeleton dynamics, gametogenesis, floral pigmentation, trichome and root hair formation, and seed development (Gachomo et al. 2014; Gao et al. 2012; Pattanaik et al. 2014; Zeng et al. 2009; Zhao et al. 2013).
WD40 proteins are responsive to hormones (Chen et al. 2006; Ullah et al. 2003) and different environmental stresses (Jiang et al. 2012; Shi et al. 2011; Zhu et al. 2008). All these reports suggest that each WD40 protein might play an important role in stress tolerance and hormone regulation. However, the response mechanism of WD40 proteins to glucose (Glc), which is an important signaling factor affecting plant development, has yet to be studied; a previous study reported that the WD40 repeat region of a myoinositol polyphosphate 5-phosphatase interacts with SnRK1, which is a central integrator of sugar, metabolic, stress, and developmental signals (Ananieva et al. 2008). High levels of exogenous Glc cause ABA accumulation, which results in a delay in germination and inhibition of early seedling development (Gibson 2005; Leόn and Sheen 2003; Rolland et al. 2002). Similarly, high Glc concentration represses cotyledon development and hypocotyl elongation. In terms of gene regulation, high Glc concentration induces the expression of ABA biosynthesis and signaling genes, whereas the ABA-deficient aba2 mutant is not arrested at 6 % Glc (Cheng et al. 2002). Most of the ABA biosynthesis- and ABA-insensitive mutants described thus far are insensitive to high Glc concentrations. Several negative and positive regulators controlling ABA-signaling pathways have recently been identified through the signal transduction pathway (Bu et al. 2009; Carvalho et al. 2010; Guo et al. 2009; Huang et al. 2010; Lee et al. 2011; Stone et al. 2006). The identification of these regulators emphasizes the complexity of the plant signaling transduction network.
We report the phenotypic and molecular analyses of a Glc-hypersensitive mutant, namely, ghs40-1, in which the mutated gene encodes a WD40 protein. AtGHS40 mutation showed defects in early seedling growth, and gene expression in the Glc signaling network. AtGHS40 down-regulated both ABA degrading and signaling genes, thereby indicating the occurrence of a fine-tuning modulation of Glc-induced ABA signaling in plants. The AtGHS40 protein might play an important role in Arabidopsis in response to sugar and involve in Glc- and ABA-signaling during germination and seedling growth.
Materials and methods
Plant materials, growth conditions and stress treatments
All Arabidopsis (A. thaliana) plants used in this study were in the Col-0 ecotype background. Seeds of ghs40-1 and ghs40-2 are T-DNA insertion lines (SALK_073682C and SALK_052897, respectively), were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus, OH). hxk1 (SALK_070739), abi4-1 (CS8104), and aba2 (also known as glucose-insensitive1, gin1) (Cheng et al. 2002) were also used in this study. The hxk1 mutant has a T-DNA insertion in the first intron and shown no hexokinase1 (HXK1) product tested by HXK1 polyclonal antibodies. Wild type (WT) and mutant seeds were sterilized and kept for 3 days at 4 °C in the dark to break dormancy. Seeds were grown at 22 °C and 60 % relative humidity under long-day conditions (16-h-light/8-h-dark cycle) aseptically or on soil. For soil growth, seed were sown on a 1:1:8 mixture of vermiculite, perlite, and peat moss and watered every other day.
Seed germination and post-germination assays
The mutant and WT seeds (approximately 100 seeds each) were aseptically treated and rinsed according to Yang et al. (2005). To measure Glc and ABA sensitivity, seeds were sown in sugar-free MS plates and placed at 22 °C with a 16-h light photoperiod. Various indicated concentrations of D-Glc, NaCl, and mannitol (Mtl), ABA, and 1 μM fluridone (Wako) were added where indicated. Unless indicated otherwise, three replicate plates were performed for each treatment. Germination (defined as the protrusion of the radicle through the seed coat), cotyledon greening, and cotyledon expansion were measured at indicated days. The number of germinated seeds was expressed as a percentage of the total number of seeds plated. The number of cotyledon greening and expansion was expressed as a percentage of the total number of germinated seeds. For the measurement of hypocotyl length, germinated seeds were grown vertically on solid sugar-free MS with Glc and Mtl in complete darkness at 22 °C. Hypocotyl length was measured 9 days after stratification.
Genomic DNA isolation and genotyping of mutant plants
Arabidopsis DNA was isolated from seedling according to Maliga et al. (1995). Mutant plants with the insertion of T-DNA were identified by PCR analysis. For genotyping, PCR analysis was carried out using AtGHS40-specific primers for the WT and in combination with T-DNA-specific primers (Table S1) for the mutants. The DNA fragments were analyzed by 1.5 % agarose gel and stained with ethidium bromide (EtBr).
Construction of 35S::AtGHS40 and 35S::AtGHS40-complemented lines
Genomic DNA was isolated from 9-day-old WT seedlings. An entire coding region of AtGHS40 gene was amplified by PCR with BglII-AtGHS40 and PmlI-AtGHS40 primer pair (Supplementary Table S1). The amplified AtGHS40 DNA fragment was then excised with BglII and PmlI and inserted into the binary pCAMBIA1301 vector, confirmed by DNA sequencing and introduced into Agrobacterium tumefaciens for floral dip transformation (Clough and Bent 1998) of WT and homozygous ghs40-1 plants. The medium containing hygromycin was used to select plants and T1 plants were grown to maturity and selfed. More than three independent lines in the ghs40-1 mutant background were selected from each transformation.
