Abstract
The moss-like river-weeds or Podostemaceae offer a special opportunity to study the diversity and evolution of plants that are adapted to extreme environments. This paper reviews multidisciplinary studies on this subject. Based on field work in the four continents, we discovered many species and several genera that are new components of biodiversity, and revealed the Podostemaceae floras of East Asia, Southeast Asia, and Australia. The historical biogeography of the family, i.e., the change in distribution in space and time, is characterized by a few dispersals between continents, followed by diversification within each continent. Local species may be derived from parts of separated populations of parental species, which consequently are paraphyletic. The remarkable morphological adaptations of Podostemaceae include the development of the horizontal axis in plant body, with which the plants adhere to rock surfaces under violent current. The vertical axis is reduced or lost and the horizontal axis develops in the embryo and seedling. We also found saltational organ-level variation, such as presence or absence of shoot, shoot apical meristem, root, and root cap; the form of shoot and root; the mode of root branching and leaf production; and the number of cotyledons. Morphological evolution may not be always adaptive to the habitats, which are rocks periodically submerged across the distribution range. Analyses of shoot regulatory gene expression found that, in contrast to the expression pattern in primitive species with ordinary shoots, which is comparable with Arabidopsis, the unique pattern in derived species may result in ‘fuzzy’ morphology of the shoot and leaf. Finally, problems for future study are pointed out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Unique aquatic plants
Plant evolution and adaptation to the environment is a central issue for evolutionary biology. Plants are usually terrestrial and can also grow in extreme environments, such as deserts, sea water, fresh water, hot springs, on rocks, on trees, on high mountains, and in severely cold regions (e.g., Sculthorpe 1967; Borgen and Jonsell 1997). Plants living in marginal environments have remarkable characters that are products of unique evolution under strong and specific selection pressure. Information gained by studying such plants significantly expands and deepens our knowledge of plant evolution, which has been accumulated mostly from the study of plants adapted to ordinary environments. Unique aquatic plants that are adapted to the most extreme environments include Hydrostachyaceae, Lemnaceae and Podostemaceae (Landolt et al. 1988).
Podostemaceae are the largest, exclusively aquatic family of the 413 angiosperm families recognized in the third Angiosperm Phylogeny Group (APG III) classification (Cook 1996; Haston et al. 2009). Plants of Podostemaceae grow in waterfalls and rapids in the tropics and subtropics worldwide, rarely in temperate regions (Japan and eastern North America), where water levels rise or drop seasonally; they never grow outside these habitats. In these habitats other macrophytes hardly survive. Currently, aquatic podostemads are confronted with extinction caused by destruction and change of their irreplaceable habitats due to human activities, such as dam construction, water pollution, and deposition of sediment onto rocks (Philbrick et al. 2010; Lansdown 2012). The plants adhere to submerged rock surfaces, resisting violent current during the rainy season, then flower and fruit shortly after being exposed to air during the following dry season (Fig. 1a‒f). Exposure is necessary for reproduction. Podostemaceae can live in a thin layer sandwiched between the swift current and the rock surface. Although angiosperms have vascular tissue for support against gravity and conduction for aerial parts, the vascular tissue of Podostemaceae is reduced or lost under the absence of those stresses in their aquatic habitat. Major transportation of water and solutes is probably symplastic. Furthermore, they hardly tolerate desiccation and dry quickly when exposed to air. Thus, morphologically Podostemaceae are dissimilar to terrestrial angiosperms, and partly resemble liverworts, mosses, or seaweeds. They are called river-weeds, or moss-like river-weeds in Japan and China.
Podostemaceae in natural habitats. a Cladopus doianus with ribbon-like roots adhering to submerged rock at vegetative stage, photographed in Sendai River, Kagoshima, Japan by S. Koi. b Hydrobryum japonicum with crustose root adhering to submerged rock, photographed in Okawa River, Kagoshima, Japan by S. Koi. Filiform tufted leaves are scattered on root. Inset, exposed minute flowers (each flower has two stamens and one ellipsoid ovary). c Hydrodiscus koyamae, which is rootless and has a floating shoot, photographed in Phu Khao Khouay National Protection Area, Laos by D. Juffe. Inset Basal portion of dried shoot with basal disk adhering to rock surface. d Dalzellia zeylanica, which is rootless and has submerged lobed crustose shoot with leaves dense on dorsal surface (minute) and margin (pale), photographed in Kerala, India by MK. e Terniopsis minor with ribbon-like roots adhering to rock surface and short shoots on flanks of roots, photographed in Klong Yai, Chanthaburi, Thailand by MK. f Mourera fluviatilis with emerging inflorescences and submerged leaves, photographed in Essequibo River, Guyana by MK. It is apparently stemless. Scale bars = 1 cm in a, b, b inset, c inset, e; approx. 20 cm in c, f; 5 mm in d
Podostemaceae live on rocks in torrents throughout their life history. Their life cycle proceeds in response to the seasonal change of water levels. They produce small seeds (generally up to 0.3 mm long) shortly after the dry season begins. The seeds are dispersible over long distances. When wet (e.g., at the beginning of the rainy season), the seed coat imbibes water, becomes sticky and adheres to rock surfaces. In most species, neither the plumule nor radicle develops, but instead an adventitious root forms in the seedling and attaches to the rock surface (Philbrick 1984; Mohan Ram and Sehgal 1997; Suzuki et al. 2002). During the rainy season, the plants grow submerged exclusively in the vegetative phase, but growth in response to seasonal change is not well documented and requires close field observations. In the early dry season, the plants form flower buds underwater, perhaps in response to changes in light intensity, and come into flower when exposed to the air. In Hydrobryum japonicum the flowers are formed endogenously under the vegetative leaf scars (Katayama et al. 2008) and are dense on the dorsal surface of the crustose root (Fig. 1b). Generally Podostemaceae are annual. In the field, drying begins from the top of rocks downward, while plants at the bottom and in water are still fresh. In sympatric localities in Asia, species of Tristichoideae occur on deeper rocks than do other species of Podostemoideae, indicating that Tristichoideae remains submerged longer. There is moderate habitat segregation in some species.
The flowers in Podostemaceae generally are small with little attractive tepals, semiapetalous, probably anemophilous, and single (Fig. 1b) or sometimes in inflorescences (Fig. 1f). A few genera, Apinagia, Mourera and Rhyncholacis, have reduced tepals but instead have many colored stamens and colored pedicels, which attract insects (Fig. 1f); these are secondarily entomophilous. In the 3 or 4-celled embryo sac, double fertilization does not occur and as a result, endosperm is not produced; this is a unique feature of Podostemaceae (Cook and Rutishauser 2007; Sehgal et al. 2011). A large number of such small seeds are dispersed by wind and also other vectors, e.g., birds. However, many species are local endemics or even single-river endemics, suggesting low long-distance dispersal success.