RNA isolation, RT-PCR, and qRT-PCR
For different abiotic stresses, 9-day-old seedlings of WT were at cold condition (4 °C) or exposed to drying at room temperature or incubated with shaking in sugar-free MS medium either containing 4.5 % Glc or 300 mM NaCl or 50 μM ABA for indicated time intervals before harvest. Total RNA were extracted from each treatment of WT seedlings and also from floral and vegetative organs of WT plants. For RT-PCR, the first-stranded cDNA was synthesized with 3 μg total RNA using oligo(dT) primer according to the manufacturer’s protocol (Invitrogen). RT-PCR analyses were performed using the gene-specific primer pairs such as AtGHS40-A, -B, and -partial listed in Table S1. The qRT-PCR analysis was performed according to the procedures described by Hsu et al. (2011). The cDNA was amplified in the presence of SYBR Green I Nucleic Acid Stain (Cambrex 50513) 104 × dilution from stock and using a Rotor-Gene 3000 (Corbett, Australia). In this experiment, amplification of actin cDNA under identical conditions was used as an internal control to adjust the level of cDNA. The qRT-PCR results were obtained according to the Comparative Quantification (CQ) method described by McCurdy et al. (2008). The CQ method generated a Relative Quantity (RQ) value for each individual sample using Rotor-Gene 6.0 software equipped within Rotor-Gene 3000. The value of RQtarget genes/RQactin represents the expression level of target gene in the sample. The qRT-PCR data were finally obtained by the value of RQtarget genes/RQactin from each sample over that of the WT under control condition; the sample of WT under control condition is regarded as one arbitrary unit. For a short period of induction, the 9-day-old of WT, ghs40-1, abi4, and hxk1 seedlings were incubated with shaking in sugar-free MS medium with or without containing 4.5 % Glc or Mtl for indicated time intervals before harvest. The primer sets for qRT-PCR were listed (Table S1). The melting temperatures to generate specific product were obtained as such: CAB1 (88 °C), PC2 (86 °C), CHS (86 °C), APL3 (84 °C), ASN1 (87 °C), ABA3 (85 °C), AAO3 (84 °C), NCED3 (83 °C), ABI1 (79 °C), ABI3 (83 °C), ABI4 (89 °C), ABI5 (87 °C), SnRK2.6 (80 °C), HXK1 (86 °C), AtGHS40 (80 °C), CYP707A2 (79 °C), CYP707A3 (86 °C), CYP707A4 (83 °C), and ACTIN1 (85 °C). The above experiments were repeated three times independently, and the data were averaged.
Measurement of ABA
For quantification of ABA content, 9-day-old seedlings of WT and ghs40-1 and aba2 mutants were transferred to sugar-free MS medium solutions supplemented with or without 4.5 % Glc or Mtl, and incubated for 4 h before harvest. ABA measurement is according to the method of Xiong et al. (2001). ABA in the solution was measured using the Phytodetek ABA immunoassay kit (Idetek).
Construction of EGFP, AtGHS40-mRFP, AtRH57-EGFP, AtGHS40-EGFP and EGFP-AtGHS40, and driven by 35S promoter
To make the modified pUC18 (Genemark) construct containing 35S promoter, the 35S-GUS (β-glucuronidase) fragment was released from pBI121 vector (BD Biosciences Clontech) with HindIII and EcoRI and ligated into pUC18. To create constructs of enhanced green fluorescent protein (EGFP) and monomeric red fluorescent protein (mRFP) gene sequences in the modified pUC18, the complete open reading frame (ORF) of EGFP or mRFP was amplified by PCR, respectively with a primer pair (Table S1) of which the 5′-primer of EGFP or mRFP contains two consecutive restriction sites KpnI and NotI and the 3′-primer of EGFP or mRFP contains two consecutive restriction sites SmaI and BamHI. To further generate the construct of AtGHS40-mRFP or AtGHS40-EGFP in the modified pUC18, the complete ORF of AtGHS40 was amplified by PCR with a KpnI-AtGHS40 and NotI-AtGHS40 primer pair (Table S1). The PCR-amplified fragment of AtGHS40 was restricted with KpnI and NotI, then was ligated into pUC-mRFP or pUC-EGFP that was also cut with the same restriction sites. To generate the construct of EGFP-AtGHS40 in the modified pUC18, the complete ORF of AtGHS40 was amplified by PCR with a SmaI-AtGHS40 and BamHI-AtGHS40 primer pair (Table S1). The PCR-amplified fragment of AtGHS40 was restricted with SmaI and BamHI, then was ligated into pUC-EGFP that was also cut with the same restriction sites. The sequences of EGFP, AtGHS40-mRFP, AtGHS40-EGFP, and EGFP-AtGHS40 driven by 35S promoter were confirmed by DNA sequencing.
The method of particle bombardment is according to Sanford et al. (1993) with some modifications. AtRH57-EGFP, AtGHS40-mRFP, and EGFP constructs were coated onto gold particles (1 μm) and introduced into onion cells with Biolistic PDS-1000-He apparatus (Bio-Rad) under a vacuum of 27 in of Hg. Onion epidermal cells were placed into 1/2 MS medium with 1 % (w/v) sucrose before bombardment and incubated in the dark at 24 °C for 16–24 h after particle delivery. The nuclei were stained with 0.2 μg mL−1 4′,6-diamidino-2-phenylindole (DAPI) for 30 min at room temperature. Plasmids carrying AtGHS40 tagged with EGFP were transiently expressed in leaf protoplasts according to Yoo et al. (2007). EGFP or DAPI fluorescence was observed with confocal laser scanning biological microscope FV1,000 (Olympus). Excitation wavelengths and emission filters were 488 nm/bandpass 505–530 nm for EGFP, 584 nm/bandpass 605–630 nm for mRFP, and 358/461 nm for DAPI.