The ancestor of Podostemaceae was terrestrial and invaded extreme aquatic habitats. It is likely that the invasion involved remarkable adaptations, because violent currents sweep plants from rock surfaces. After the invasion, the family repeatedly speciated and diversified in similar environments worldwide. The special habitat does not appear to allow coexistence of competitors. There are great variations at the genus and species levels, and some of them may be additional adaptations while others may not be novel adaptations. More than a century ago, Willis (1914, earlier papers) claimed that variation happened in the absence of adaptation, and “there must have been evolution by means of mutations, and without natural selection”.
As described above, the ecology, distribution and morphology of Podostemaceae are extreme and hardly comparable to other plants. However unique, they must have been derived from a terrestrial ancestor and then distributed and diversified. This unique adaptations have attracted evolutionary botanists. The processes of evolution, however, has remained uncertain, partly because of the difficulty in applying general botanical knowledge. Therefore, we have performed and developed multidisciplinary studies to investigate Podostemaceae and to provide new insights into the biogeography, morphology, evolution, and ecology of members of the family. This article reviews those and other relevant studies and presents questions to be solved.
Discovering diversity
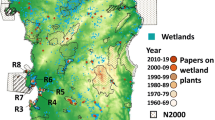
Species are components of biodiversity. Information on where and how species live is basic to understanding plant diversity, which is distributed unevenly over the surface of the earth and has changed in the course of time. Full understanding of biodiversity requires reduction in the number of unknown species, and increased species data facilitated by research on the diversity and evolution of Podostemaceae (Kato 2013). The first member of Podostemaceae discovered (from Suriname in 1775) was the colorful, prominent Mourera fluviatilis (Fig. 1f). Subsequently, particularly in the last three decades, a number of species were described from around the world (Cook and Rutishauser 2007). In Japan, Podostemaceae were discovered for the first time in 1927 by Shun-ichiro Imamura, a student of Kyoto University (Imamura 1927, 1928a, b). During the few years after his discovery, most species were found from southern Kyushu (Fig. 2). Nonetheless, further exploration is necessary to discover unknown species, which are likely to be local endemics.
Distribution and habitat of Podostemaceae in Japan. a Map showing localities in Miyazaki (1) and Kagoshima Prefectures (2‒19): 1 Iwase River at Kobayashi; 2 Sendai River at Satsuma; 3 Kubuki River, tributary of Sendai River at Yamazaki, Satsuma; 4 Amori River at Shiobitashi, Kirishima; 5 Inukai Waterfall in Nakatsu River, tributary of Amori River, Kirishima; 6 Ryumon Waterfall in Amikake River, Aira; 7 Manose River, Minami-Satsuma; 8 Mawatari River, Minami-Kyushu; 9 Anraku River, Shibushi; 10 Mae River, Shibushi; 11 Okawa River at Haendani, Kinko; 12 Urushiyama Waterfall in Kami River, Kinko; 13 Tomano River at Aira, Kanoya; 14 Takasu River at Gonohara, Kanoya; 15 Kamikawa-Ohtaki Waterfall in Kami River, Kinko; 16 Okawa River at Minami-Osumi; 17 Okawa River at Hanaze, Kinko; 18 Okawa River at Udono, Kinko; 19 Isso River, Yaku Island. No. 1 is inhabited by Hydrobryum koribanum; 2‒8, Cladopus doianus; 9, C. doianus and H. floribundum; 10, H. floribundum; 11, C. fukienensis; 12‒18, H. japonicum; 19, H. puncticulatum. Cladopus doianus also occurs on concrete bottom of manmade channel at Kijo, Miyazaki Prefecture (Seno and Hattori 2013). b Welded tuff bank of Iwase River, Kobayashi, where H. koribanum grows (No. 1 in a)
To investigate the diversity of Podostemaceae, I explored various Asian regions, Australia, tropical Africa (Cameroon, Gabon), Central America (Mexico, Panama), and South America (Guyana, Brazil). Some other areas were explored by my colleagues: South (Ecuador, Venezuela), Central (Costa Rica) and North America, Madagascar, Southeast Asia (Myanmar, Papua New Guinea), and Africa (Ghana, South Africa, Tanzania). Furthermore, I commenced a floristic study of Podostemaceae in Thailand for the Flora of Thailand project in 1999. Cusset (1992 and previous publications cited therein) had enumerated seven genera and 10 species from Thailand, but we have recorded 10 genera and 50 species across Thailand so far (Kato 2004, 2006; Koi et al. 2008; Kato and Koi 2009; Werukamkul et al. 2012). Among them, 34 species were new to science, and Cussetia, Paracladopus and Thawatchaia were described as new genera (Kato et al. 2004; Kato 2006). The floristic study was extended to the Podostemaceae of Laos, resulting in enumerating nine genera and 35 species (Koi and Kato 2012, 2015a, b) in that country. It is a remarkable increase from the 2002 count of four genera and seven species (Kato and Fukuoka 2002) and includes 17 new species and the new genus Hydrodiscus. The discovery of a new species of Hydrobryum with ribbon-like roots changed the concept of the genus, which has been characterized by its crustose root (Koi and Kato 2012). Hydrodiscus is characterized by an elongate shoot with an anchoring basal disk, being rootless and monocotyledonous (Fig. 1c). The genera Dalzellia and Terniopsis (as Malaccotristicha) had been recognized to be small and restricted in distribution outside Southeast Asia (Cusset and Cusset 1988a), but we found remarkable diversity of the genera in Southeast Asia (Kato 2006; Kato and Koi 2009; Koi and Kato 2015a). This increased knowledge is useful for understanding the biogeography and evolution of Podostemaceae. The Podostemaceae of Cambodia and Vietnam have been poorly investigated. Recent field work resulted in finding many more species than indicated in previous reports (Kato 2011; M. Kato unpubl. data).