Results
ghs40-1 is hypersensitive to Glc-dependent inhibition of germination and early seedling growth
Thousands of T-DNA lines were screened to identify Glc-hypersensitive mutants, one of which was named as ghs40-1. The seedlings of T-DNA insertion mutant ghs40-1 (SALK_073682C) were smaller and slightly pale in color compared with WT of A. thaliana under normal growth conditions (Fig. 1b). Addition of high Glc concentrations to the MS (Murashige and Skoog 1962) medium decreased germination percentage and induced a post-germinative growth block in cotyledon greening and expansion in WT Arabidopsis seedlings (Zhou et al. 1998). Therefore, quantitative analysis was taken to examine seed germination and cotyledon development of the mutant. When grown in MS medium for 9 days, ghs40-1 mutant cotyledon greening and expansion were greatly impaired by the addition of Glc concentrations more than 3 % (Fig. 1a). At 4.5 % Glc, only 18 % of ghs40-1 seedlings were able to undergo greening and 24 % of their cotyledons expanded while both greening and expansion of WT cotyledons remained higher than 90 % (Fig. 1a). Their cotyledons remained white or dark brown and no true leaves were observed in ghs40-1 seedlings. Similarly, in the presence of either 150 or 200 mM NaCl, seed germination and cotyledon development of the mutant were severely impaired (Fig. 1a, c). In contrast, no visible phenotypic difference was observed in ghs40-1 compared with 9-day-old WT seedlings in the presence of either 3 or 4.5 % Mtl. These observations suggested that the Glc-hypersensitivity of ghs40-1 might be caused by mutation of AtGHS40 (At5g11240).
Effects of glucose (Glc) and NaCl on seed germination and early seedling development of the ghs40-1 mutant. a Wild-type (WT, white bars) Arabidopsis thaliana and ghs40-1 (SALK_073682C, black bars) seedlings were grown in sugar-free MS medium with or without the indicated concentrations of Glc, NaCl, and mannitol served as a control. The seed germination (radicle emergence) and early seedling development (cotyledon greening and expansion) rates were scored 9 days after stratification. b, c Representative images of WT and ghs40-1 seedlings. Approximately 100 seeds of each genotype were sown for each experiment. Data were obtained from three biologically independent experiments. Error bars represent standard deviation (SD; t test: *P < 0.05, **P < 0.01). bar = 5 mm
On the other hand, the T2 transgenic lines of Arabidopsis seedlings expressing 35S::AtGHS40 were examined. The AtGHS40 gene was successfully expressed in T2 transgenic lines (Fig. S2). No obvious difference was observed in 35S::AtGHS40 seedlings compared with the WT. However, with an increasing concentration of Glc, the 35S::AtGHS40 cotyledons grew better than the counterparts of WT. In the presence of 4.5 % Glc, the 35S::AtGHS40 roots grew longer than those of WT (Fig. 2a), suggesting that the transgenic plants expressing 35S::AtGHS40 are hyposensitive to high Glc conditions. The hyposensitive phenotype of 35S::AtGHS40 seedlings correlates with the hypersensitive feature of ghs40-1.
Effects of glucose (Glc) on Arabidopsis seedling development. a The Arabidopsis 35S::AtGHS40 lines compared with wild type (WT) in the presence of 3 and 4.5 % Glc conditions. b The 35S::AtGHS40-complemented lines in a ghs40-1 mutant background compared with the ghs40-1 mutant and WT. For each line, about 50 seedlings were grown on sugar-free MS medium containing 4.5 % Glc for 9 d after stratification. The experiment was repeated three times. Bar 5 mm
ghs40-1 mutation also had an inhibitory effect on hypocotyl growth of dark-grown seedlings under relatively high Glc conditions (Fig. S1). The length of ghs40-1 hypocotyls was greatly decreased by the addition of Glc higher than 3 %. In contrast, the length of ghs40-1 hypocotyls remained similar to WT in the presence of various concentrations of Mtl (Fig. S1).
Phenotype recovery of ghs40-1 by complementation with AtGHS40
To further confirm that the abnormal phenotypes of ghs40-1 might be due to the absence of AtGHS40, we examined the T3 transgenic seedlings expressing 35S::AtGHS40 in a ghs40-1 mutant background. Eight transgenic lines corresponding to independent transformation events were generated. The AtGHS40 gene was successfully expressed in the three complementation lines presented in Fig. S2. The three transgenic lines presenting different levels of recovery were presented (Fig. 2b). Thus, the expression of AtGHS40 driven by a 35S promoter indeed recovered phenotypes similar to the WT, thereby indicating that the AtGHS40 protein is essential for normal seedling growth.
Molecular characterization of ghs40 mutants
In addition to ghs40-1, another T-DNA insertion mutant ghs40-2 (SALK_052897) was examined (Fig. S3a). Polymerase chain reaction (PCR) analysis with the T-DNA-specific primer (BP) and AtGHS40-specific primer (RP) amplified a product from the mutants of ghs40-1 and ghs40-2, but not from WT (Fig. S3b), confirming that insertion occurs in AtGHS40. Further, PCR with the AtGHS40-specific primer pair (LP + RP) flanking the T-DNA insertion site amplified the predictable size of the gene product from WT but not from ghs40-1, confirming that the mutant is a homozygous; however, ghs40-2 is heterozygous given that the gene product was detected both in WT and in ghs40-2 (Fig. S3b). The ghs40-2 seeds, some of which were undeveloped ovules in the silique (Fig. S3c), also suggesting that ghs40-2 is heterozygous. Genotype analysis of a total number of 88 progenies indicated that the segregation of ghs40-2 heterozygous plants to WT was close to 2:1 (58/30). The homozygous ghs40-2 seeds could not be obtained, indicating that they are lethal because the fragment of T-DNA is inserted at the third exon of AtGHS40. It is different from ghs40-1 where the T-DNA fragment resides at the last intron of the gene. Thus, the homozygous ghs40-1 seeds are available. Portions of the 1845 bp open reading frame of ghs40-1 were amplified by reverse transcription-polymerase chain reaction (RT-PCR) to help define the physiological function of AtGHS40 and accumulation of the AtGHS40 transcript. The AtGHS40 transcript levels were absent or markedly reduced in ghs40-1. Significant reduced levels of mRNAs were detected with a primer pair that amplified a region upstream of the insertion site (primer pair A) in ghs40-1, but no product was detected with primer pair B, which spanned the insertion site (Fig. S3d). Quantitative real time PCR (qRT-PCR) analysis of the AtGHS40 transcripts in various organs of A. thaliana indicated that the gene was differentially expressed in various organs with high expressions in root (43-fold) and silique (24-fold) (Fig. S4).
AtGHS40 encodes a WD40 protein localized in the nucleus and nucleolus
The AtGHS40 gene (At5g11240) encodes a WD40 protein. Four WD40 repeats were found in the N-terminal half of the AtGHS40 protein by profile searching (Fig. S5). The two dipeptides GH and WD at both ends of a WD repeat conserved in WD1 and WD3 domains and alternative conserved amino acids among the other WD repeats were indicated by asterisks (Smith et al. 1999). AtGHS40 shares 58, 54, and 45 % identities with the unnamed or hypothetical proteins in Vitis vinifera (CBI22945.3), Glycine max (XP_003550446.1), and Oryza sativa (EEC75066.1), and 25 and 23 % identities with the WD repeat-containing proteins 43 in Danio rerio (AAI55127.1) and Homo sapiens (BAA05499.1), respectively (Fig. S5). Obviously, the protein is phylogenically closer to plant WD40 proteins. In addition, all plant representatives contain a putative nuclear localization signal (NLS, 76 to 84 amino acid residues) sequence that is absent in those sequences of animal WD40 proteins (Fig. S5).
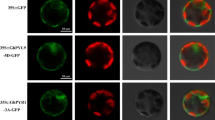
Because the AtGHS40 protein contains a bipartite nuclear localization sequence (NLS, 95 to 99 residues), the subcellular localization of AtGHS40 was examined. We generated a construct of AtGHS40 fused with the sequence of mRFP gene under the control of a 35S promoter (Fig. 3). AtRH57 fused with EGFP gene (AtRH57-EGFP) driven by a 35S promoter was used as an indicator of nucleolar localization (Hsu et al. 2014). The two constructs were then introduced into onion epidermal cells by particle bombardment. Fluorescence microscopy revealed that the AtGHS40-mRFP protein was localized in the nucleus, with a number of dense spots colocalized with the nucleolus in expressing onion cells (Fig. 3). The nucleolar localization of AtGHS40-mRFP was confirmed by colocalization of the protein with AtRH57-EGFP (Fig. 3). The use of DAPI only stained the nucleus, but not the nucleolus. EGFP protein, a control, was detected in the cytoplasm and nucleus. The cell protoplast isolated from A. thaliana was also used for subcellular localization of AtGHS40. AtGHS40 was fused with EGFP gene (AtGHS40-EGFP and EGFP-AtGHS40) and driven by a 35S promoter. The transient expression of the two constructs showed that the AtGHS40 protein was primarily localized in the nucleus (Fig. S6).
Subcellular localization of AtGHS40 in onion epidermal cells. Top panel indicates the constructs of AtGHS40–mRFP (monomeric red fluorescent protein), AtRH57–EGFP (enhanced green fluorescent protein), and EGFP only. Transient expression of these constructs in the onion epidermal cells. DAPI (4′,6-diamidino-2-phenylindole) or EGFP fluorescent field (left) and its bright fields (right) are indicated. The images of green and red fluorescent fields and a merge (right) of both are also indicated. The nucleolar AtRH57–EGFP proteins (Hsu et al. 2014) were used as a positive control for nucleolar location indicated by arrowheads. N nucleus; bar 50 μm
ghs40-1 exhibits ABA hypersensitivity may mainly through Glc-mediated ABA accumulation
Previous studies have established pivotal roles for ABA in plant Glc responses (Cheng et al. 2002; Carvalho et al. 2010). Exogenous application of ABA significantly affected germination and early growth of mutant cotyledons (Fig. 4a). In 0.25 μM ABA, little effect occurred on greening and expansion of WT cotyledons whereas approximately 88 and 77 % of the mutant cotyledons achieved greening and expansion, respectively. Application of higher ABA concentrations resulted in a more pronounced decrease in cotyledon greening and expansion of mutant seedlings compared with WT, indicating that ghs40-1 exhibited hypersensitivity to ABA. Previous studies reported that ABA accumulation is induced by Glc during early stages of seedling growth (Carvalho et al. 2010). This indication is armored by the observation that WT seedlings fail to arrest growth in 6 % Glc supplemented with fluridone (Ullah et al. 2002). Fluridone, an inhibitor of ABA synthesis, interrupts carotenoid synthesis generating an albino-like seedling and so only cotyledon expansion was measured. Without fluridone addition, the presence of Glc did not significantly affect early development of WT seedlings, but adverse effects on the development of ghs40-1 seedling were obvious (Fig. 4b). The significant developmental arrest observed in ghs40-1 seedlings in the medium containing 3 or 4.5 % Glc was greatly reverted when supplemented with 1 μM fluridone (Fig. 4b). Therefore, this result suggested that seedling growth arrest in the mutant may mainly function through Glc-mediated ABA biosynthesis.