South Asia, i.e., India and Sri Lanka, where they were extensively investigated by Willis (1902) and his followers, is another biodiversity hotspot for Podostemaceae. We also botanized in northeast, central and southern India, and Sri Lanka, with the results that the Podostemaceae are more diverse than we were aware (M. Kato unpubl. data). Our contribution is a proposal for a new genus, Indodalzellia (1 species), with crustose dorsiventral shoots borne on the flank of the capless root (Koi et al. 2009; see also Fig. 5a). As a result, 17 genera, five of which were described recently by us (Fig. 3), and 80 species or more are distributed in tropical and subtropical Asia, particularly in southern India, Thailand, and Laos (Kato 2013). In contrast to the Asian flora, only two species are distributed in narrow areas in north Australia: the Australian Cladopus queenslandicus and Terniopsis australis were transferred from Torrenticola and Tristicha, respectively, based on our phylogenetic findings (Kita and Kato 2001; Kato et al. 2003).
Phylogeny of Podostemaceae reconstructed from data of Koi et al. (2012a). Arrow indicates Diamantina (Philbrick et al. 2004), and asterisks indicate genera described as new by us (Kato et al. 2004; Kato 2006; Koi et al. 2009; Koi and Kato 2010b). Most species of Terniopsis and Cladopus are Asian, and only T. australis and C. queenslandicus are Australian and Australian-Papua New Guinean, respectively. Cussetia* has not been examined and is not shown. Black, American clade; gray, African clade; white, Asian clade
In comparison, the Podostemaceae in the tropics and subtropics of America consist of 20 genera and 155 species, the largest number in the world (Philbrick et al. 2010). In particular, they are most diverse in central and southern Brazil with extensive cerrado. A recent noteworthy finding is the Brazilian genus Diamantina (Philbrick et al. 2004), which was later found to be sister to the rest of the subfamily Podostemoideae (Fig. 3; Ruhfel et al. 2011; Koi et al. 2012a). The discovery of this key species greatly contributed to the knowledge of the diversity, phylogeny and biogeography of Podostemaceae. In tropical and southern Africa are 14 genera and 75 species; five genera and six species are in Madagascar (Rutishauser et al. 2007).
Biogeography
The biogeography of Podostemaceae is an interesting issue because of their contrasting scattered distribution and unique ecology. The small seeds are dispersible over long distances by wind and other vectors between the habitats that are restricted and widely isolated. Furthermore, Podostemaceae occur on four continents, although the Australian species are quite few. Contrary to dispersal-based biogeography, Cusset and Cusset (1988c) interpreted that the distribution changed primarily due to the breakup and drift of continents, or geodispersal (Lieberman and Eldredge 1996), and Tristichoideae originated in Gondwanaland and the Asian-Australian genera were derived. However, this Gondwanian hypothesis was not based on accurate phylogeny and divergence times, and is rejected by molecular analyses (Kita and Kato 2001, 2004a).
Any biogeographic analysis depends on an accurate phylogeny. The phylogenetic classification of Podostemaceae had been primarily based on comparative morphology (Rutishauser 1997), but molecular studies revealed significant alterations (Kita and Kato 2001; Koi et al. 2009, 2012a; Thiv et al. 2009; Ruhfel et al. 2011; Khanduri et al. 2015). Compared with traditional classifications recognizing two subfamilies, the family is divided into three subfamilies; subfamily Podostemoideae is sister to the South American Weddellinoideae, although Weddellina (the sole genus of the subfamily) had traditionally been assigned to Tristichoideae (Kita and Kato 2001). Most (51/54, ca. 94 %) genera are restricted within one continent, indicating that they diversified on the original continent in which they occur. In subfamily Podostemoideae, most American genera including Diamantina are basal, while the Asian genera are derived. Many genera are clustered into generic groups, which are also characterized by the regions of distribution (America, Africa or Asia) (Fig. 3; Koi et al. 2012a). In comparison, most Tristichoideae are Asian (and also Australian).
A Maximum Likelihood analysis of the biogeography suggests an American origin of the family, whereas an alternative Asian origin was suggested by comparative morphology (Koi et al. 2015). In either scenario, long distance dispersal shaped the biogeography. The earliest dispersal of subfamilies between America and Asia (including India) is still a puzzle (Fig. 3). Long distance dispersal may have happened in the Paleogene when America and India, which had been (sub)continents of Gondwanan origin, were closer than at present. Instead, the migration of Tristichoideae from Africa to America across the widened Atlantic Ocean occurred once and recently (late Neogene), resulting in rapid colonization by a single species (Tristicha trifaria) across South and Central America (Kita and Kato 2004b). In comparison, Podostemoideae originated and diversified first in America, followed by dispersal to Africa and Asia, and subsequent diversification on each continent. Return dispersal from Africa to America happened once, giving rise to a few ‘odd genera’ in America, Ceratolacis and Podostemum (Cipoia, a third American genus with dyad pollen (Bove et al. 2006), waits molecular phylogenetic analysis). This is consistent with pollen morphology: the dyad pollen is shared with the African and Asian podostemads and differs from the monad pollen of American ones. Thus, the biogeography of each continent is characterized by a few events of emigration and immigration. Podostemoideae and Tristichoideae immigrated to common habitats at different times in the overlapping distributional regions, and the migration may or may not have been accompanied by competition between immigrants and existing species.
Nothing is known about geological impact on the origin and diversification of Podostemaceae. Rocky rapids and waterfalls are abundant in the biodiversity hotspots, i.e., Brazil in South America, Cameroon in Africa, south India and southeast Asia (Laos and Thailand) in Asia. Many species grow on igneous rocks, while others grow on metamorphic or sedimentary rocks. It is possible that a past large-scale catastrophe, e.g., volcanic activity, produced abundant habitats for the Podostemaceae to invade (Koi et al. 2015). In Japan, all of the six species except one occur exclusively on igneous welded tuff formed by recent eruptions, rarely on basalt (Fig. 2; Katayama et al. 2016). It is likely that the Japanese species colonized igneous rocks that were formed or appeared recently (later than 150‒18 thousand years ago, rarely later than 700‒150 thousand years ago).