Abscisic acid (ABA) phenotypes of the ghs40-1 mutant. a ghs40-1 seedlings are hypersensitive to ABA. Wild type (WT) and ghs40-1 seeds were sown either in sugar-free medium or in medium containing the indicated ABA concentrations. Seed germination (radicle emergence) and early seedling development (cotyledon greening and expansion) percentages were scored 9 d after stratification. b The addition of fluridone reduces the Glc-induced inhibition of cotyledon expansion. The WT and ghs40-1 seeds were sown in sugar-free medium or in the presence of Glc supplemented with or without 1 μM fluridone. Cotyledon expansion percentages of WT and ghs40-1 were scored 9 d after stratification. Approximately 100 seeds of each genotype were sown for each experiment. Data were obtained from three biologically independent experiments. Error bars represent standard deviation (SD; t test: *P < 0.05, **P < 0.01)
Transcripts of photosynthesis genes and a gene related to nitrogen metabolism are repressed in ghs40-1 whereas transcripts of ABA degrading, and signaling genes, and two genes related to starch and anthocyanin biosynthesis are significantly induced by Glc
Given that seedling growth arrest in the mutant mainly functions through Glc-induced ABA biosynthesis, we examined the regulation of ABA biosynthesis gene expression under 4.5 % Glc conditions in WT and mutant seedlings using qRT–PCR analysis. Exposure of 9-day-old WT seedlings and ghs40-1 mutants to 4.5 % Glc for a short induction period resulted in induced expressions of AAO3, ABA3, and NCED3 in both plants. However, the induced transcripts of ABA biosynthesis genes in ghs40-1 were similar to those in the WT (Fig. 5b). This finding seemed to contradict the Glc hypersensitivity feature of ghs40-1. The ABA endogenous levels in Col-0 and ghs40-1 seedlings grown in the presence of either 4.5 % Glc or 4.5 % Mtl were further determined. The ABA-deficient aba2 mutant (Cheng et al. 2002) was included as a control. Table 1 shows no significant difference in ABA content between WT and mutant seedlings in the absence of sugar. The presence of 4.5 % Glc induced a 1.5- and 1.3-fold increase in endogenous levels of ABA in WT and ghs40-1 seedlings, respectively. Similarly, an exogenous addition of 4.5 % Mtl induced a 1.8- and 1.7-fold increase in endogenous levels of ABA in WT and ghs40-1 seedlings, respectively (Table 1). It is noted that the increased ABA level in ghs40-1 is much less than that in WT seedlings under 4.5 % Glc or 4.5 % Mtl treatment. To realize the mystery, ABA degrading genes were examined using qRT–PCR analysis. The transcripts of three ABA degrading genes, namely, CYP707A2, CYP707A3, and CYP707A4, were significantly increased by 4 h of inductive treatment with either 4.5 % Glc or Mtl in ghs40-1 (Figs. 5b, S7). The increased transcripts of those ABA degrading genes under high Glc or Mtl conditions could balance the accumulation of ABA generated by ABA biosynthesis genes, resulting in a decreased ABA level in ghs40-1 compared with WT seedlings.
Quantitative real-time PCR (qRT-PCR) analysis of glucose (Glc)-regulated gene expression in the ghs40-1 mutant. RNA levels of a Glc-responsive and b ABA biosynthesis, degrading, and signaling genes were determined by qRT-PCR analysis using total RNA isolated from nine-day-old seedlings of wild type (WT) and ghs40-1. The seedlings were incubated with shaking in sugar-free MS containing 4.5 % Glc or mannitol (Mtl) for indicated time intervals before harvest. The y-axis represents the relative transcript abundance ratios expressed in arbitrary units. Primers for qRT-PCR analysis were listed in Table S1. The analyzed Glc-responsive genes: CAB1, chlorophyll a/b-binding protein 1; PC2, plastocyanin 2; CHS, chalcone synthase; APL3, ADP-Glc pyrophosphorylase large subunit; and ASN1, asparagine synthetase 1. ABA biosynthesis genes: ABA3, NCED3, and AAO3; ABA degrading genes: CYP707A2, CYP707A3, and CYP707A4; ABA signaling genes: ABI1, ABI3, ABI4, and ABI5 and a protein kinase gene, SnRK2.6. ACTIN1 was used as an internal control for qRT-PCR analysis. Data were obtained from three biologically independent experiments. Error bars represent standard deviation (SD; t test: **P < 0.01)
ABI3 acts upstream of ABI5 to execute seedling growth arrest during germination (Lopez-Molina et al. 2002). ABI4 also induces ABI5 gene expression in response to sugar (Arroyo et al. 2003). The ABA signaling genes are also activated by ABA, salt, and sugar (Lopez-Molina et al. 2001; Cheng et al. 2002; Bossi et al. 2009). Thus, the transcription of the three ABA signaling genes in ghs40-1 seedlings was examined by qRT–PCR. The transcripts of ABI3, ABI4, and ABI5 were significantly increased by 4.5 % Glc in ghs40-1 after a short period of induction (Fig. 5b). However, the transcripts of ABI1 and SnRK2.6, a protein kinase gene, were not appreciably changed in ghs40-1 seedlings after a short period of high Glc treatment (Fig. 5b). These results are consistent with the ABA-hypersensitive feature of the ghs40-1 mutant, thereby indicating that AtGHS40 could be involved in the transcriptional down-regulation of these ABA signaling genes under high Glc conditions.
Several other sugar-responsive genes were also analyzed. APL3, which encodes a large subunit of ADP-Glc pyrophosphorylase, a key enzyme for starch synthesis, and CHS, which encodes a chalcone synthase, a key enzyme for anthocyanin biosynthesis, were significantly induced in ghs40-1 compared with the WT seedlings under the treatment of 4.5 % Glc (Fig. 5a). On the other hand, 4.5 % Glc greatly repressed photosynthetic genes CAB1, which encodes chlorophyll a/b-binding protein 1, and PC2, which encodes a plastocyanin protein in ghs40-1 compared with the WT seedlings. 4.5 % Glc also significantly repressed nitrogen metabolism related gene ASN1, which encodes asparagine synthetase 1 (Fig. 5a). No appreciable change in gene expression was observed between ghs40-1 and WT seedlings when 4.5 % Mtl was used as a control. These altered Glc responses may not attribute to osmotic stress, but rather to the loss of function of ghs40-1.
AtGHS40 transcripts are significantly induced by high Glc, ABA, and various stresses
Expression of AtGHS40 under various stress conditions was examined by qRT-PCR analysis. The AtGHS40 transcript was strongly induced when 9-day-old WT seedlings were incubated in medium containing either 4.5 % Glc or 50 μM ABA for a short period of induction (Fig. 6a). Significant induction was also observed when 9-day-old WT seedlings were incubated in medium containing 300 mM NaCl or under either cold or drying treatments for a short induction period (Fig. 6a).
Expression profiles of transcript in Arabidopsis thaliana and various mutants. a AtGHS40 transcript in A. thaliana analyzed by quantitative real-time PCR (qRT-PCR). Determination of the effect of various stresses on AtGHS40 expression at various time points. ACTIN1 was used as an internal control for qRT-PCR analysis. Data were obtained from three biologically independent experiments. b AtGHS40 acts in a signaling network downstream of HXK1. RNA levels of AtGHS40, HXK1, and ABI4 were determined by qRT-PCR using total RNA isolated from nine-day-old seedlings of WT, ghs40-1, hxk1 (SALK_070739), and abi4 (CS8104) that were incubated with shaking in sugar-free MS medium containing 4.5 % Glc for indicated time intervals before harvest. ACTIN1 was used as an internal control for qRT-PCR analysis. The y axis represents the relative transcript abundance ratios expressed in arbitrary units. ABI4 is an ABA signaling gene. HXK1, the hexokinase1 gene. Data were obtained from three biologically independent experiments. Error bars represent standard deviation (SD; t test: *P < 0.05, **P < 0.01)
AtGHS40 acts downstream of HXK1 and is activated by ABI4 while ABI4 expression is negatively modulated by AtGHS40
To further elucidate the role of AtGHS40 in sugar signaling, qRT–PCR was performed using total RNA obtained from 9-day-old seedlings of WT, ghs40-1, hxk1 and abi4, which were incubated with shaking in the medium containing 4.5 % Glc for the indicated time intervals. Since both HXK1 and ABI4 are essential for sugar and ABA signaling (Dekkers et al. 2008; Lee et al. 2012), the relationships of HXK1 and ABI4 to AtGHS40 were examined. As shown in Fig. 6b, no change in HXK1 expression was observed in ghs40-1 mutants compared with the WT, whereas AtGHS40 expression was significantly reduced in hxk1 mutants under 4.5 % Glc condition for 6 h. It is in accordance with the observation that the exogenous addition of 50 µM ABA or 300 mM NaCl significantly induced AtGHS40 expression (Fig. 6a). These findings suggest that AtGHS40 acts in a signaling network downstream of ABA. Similarly, no change in HXK1 expression was observed in abi4 compared with WT, whereas ABI4 expression was significantly reduced in hxk1 mutants (Fig. 6b). Therefore, ABI4 acted downstream of HXK1, and this is consistent with the result of a previous report, which established that ABI4 acts downstream of HXK1 Glc sensors (Arenas-Huertero et al. 2000; Leόn and Sheen 2003).
qRT–PCR analysis was also used to examine the relationship between ABI4 and AtGHS40. AtGHS40 expression was markedly repressed in abi4 mutants compared with WT, whereas ABI4 expression was significantly induced in ghs40-1 (Fig. 6b). This finding suggests that a fine-tuning modulation occurs in plants under high Glc conditions where ABI4 expression was negatively modulated by AtGHS40 while ABI4 activates AtGHS40 expression in the ABA-mediated Glc signaling pathway (Fig. 7).
Discussion
WD40 proteins are responsive to hormones and different environmental stresses (Ananieva et al. 2008; Shi et al. 2011). We have identified a Glc- and ABA- hypersensitive mutant, ghs40-1, thereby suggesting that AtGHS40 may be a crucial player in the sugar-signaling pathway during early seedling growth in Arabidopsis. With the analysis of qRT–PCR, AtGHS40 is activated by ABI4 while ABI4 expression is negatively modulated by AtGHS40 in the Glc signaling network. However, the final proof is relied on genetic analysis. As proposed in Fig. 7, high Glc levels up-regulate the HXK1-mediated expression of ABA biosynthesis genes and an increase in ABA levels enhances ABA signaling gene expression, resulting in sugar-mediated seedling growth arrest. The AtGHS40 gene is also up-regulated as a consequence of ABA accumulation. The increased AtGHS40 not only inhibits the expression of ABA signaling genes such as ABI3, ABI4 and ABI5, thereby counteracting growth arrest but inhibits ABA degrading gene expression, thereby assisting ABA accumulation and growth arrest. However, AtGHS40 may not affect ABI1 and SnRK2.6 gene expression. It is in agreement with the concept that ABI1 does not likely play a critical role in transmitting sugar signals during seed germination (Price et al. 2003). ABI1 inactivates SnRK2.6 kinase via dephosphorylation of Ser/Thr residues in the activation loop (Xie et al. 2012). SnRK2.6 is not only known as a positive regulator of ABA signaling mediating the control of stomatal aperture, but has a role in the regulation of carbon and energy supply (Zheng et al. 2010).
Thus, a circuit of fine-tuning mechanism, by which ABA and ABA signaling gene expression are modulated in balance, occurs in plants (Fig. 7). The AtGHS40 protein acts as a modulator, ensuring appropriate generation of ABI4, which plays a crucial role in ABA signaling during early seedling development (Finkelstein et al. 1998). Upon AtGHS40 mutation, the relief of inhibition of ABA degrading genes may result in a decrease in the ABA content to a level lower than that in the WT under 4.5 % Glc conditions (Table 1); however, the ghs40-1 seedlings remain hypersensitive. Thus we report a new player, AtGHS40, in the sugar signaling transduction pathway. Other transcription factors play varied roles in connecting proline accumulation to sugar sensing (Verslues and Bray 2006), in chloroplast retrograde signaling (Koussevitzky et al. 2007), lipid mobilization from the embryo (Penfield et al. 2006), and in bidirectional communication between the plastid and nucleus (Lee et al. 2012).