The habitats of Podostemaceae are strict and widely separated from each other by extensive unsuitable areas. Dispersal to unoccupied localities and geographical isolation may provide opportunities for new colonies to develop into new species. Therefore, the island biogeography model (Stuessy et al. 1990; Whittaker and Fernández-Palacios 2007) is applicable to the mode of speciation in Podostemaceae. One product of such speciation is Terniopsis australis of northwestern Australia, which has the same haplotype as one of the matK haplotypes of T. malayana distributed in Peninsular Thailand and Malaysia (Kato et al. 2003; Koi et al. 2012a, 2015), but they are distinct morphologically and geographically. This finding also improved previous taxonomic and biogeographic interpretations of Terniopsis australis, because it had been assigned to another genus Tristicha (Cusset and Cusset 1988a; Aston 1990). There are other species pairs showing such biogeography and speciation (Koi et al. 2015). By contrast, American Tristicha trifaria is a recent colonizer from Africa, and has not yet differentiated into a distinct species (Kita and Kato 2004b),
Discrepancy of molecular phylogeny and morphology-based classification
Phylogenies and classifications deduced from different sources of evidence are not always consistent. The discrepancy is greatest in Podostemaceae (Thiv et al. 2009), in which the plants are reduced, simplified and specialized to adapt to the torrents. This morphology has caused the unstable taxonomic status of the family. It was considered to be related to various families, e.g., Saxifragaceae or Crassulaceae (for historical review, see Cook and Rutishauser 2007), or even separated as the subclass Podostemopsida at the same high taxonomic rank as Dicotyledopsida and Monocotyledopsida (Cusset and Cusset 1988b). Molecular phylogenetic analysis, with considerably more data than is available from morphological characters, can yield a more reliable phylogeny. Recent molecular phylogenetic analyses consistently showed Podostemaceae to be sister to Hypericaceae of the eudicot rosids II Malpighiales (Savolainen et al. 2000; Soltis et al. 2000, 2011; Gustafsson et al. 2002; Davis et al. 2005; Tokuoka and Tobe 2006; Korotkova et al. 2009; Wurdack and Davis 2009; Ruhfel et al. 2011). Nevertheless, there are quite a few characters shared by the families, e.g., tenuinucellate ovules (Ruhfel et al. 2011). The discrepancy between the morphology-based and molecular phylogenies indicates that the evolution of the aquatic Podostemaceae from a terrestrial ancestor was apparently drastic.
The morphology-based infrafamilial classification of Podostemaceae may not agree with molecular phylogenetic relationships partly because shared simplified morphologies include analogy as well as homology. In the current classification, the family comprises about 54 genera and 300 species or more. Among the genera, Apinagia includes 51 species; Ledermanniella, 26 species; Hydrobryum, 26 species; Rhyncholacis, 22 species; and Inversodicraea, 20 species (Koi et al. 2012a). By contrast, about 49 (91 %) of the other genera are small, consisting of 10 or fewer species. The mean species number per genus is ca. 5.6, which is small compared to >10 for angiosperms (http://www.theplantlife.org), and 22 (41 %) genera are monospecific, i.e., 1 species/genus. Given that phenotypic differences at the genus level are larger than at the species level, the small genus size reflects large or distinct differences between species; they are extreme in the mono- and oligospecific genera. Furthermore, molecular analyses demonstrated that some small genera are nested within large genera (Moline et al. 2007; Koi et al. 2012a), indicating that, although the classification with nested genera should be revised by adding molecular data (Thiv et al. 2009), rapid evolution characterizes part of the evolution of Podostemaceae.
The discrepancies and small genus size indicate that in Podostemaceae the extents of morphological differentiation are not always proportional to phylogenetic distances. Evolution is rapid or saltational in some lineages (see below; Rutishauser 1995), while it is slow in others, e.g., Tristicha trifaria having as great molecular variation as groups of genera (Koi et al. 2012a). Comparison of the morphological differentiation and phylogeny will be useful to reveal the pattern of morphological evolution in Podostemaceae.
Saltational evolution
Body plan is constructed by the composition of organs. Typically, the body of terrestrial flowering plants consists of the upper aerial shoot system with leaves and flowers and the lower subterranean root system. This vertical shoot–root axis develops from the plumule-hypocotyl-radicle axis in the seedling (Fig. 4a; Steeves and Sussex 1989). In contrast to angiosperms in general, the aquatic epilithic Podostemaceae have particular horizontal axes. In many podostemads, the roots creep and adhere to rock surfaces and produce usually short or reduced adventitious shoots (Fig. 1a, b, e), while in the rootless Dalzellia the shoot is crustose and adhering. In the primitive Tristichoideae (e.g., Terniopsis) the vertical axis is maintained even though reduced. Besides, the adventitious root is produced from the side of the hypocotyl and makes the horizontal axis (Kita and Kato 2005), while the vertical axis does not develop in the derived subfamily Podostemoideae (Philbrick 1984; Mohan Ram and Sehgal 1997; Suzuki et al. 2002; Kita and Kato 2005; Koi et al. 2012b). In comparison, Hypericum perforatum (Hypericaceae sister to Podostemaceae) has a hypocotyl-derived adventitious root, in addition to the well-developing primary shoot–root system, which is a possible archetype for Podostemaceae (Fig. 4a; S. Koi unpubl. data). Thus, transition from the vertical axis to the horizontal axis seems to be the result of both reduction of the vertical axis and innovation of the horizontal axes. Comparison using available seedling morphological data suggests that complete change of the axes took place at the divergence of Podostemoideae from the other two subfamilies. In most species, the root is formed adventitiously from the lateral part of the hypocotyl (Fig. 4b). The radicle and plumule are extremely reduced or lost and do not develop into a mature root and a shoot, respectively, with the result that the vertical axis is not formed or is rudimentary at the seedling stage. Rarely, instead of the root, the crustose dorsiventral shoot plays such a horizontal adhering organ in Dalzellia and Indodalzellia (Fig. 1d; Tristichoideae). It develops from near the axil of the cotyledon (Imaichi et al. 2004; Koi and Kato 2010a). Therefore, organographically the horizontal axis does not represent a different habit of the same axis, but is an organ complex different from the vertical axis.