Many genes that encode WD40 proteins in Arabidopsis are diverse in function and modulate different biological processes. The exact role of most plant WD40-containing proteins remains unclear and requires further study. AtGHS40 is a predicted ortholog of UTP5, which is a component of the t-UTP complex involved in ribosome assembly in yeast (Pérez-Fernández et al. 2007). That rRNA biogenesis mainly occurs in the nucleolus is all in agreement with the nucleolar localization of the AtGHS40 protein. Similar to AtGHS40, SLOW WALKER1 encodes a WD40 protein that is reportedly involved in 18S ribosomal RNA biogenesis (Shi et al. 2005). Recently, a new member of WD40 repeat proteins in A. thaliana, known as GTS1, reportedly interacts with two ribosomal protein partners (i.e., a ribosome-biogenesis factor L19e and a component of ribosome Nop16) in controlling plant growth development (Gachomo et al. 2014).
In conclusion, the ghs40-1 seedlings exhibit enhanced Glc- and ABA-hypersensitivity in Arabidopsis. The AtGHS40 gene encodes a WD40 protein localized in the nucleus and nucleolus. AtGHS40 acts in a signaling network downstream of HXK1 and is activated by ABI4 while ABI4 expression is negatively modulated by AtGHS40. Both ABA signaling and degrading genes are down-regulated by AtGHS40, thereby generating a fine-tuning modulation in the Glc responses in plants. Thus, AtGHS40 may play an important role in ABA-mediated Glc signaling during early seedling development.
Abbreviations
- ABA:
-
Abscisic acid
- ABI :
-
ABA insensitive
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- EGFP:
-
Enhanced green fluorescent protein
- EtBr:
-
Ethidium bromide
- ghs :
-
Glucose hypersensitive
- Glc:
-
Glucose
- HXK1 :
-
Hexokinase1
- mRFP:
-
Monomeric red fluorescent protein
- Mtl:
-
Mannitol
- qRT-PCR:
-
Quantitative real-time-PCR
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- WT:
-
Wild type
References
Ananieva EA, Gillaspy GE, Ely A, Burnette RN, Erickson F (2008) Interaction of the WD40 domain of a myoinositol polyphosphate 5-phosphatase with SnRK1 links inositol, sugar, and stress signaling. Plant Physiol 148:1868–1882
Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P (2000) Analysis of Arabidopsis glucose-insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14:2085–2096
Arroyo A, Bossi F, Finkelstein RR, Leόn P (2003) Three genes that affect sugar sensing (abscisic acid insensitive 4, abscisic acid insensitive 5, and constitutive triple response 1) are differentially regulated by glucose in Arabidopsis. Plant Physiol 133:231–242
Bossi F, Cordoba E, Dupré P, Mendoza MS, Román CS, León P (2009) The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59:359–374
Bu Q, Li H, Zhao Q, Zhang J, Wu X, Sun J et al (2009) The Arabidopsis RING finger E3 ligase RHA2a is a novel positive regulator of abscisic acid signaling during seed germination and early seedling development. Plant Physiol 150:463–481
Carvalho RF, Carvalho SD, Duque P (2010) The plant-specific SR45 protein negatively regulates glucose and ABA signaling during early seedling development in Arabidopsis. Plant Physiol 154:772–783
Chen JG, Ullah H, Temple B, Liang J, Guo J, Alonso JM et al (2006) RACK1 mediates multiple hormone responsiveness and developmental processes in Arabidopsis. J Exp Bot 57:2697–2708
Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A et al (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14:2723–2743
Chothia C, Hubbard T, Brenner S, Barns H, Murzin A (1997) Protein folds in the all-beta and all-alpha classes. Annu Rev Biophys Biomol Struct 26:597–627
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Dekkers BJ, Schuurmans JA, Smeekens SC (2008) Interaction between sugar and abscisic acid signaling during early seedling development in Arabidopsis. Plant Mol Biol 67:151–167
Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10:1043–1054
Gachomo EW, Jimenez-Lopez JC, Baptiste LJ, Kotchoni SO (2014) GIGANTUS1 (GTS1), a member of Transducin/WD40 protein superfamily, controls seed germination, growth and biomass accumulation through ribosome-biogenesis protein interactions in Arabidopsis thaliana. BMC Plant Biol 14:37. doi:10.1186/1471-2229-14-37
Gao X, Chen Z, Zhang J, Li X, Chen G, Li X et al (2012) OsLIS-L1 encoding a lissencephaly type-1-like protein with WD40 repeats is required for plant height and male gametophyte formation in rice. Planta 235:713–727
Gibson SI (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8:93–102
Guo J, Wang J, Xi L, Huang WD, Liang J, Chen JG (2009) RACK1 is a negative regulator of ABA responses in Arabidopsis. J Exp Bot 60:3819–3833
Hsu YF, Yu SC, Yang CY, Wang CS (2011) Lily ASR protein-conferred cold and freezing resistance in Arabidopsis. Plant Physiol Biochem 49:937–945
Hsu YF, Chen YC, Hsiao YC, Wang BJ, Lin SY, Cheng WH et al (2014) AtRH57, a DEAD-box RNA helicase, is involved in feedback inhibition of glucose-mediated abscisic acid accumulation during seedling development and additively affects pre-ribosomal RNA processing with high glucose. Plant J 77:119–135
Huang Y, Li CY, Pattison DL, Gray WM, Park S, Gibson SI (2010) SUGAR-INSENSITIVE3, a RING E3 ligase, is a new player in plant sugar response. Plant Physiol 152:1889–1900
Jiang L, Wang Y, Li QF, Björn LO, He JX, Li SS (2012) Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res 22:1046–1057
Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M et al (2007) Signals from chloroplasts converge to regulate nuclear gene expression. Science 316:715–719
Lee JH, Terzaghi W, Deng XW (2011) DWA3, an Arabidopsis DWD protein, acts as a negative regulator in ABA signal transduction. Plant Sci 180:352–357
Lee SA, Yoon EK, Heo JO, Lee MH, Hwang I, Cheong H et al (2012) Analysis of Arabidopsis glucose insensitive growth mutants reveals the involvement of the plastidial copper transporter PAA1 in glucose-induced intracellular signaling. Plant Physiol 159:1001–1012
Leόn P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8:110–116
Li D, Roberts R (2001) WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases. Cell Mol Life Sci 58:2085–2097
Lopez-Molina L, Mongrand S, Chua NH (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98:4782–4787
Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH (2002) ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 32:317–328
Maliga P, Klessig DF, Cashmore AR, Gruissem W, Varner JE (1995) Methods in plant molecular biology: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Mccurdy RD, Mcgrath JJ, Mackay-sim A (2008) Validation of the comparative quantification method of real-time PCR analysis and a cautionary tale of housekeeping gene selection. Gene Ther Mol Biol 12:15–24
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco. Physiol Plant 15:473–497
Pattanaik S, Patra B, Singh SK, Yuan L (2014) An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Front Plant Sci 5:259. doi:10.3389/fpls.2014.00259
Penfield S, Li Y, Gilday AD, Graham S, Graham IA (2006) Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18:1887–1899
Pérez-Fernández J, Román A, De Las Rivas J, Bustelo XR, Dosil M (2007) The 90S preribosome is a multimodular structure that is assembled through a hierarchical mechanism. Mol Cell Biol 27:5414–5429
Price J, Li TC, Kang SG, Na JK, Jang JC (2003) Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol 132:1424–1438
Ramsay NA, Glover BJ (2005) MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci 10:63–70
Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14(Suppl):S185–S205
Sanford JC, Smith FD, Russell JA (1993) Optimizing the biolistic process for different biological applications. Methods Enzymol 217:483–509
Shi DQ, Liu J, Xiang YH, Ye D, Sundaresan V, Yang WC (2005) SLOW WALKER1, essential for gametogenesis in Arabidopsis, encodes a WD40 protein involved in 18S ribosomal RNA biogenesis. Plant Cell 17:2340–2354
Shi S, Chen W, Sun W (2011) Comparative proteomic analysis of the Arabidopsis cbl1 mutant in response to salt stress. Proteomics 11:4712–4725
Smith TF, Gaitatzes C, Saxena K, Neer EJ (1999) The WD repeat: a common architecture for diverse functions. Trends Biochem Sci 24:181–185
Stirnimann CU, Petsalaki E, Russell RB, Müller CW (2010) WD40 proteins propel cellular networks. Trends Biochem Sci 35:565–574
Stone SL, Williams LA, Farmer LM, Vierstra RD, Callis J (2006) KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell 18:3415–3428
Ullah H, Chen JG, Wang S, Jones AM (2002) Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol 129:897–907
Ullah H, Chen JG, Temple B, Boyes DC, Alonso JM, Davis KR et al (2003) The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15:393–409
van Nocker S, Ludwig P (2003) The WD-repeat protein superfamily in Arabidopsis: conservation and divergence in structure and function. BMC Genom 4:50
Verslues PE, Bray EA (2006) Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J Exp Bot 57:201–212
Xie T, Ren R, Zhang YY, Pang Y, Yan C, Gong X et al (2012) Molecular mechanism for inhibition of a critical component in the Arabidopsis thaliana abscisic acid signal transduction pathways, SnRK2.6, by protein phosphatase ABI1. J Biol Chem 287:794–802
Xiong L, Ishitani M, Lee H, Zhu JK (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13:2063–2083
Yang CY, Chen YC, Jauh GY, Wang CS (2005) A lily ASR protein involves abscisic acid signaling and confers drought and salt resistance in Arabidopsis. Plant Physiol 139:836–846
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Zeng CJ, Lee YR, Liu B (2009) The WD40 repeat protein NEDD1 functions in microtubule organization during cell division in Arabidopsis thaliana. Plant Cell 21:1129–1140
Zhao L, Gao L, Wang H, Chen X, Wang Y, Yang H et al (2013) The R2R3-MYB, bHLH, WD40, and related transcription factors in flavonoid biosynthesis. Funct Integr Genomics 13:75–98
Zheng Z, Xu X, Crosley RA, Greenwalt SA, Sun Y, Blakeslee B et al (2010) The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol 153:99–113
Zhou L, Jang JC, Jones TL, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95:10294–10299
Zhu J, Jeong JC, Zhu Y, Sokolchik I, Miyazaki S, Zhu JK et al (2008) Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc Natl Acad Sci USA 105:4945–4950
Acknowledgments
This study was supported by the Ministry of Science and Technology, Taiwan, Republic of China (NSC-103-2911-I-005-301, NSC-102-2911-I-005-301) and the Ministry of Education, Taiwan, R.O.C. under the ATU plan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y.-C. Hsiao, Y.-F. Hsu and Y.-C. Chen have equal contribution in this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hsiao, YC., Hsu, YF., Chen, YC. et al. A WD40 protein, AtGHS40, negatively modulates abscisic acid degrading and signaling genes during seedling growth under high glucose conditions. J Plant Res 129, 1127–1140 (2016). https://doi.org/10.1007/s10265-016-0849-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0849-5