Vertical axis in angiosperms in general (a) and horizontal axis in Podostemaceae (b). In a, upward and downward arrows indicate plumule and radicle development, and horizontal arrow indicates adventitious root in Hypericum (S. Koi unpubl. data). In b, which is applicable for most Podostemoideae, horizontal arrow indicates adventitious root development; broken arrows indicate reduced or lost plumule and radicle development. C cotyledon, H hypocotyl
Besides the transition of plant axis, remarkably different body plans or compositions of organs are developed in Podostemaceae (Table 1). In many genera (e.g., Cladopus, Hydrobryum, Polypleurum, Zeylanidium) the shoot is usually rudimentary so that the plant comprises tufts of leaves borne on the creeping root (Fig. 1a, b). By contrast, the shoots borne on the roots are elongate in some podostemads (Kato 2006; Koi and Kato 2012). Nonetheless, the shoot lacks a shoot apical meristem (SAM) in Cladopus queenslandicus, which, therefore, has apparent shoots and the ‘shoot’ comprises a chain of leaves (Koi et al. 2005). It is probably the case with other apparently shoot-bearing Podostemoideae (Imaichi et al. 2005). In Dalzellia, Indotristicha tirunelveliana and Hydrodiscus, the plant is rootless and comprises a shoot with leaves and flowers (Figs. 1c, d, 5a, b; Koi and Kato 2010b). In other genera, such as Mourera, the plant consists of only leaves and flowers, that is, vegetatively it is stemless and rootless (Fig. 1f). Transformation of body plan is caused partly by loss of organs (Table 1). For example, it may be interpreted that, in Hydrodiscus of the Hydrobryum clade, the adventitious root hardly develops and the shoot develops adventitiously within the hypocotyl (S. Koi unpubl. data); consequently the Hydrodiscus plant is rootless and comprises only a shoot (more precisely SAM-less pseudoshoot) with an anchoring basal disk (Fig. 5b; Koi and Kato 2010b).
Phylogenies of the Dalzellia clade (a) and the Cladopus‒Hydrobryum clade (b), showing discrepancy of morphology and phylogeny and saltational evolution. Tree a is a combined tree deduced from Koi et al. (2012a) and Khanduri et al. (2015). Indotristicha is not monophyletic. Tree b is simplified from Koi et al. (2012a). Ramulus is a short leafy shoot-branch (Fig. 1e); primary shoot is a shoot in embryo/seedling. (+), present (for primary shoot, shoot between cotyledons is rudimentary); (−), absent; crust, crustose; 3-D, 3-dimensionally branched. Asterisks indicate newly described genera (see Fig. 3)
In some species and genera, phylogenetic relationships are not in accordance with morphological similarities/differences. Among the most conspicuous taxa are Dalzellia, Indodalzellia and Indotristicha belonging to a monophyletic clade (Fig. 5a; Koi et al. 2012a; Khanduri et al. 2015). The shoots of Indotristicha ramosissima are adventitious, 3-D branched, and borne on the flanks of the root. Indotristicha tirunelveliana has been treated as a congener (Sharma et al. 1974), but a recent ITS analysis showed that it is sister to Dalzellia (Khanduri et al. 2015). This species is rootless (but it has root-like elongate holdfasts; Sharma et al. 1974) and has crustose shoots. The shoot of the rootless Dalzellia is also crustose, dorsiventral, and leafy (Fig. 1d; Mathew and Satheesh 1997; Imaichi et al. 2004). Indodalzellia gracilis was assigned first to Dalzellia as D. gracilis, because they share the crustose shoot, but a recent molecular analysis showed that it is related to the subclade of Indotristicha and Dalzellia, and was separated as an independent genus (Koi et al. 2009). Indodalzellia shares the subcylindrical roots with Indotristicha ramosissima and the crustose shoots with Dalzellia. The phylogeny suggests the following saltational evolution of morphology: the common ancestor of the three genera had roots and flattened dorsiventral shoots, which had been derived by congenital fusion of the subcylindrical tristichous shoots (Fujinami and Imaichi 2015); the shoot became radially symmetric in Indotristicha ramosissima; the root was lost in the clade of Indotristicha tirunelveliana and Dalzellia; and short shoots (called ramuli) were lost in Indodalzellia and Dalzellia.
Koi et al.’s (2012a) phylogenetic tree indicates a remarkable difference in the number of cotyledons in the eudicot Podostemaceae. In the Hydrodiscus‒Hydrobryum clade (Fig. 5b), the primary shoot apex does not develop at all (Suzuki et al. 2002; Koi et al. 2012b). Hydrodiscus (Koi and Kato 2010b) and a few species of Hydrobryum (S. Koi unpubl. data) have a single cotyledon. The monocotyly may be caused partly by congenital fusion of two cotyledons in the embryo that does not develop a SAM between the cotyledons. A few monocotyledonous species of Hydrobryum are sister to different dicotyledonous species, suggesting recurrent origins (S. Koi unpubl. data). However, many other SAM-less species of the clade are dicotyledonous (Suzuki et al. 2002; Koi et al. 2012b). In contrast, there is no monocotyledonous species in other genera, e.g., Cladopus, perhaps owing to the presence of a rudimentary primary shoot between the cotyledons.
It is well known that the root morphology of Podostemaceae is variable. The root adhering to rock surfaces is a leading organ of this aquatic lithophyte, and the diversification of the root is significant for evolution and adaptation. Compared with most angiosperm roots, which anchor the aerial part and absorb water and solutes (Esau 1965), the roots of Podostemaceae are subcylindrical, ribbon-like or crustose (leaflike) (Cook and Rutishauser 2007). The subcylindrical root of Tristichoideae, like that of other angiosperms, has a nearly radially symmetric root apical meristem (RAM) (Koi et al. 2006). In comparison, the ribbon-like root of Podostemoideae (Fig. 1a) has a central RAM and marginal meristems lateral to the RAM. The crustose root (Fig. 1b), which is most extreme in vascular plants, has a wide, uniform marginal meristem along the margin of the root lobes (Ota et al. 2001). The crustose root has traditionally been estimated to be uniform in the Hydrobryum clade except in Hydrodiscus (Podostemoideae). However, a morphological and phylogenetic study shows that the root is variable in the Hydrobryum clade; crustose, ribbon-like, or flattened subcylindrical (Koi and Kato 2012). It is suggested that the crustose root changed to a ribbon-like or subcylindrical root, due to a change of RAM, showing recurrent returns to the general root morphology. Other genera, such as Zeylanidium, Ledermanniella, Macropodiella and Sphaerotylax, also comprise species with ribbon-like roots and those with crustose roots (Mathew and Satheesh 1997; Rutishauser et al. 2007), suggesting that the RAM changed independently. The root cap, whose structure is the result of organization of the RAM, is also characteristic and variable. The root cap of Tristichoideae and Weddellinoideae is nearly radially-symmetric and cup-like, while the cap of the ribbon-like root of Podostemoideae is remarkably dorsiventral and recurved on the dorsal surface (Koi et al. 2006). The cap of the crustose root comprises layers of cells that are produced outward from the marginal root meristem and are long along the meristem. Thus, the RAM structure and resulting root-cap morphology are dorsiventral to various degrees. The root is capless in some species that are not closely related to each other. It may be that the root cap has no salient role or a role other than protection and geotropism.
Endogeny is a common mode of root branching in angiosperms (Esau 1965). By contrast, root branching is variable in Podostemaceae: it is endogenous in many species, but exogenous in others (Rutishauser 1997; Cook and Rutishauser 2007). Furthermore, in the exogenously-branched Hydrobryum (Ota et al. 2001) the adventitious root that develops from the hypocotyl in the seedling is also exogenous, while in the other exogenous Cladopus, Polypleurum and Zeylanidium (Hiyama et al. 2002; Suzuki et al. 2002; Koi and Kato 2003) it is endogenous (Suzuki et al. 2002). Irrespective of the variation at the seedling stage, the exogenous root may be a fuzzy organ or analogous to shoot, and may not be a typical root.
Fuzzy morphology and gene expression
Terrestrial vascular plants produce primary organs (stem, leaf, root). By contrast, as a result of invading aquatic environments, the organs of some Podostemaceae are ill-differentiated and borderless or fuzzy (Rutishauser 1995). The stem and the leaf are well differentiated in subfamilies Tristichoideae and Weddellinoideae, as in vascular plants in general (Rutishauser 1997; Rutishauser and Huber 1991; Imaichi et al. 1999; Koi and Kato 2007). Instead of the typical stem and leaf, some Podostemoideae have chains of leaves and no typical stem. The Podostemoideae morphology is constructed under the absence of the typical shoot apical meristem (SAM), regardless of presence or absence of apparent stems (Imaichi et al. 2005; Koi et al. 2005). In a developmental study of Zeylanidium (Podostemum) subulatum, Imaichi et al. (2005) criticized Jäger-Zürn’s (1999) interpretation that the single-layered apical meristem is a reduction of a typical SAM. Jäger-Zürn (1999, 2007) stressed that the SAM in Podostemoideae occurs in a modified form, but presence or absence of the typical SAM requires another source of evidence (see below). In SAM-less species, a new leaf forms within the basal part of a young leaf primordium (see Fig. 6b). The youngest leaf primordia are histologically continuous and cells present between the primordia become vacuolated and detach to separate the leaves, as in the pattern of programmed cell death (Gray 2004). This unusual development produces the leaf-on-leaf or tufted-leaf morphology characteristic of Podostemoideae. Another remarkable morphogenesis exists in Zeylanidium subulatum with not only adaxial (axillary) flowers but also atypical abaxial (extra-axillary) ones (Jäger-Zürn 1999). Imaichi et al. (2005) found that the abaxial flower develops following the adaxial one and at the abaxial base or non-SAM site of a leaf. It suggests the possibility that the leaf has a shoot characteristic as well. Similar ‘double flower’ morphology is known in many taxa (Cook and Rutishauser 2007, and references cited herein). Thus, Podostemoideae do not exhibit typical modular structure.
Diagram showing patterns of gene expression in developing organs, based on Katayama et al. (2010). Ellipses indicate organizing center. a Shoot apex of model plant. STM and WUS are expressed in shoot apex, and PHAN is expressed in leaf primordia. In Terniopsis minor (Tristichoideae), STM and WUS homologs are also expressed in shoot apex, but expression of PHAN homolog is unknown. b ‘Leaf’ primordia of shootless Cladopus doianus and Hydrobryum japonicum (Podostemoideae). STM and WUS homologs are expressed in youngest ‘leaf’ primordium produced in older one, and PHAN homolog is expressed in distal part of older ‘leaf’ primordium, where STM and WUS homologs are no longer expressed
The evolution of morphology is caused by modification of the underlying genetic system. There are distinct organogenetic shoot apices in Tristichoideae and Weddellinoideae, as in typical terrestrial plants (Imaichi et al. 1999, 2004; Koi and Kato 2007; Koi et al. 2009; Fujinami et al. 2013). In model plants, SHOOT MERISTEMLESS (STM) and WUSCHEL (WUS) are expressed in the SAM, while PHANTASTICA (PHAN) is expressed in a leaf primordium (Piazza et al. 2005). Patterns of gene expression in proper sites underline the regular organogenesis in the shoot. In a species of Tristichoideae, STM and WUS homologs are expressed in the SAM, although the expression of a PHAN ortholog was not examined (Fig. 6a; Katayama et al. 2010; Koi and Katayama 2013). In comparison, STM and WUS orthologs are expressed in a ‘leaf’ primordium in two species of Podostemoideae with tufts of ‘leaves’ on the root (Fig. 6b; Katayama et al. 2010; Koi and Katayama 2013). Subsequently, this expression disappears and instead a PHAN ortholog is expressed in the distal part of the developing ‘leaf’ primordium. Therefore, all these genes are expressed simultaneously or successively in one organ. Katayama et al. (2010) interpreted the ‘leaf’ primordium to be a mixture of shoot and leaf, or fuzzy morphology. It may be that the aquatic habitat has weaker pressure toward organ differentiation than the terrestrial habitat. Analysis of the patterns of gene expression will reveal different patterns of body plan, and eventually the great diversity and evolution of Podostemaceae.
The disappearance of the vertical axis in Podostemaceae was a necessary adaptation to the habitat, as shown above. It is manifested from the earliest stages of the life cycle. In the seedlings of Tristichoideae and Weddellinoideae, the primary shoot (plumule) is formed, while it is rudimentary or lacking in Podostemoideae. It was unclear how the primary shoot was reduced. Zeylanidium lichenoides, like many other species of Podostemoideae, has a rudimentary primary shoot, which produces only a few leaves between the cotyledons. In an analysis of gene-expression pattern in the embryo and seedling, Katayama et al. (2013) showed that expression of an STM ortholog began in the apical part of the 16-celled embryo. By the heart-shaped embryo stage, its expression is restricted to the putative organizing center (OC) and the protodermal cells, forming a cryptic embryonic shoot meristem without a typical stem cell (apical initials) layer. During seedling development, expression is shifted from the meristem to the adaxial base of the cotyledons, where plumular leaves would form. Modification of the regulatory mechanism between the OC and stem cells might be responsible for this ephemeral primary shoot meristem. By contrast, Hydrobryum does not have a primary shoot nor leaves between the cotyledons. Morphological observations revealed that cell divisions that produce the putative OC cells do not occur in the eight cell stage embryo of H. japonicum (Katayama et al. 2011). Thus, the loss of the initiation step of the OC cells may result in the absence of the primary shoot (Katayama et al. 2011).
Problems
There are remarkable morphological differences or discontinuous variation among many species. In contrast, the aquatic habitats are similar for all species. Long before modern data from morphology and phylogeny became available, Willis (1914) stressed that variation in Podostemaceae was generated in the absence of natural selection. The drastic change in the morphology of closely related taxa includes monocotyly vs. dicotyly, three-dimensional versus flattened dorsiventral shoot, elongated shoot vs. no shoot, absence versus presence of root, absence vs. presence of root cap, and subcylindrical versus crustose root. It is difficult to interpret how all these differences are products of adaptations. Neutral evolution of morphology and mutation-driven evolution are argued by molecular evolutionists (Saitou 2009; Nei 2013). It is a challenging issue whether the morphologies in Podostemaceae changed in the absence of natural selection.
The evolutionary history of Podostemaceae had three turning points; divergence from the ancestor, invasion with innovation, and diversification in particular habitats, among which the invasion, as well as the diversification (see above), most characterizes their evolution. The estimated time of divergence of Podostemaceae from Hypericaceae is 72.4 Ma (Davis et al. 2005) or later. The time of origin for most genera of Podostemoideae is 26.3 Ma (Xi et al. 2012). Invasion and a few basal divergences should have happened in this long interval between the two (Fig. 3). The Podostemaceae probably were originally terrestrial and became aquatic, although it is not excluded that they were aquatic from the beginning. The family’s unique character is absence of double fertilization (Cook and Rutishauser 2007; Sehgal et al. 2011). The 3 or 4-celled embryo sac is apparently similar to that of primitive angiosperms, although they have double fertilization (Friedman 2008). The other unique characters are related to the special ecology. Increase in growth of the adhering horizontal axis and instead suppression of growth of the vertical axis may have happened saltationally or gradually. The sticky seed coat and roots (occasionally shoots instead) are essential for the aquatic haptophytic life. A syndrome of these innovations should have appeared in an early stage of invasion. However, it is uncertain whether the shared characters are derived from the common ancestor of Podostemaceae or appeared independently in separate lineages of the family.
The scattered island-like distribution may affect speciation in Podostemaceae, and in the island-biogeography model, dispersal is the first process to give rise to a new species in a colonized habitat. Molecular phylogenies showed that several or possibly many species are paraphyletic and their populations are closely related to local species (Koi et al. 2012a, 2015). The derived local species are often morphologically distinct from the mother species, while the populations of the mother species remain uniform. Therefore, a particular population of a mother species may have differentiated into a new species due to specific factors, such as geographical isolation, reproductive isolation, founder effect, mutation, or in response to habitat differences, even if subtle.
The Japanese Podostemaceae will provide a good model for historical biogeography. They comprise two endemic and four non-endemic species in southern Kyushu (Fig. 2), although all have at times been considered to be endemic (Kato 2008, 2013). Japanese Hydrobryum japonicum (No. 12‒18 in Fig. 2) has the same matK haplotype as populations in Laos and Thailand, which is one of several haplotypes in southeast Asia. The endemic H. floribundum (No. 9, 10) is related to an extra-Japanese population of H. japonicum. Hydrobryum koribanum (No. 1) occurs in Miyazaki Prefecture and in Fujian, China (N. Katayama and M. Kato unpubl. data) and is sister to H. puncticulatum (No. 19) endemic to Yaku Island (Kita and Kato 2004a; Koi et al. 2012a). The area between Localities No. 1 and 19 is inhabited by the other congeners. The two species of Cladopus (No. 2‒9, 11) are nearly allopatric to Hydrobryum and also occur in Fujian. Cladopus doianus and C. fukienensis are sister to part of southeast Asian C. pierrei and part of south Chinese C. austrosinensis, respectively (Koi et al. 2012a, 2015). To sum up, all or most species of Japan are derived from neighboring areas and are nearly allopatric. As shown above, all Japanese species except H. puncticulatum grow on volcanic rocks that formed in the late Quaternary. Taken together, it is suggested that the Japanese species are recent recurrent immigrants. They may have invaded their present localities from outside Japan or from unlocated refugia in Japan.
References
Aston HI (1990) Podostemaceae. In: George AS (ed) Flora of Australia, vol 18. Australian Government Publishing Service, Canberra, pp 1–5
Borgen L, Jonsell B (ed) (1997) Variation and evolution in arctic and alpine plants. In: Proceedings of VI international symposium of international organization of plant biosystematics. Opera Botanica No. 132
Bove CP, Philbrick CT, Novelo RA (2006) A new species of Cipoia (Podostemaceae) from Minas Gerais, Brazil. Syst Bot 31:822–825
Cook CDK (1996) Aquatic plant book, 2nd ed. SPB Academic Publishing, The Hague
Cook CDK, Rutishauser R (2007) Podostemaceae. In: Kubitzki K (ed) The families and genera of vascular plants, vol 9. Springer, Berlin, pp 304–344
Cusset C (1992) Contribution a l’etude des Podostemaceae: 12. Les genres asiatiques. Bull Mus Natl Hist Nat Paris B Adansonia 14:13–54
Cusset C, Cusset G (1988a) Etude sur les Podostemales. 9. Délimitations taxinomiques dans les Tristichaceae. Bull Mus Natl Hist Nat Paris B Adansonia 10:149–177
Cusset G, Cusset C (1988b) Etude sur les Podostemales. 10. Structures florales et végétatives des Tristichaceae. Bull Mus Natl Hist Nat Paris B Adansonia 10:179–218
Cusset G, Cusset C (1988c) Etude sur les Podostemales. 11. Répartition et évolution des Tristichaceae. Bull Mus Natl Hist Nat Paris B Adansonia 10:223–262
Davis CC, Webb CO, Wurdack KJ, Jaramillo CA, Donoghue MJ (2005) Explosive radiation of Malpighiales supports a mid-Cretaceous origin of modern tropical rain forests. Amer Nat 165:E36–E65
Esau K (1965) Plant anatomy, 2nd edn. Wiley, New York
Friedman WE (2008) Hydatellaceae are water lilies with gymnospermous tendencies. Nature 453:94–97
Fujinami R, Imaichi R (2015) Developmental morphology of flattened shoots in Dalzellia ubonensis and Indodalzellia gracilis with implications for the evolution of diversified shoot morphologies in the subfamily Tristichoideae (Podostemaceae). Am J Bot 102:484–859
Fujinami R, Ghogue JP, Imaichi R (2013) Developmental morphology of the controversial ramulus organ of Tristicha trifaria (subfamily Tristichoideae, Podostemaceae): implications for evolution of a unique body plan in Podostemaceae. Int J Plant Sci 174:609–618
Gray J (ed) (2004) Programmed cell death in plants. Blackwell, Oxford
Gustafsson MHG, Bittrich W, Stevens PF (2002) Phylogeny of Clusiaceae based on rbcL sequences. Int J Plant Sci 163:1045–1054
Haston E, Richardson JE, Stevens PF, Chase MW, Harris DJ (2009) The Linear Angiosperm Phylogeny Group (LAPG) III: a linear sequence of the families in APG III. Bot J Linn Soc 161:128–131
Hiyama Y, Tsukamoto I, Imaichi R, Kato M (2002) Developmental anatomy and branching of roots of four Zeylanidium species (Podostemaceae), with implications for evolution of foliose roots. Ann Bot 90:735–744
Imaichi R, Ichiba T, Kato M (1999) Developmental morphology and anatomy of Malaccotristicha malayana (Podostemaceae). Int J Plant Sci 160:253–259
Imaichi R, Maeda R, Suzuki K, Kato M (2004) Developmental morphology of foliose shoots and seedlings of Dalzellia zeylanica (Podostemaceae) with special reference to their meristems. Bot J Linn Soc 144:289–302
Imaichi R, Hiyama Y, Kato M (2005) Leaf development in absence of shoot apical meristem in Zeylanidium subulatum (Podostemaceae). Ann Bot 96:51–58
Imamura S (1927) Discovery of Podostemonaceae in Japan. Proc Imp Acad Jap 3:616–618
Imamura S (1928a) Ueber Cladopus japonicus n. sp., eine Podostemonaceen in Japan. J Jap Bot 5:50–62 (in Japanese with German summary)
Imamura S (1928b) Über Cladopus japonicus n. sp., eine Podostemonaceen in Japan. Bot Mag Tokyo 42:379–387 (pl. 5, 6)
Jäger-Zürn I (1999) Developmental morphology of the shoot system of Podostemum subulatum (Podostemaceae-Podostemoideae): part V of the series ‘morphology of Podostemaceae’. Beitr Biol Pflanzen 71:281–334
Jäger-Zürn I (2007) The shoot apex of Podostemaceae: de novo structure or reduction of the conventional type? Flora 202:383–394
Katayama N, Koi S, Kato M (2008) Developmental anatomy of the reproductive shoot in Hydrobryum japonicum (Podostemaceae). J Plant Res 121:417–424
Katayama N, Koi S, Kato M (2010) Expression of shoot meristemless, wuschel, and asymmetric leaves1 homologs in the shoots of Podostemaceae: implications for the evolution of novel shoot organogenesis. Plant Cell 22:2131–2140
Katayama N, Kato M, Nishiuchi T, Yamada T (2011) Comparative anatomy of embryogenesis in three species of Podostemaceae and evolution of the loss of embryonic shoot and root meristems. Evol Dev 13:333–342
Katayama N, Kato M, Yamada T (2013) Origin and development of the cryptic shoot meristem in Zeylanidium lichenoides (Podostemaceae). Amer J Bot 100:635–646
Katayama N, Kato M, Imaichi R (2016) Habitat specificity enhances genetic differentiation in two species of aquatic Podostemaceae in Japan. Am J Bot 103. doi:10.3732/ajb.1500385
Kato M (2004) Taxonomic study of Podostemaceae of Thailand 1. Hydrobryum and related genera with crustaceous roots (subfamily Podostemoideae). Acta Phytotax Geobot 55:133–165
Kato M (2006) Taxonomic study of Podostemaceae of Thailand 2. Subfamily Podostemoideae with ribbon-like roots and subfamily Tristichoideae. Acta Phytotax Geobot 57:1–54
Kato M (2008) A taxonomic study of Podostemaceae of Japan. Bull Natl Mus Nat Sci ser B (Bot) 34:63–73
Kato M (2011) Taxonomic enumeration of Podostemaceae of Cambodia and Vietnam. Bull Natl Mus Nat Sci ser B (Bot) 37:1–8
Kato M (2013) The illustrated book of plant systematics in color: Podostemaceae of the world. Hokuryukan, Tokyo
Kato M, Fukuoka F (2002) Two new species of Diplobryum (Podostemaceae, Podostemoideae) from Laos. Acta Phytotax Geobot 53:115–120
Kato M, Koi S (2009) Taxonomic studies of Podostemaceae of Thailand. 3. Six new and a rediscovered species. Gard Bull Singapore 61:55–72
Kato M, Kita Y, Koi S (2003) Molecular phylogeny, taxonomy and biogeography of Malaccotristicha australis comb. nov. (syn. Tristicha australis) (Podostemaceae). Aust Syst Bot 16:177–183
Kato M, Koi S, Kita Y (2004) A new foliose-rooted genus of Podostemaceae (subfamily Podostemoideae) from Thailand with note on root evolution. Acta Phytotax Geobot 55:65–73
Khanduri P, Tandon R, Uniyal PL, Bhat V, Pandey AK (2015) Comparative morphology and molecular systematics of Indian Podostemaceae. Plant Syst Evol 301:861–882
Kita Y, Kato M (2001) Infrafamilial phylogenetic relationships of the aquatic angiosperm family Podostemaceae inferred from matK sequence data. Plant Biol 3:156–163
Kita Y, Kato M (2004a) Molecular phylogeny of Cladopus and Hydrobryum (Podostemaceae, Podostemoideae) with implications for their biogeography in East Asia. Syst Bot 29:921–932
Kita Y, Kato M (2004b) Phylogenetic relationships between disjunctly occurring groups of Tristicha trifaria (Podostemaceae). J Biogeogr 31:1605–1612
Kita Y, Kato M (2005) Seedling developmental anatomy of an undescribed Malaccotristicha species (Podostemaceae, subfamily Tristichoideae) with implications for body plan evolution. Plant Syst Evol 254:221–232
Koi S, Katayama N (2013) Gene expression analysis of aquatic angiosperms Podostemaceae to gain insight into the evolution of their enigmatic morphology. In: De Smet I (ed) Plant organogenesis: methods and protocols. Methods in molecular biology, vol 959. Springer Science + Business Media, New York, pp 83‒95
Koi S, Kato M (2003) Comparative developmental anatomy of the root in three species of Cladopus (Podostemaceae). Ann Bot 91:927–937
Koi S, Kato M (2007) Developmental morphology of shoot in Weddellina squamulosa (Podostemaceae) and implications for shoot evolution in the Podostemaceae. Ann Bot 99:1121–1130
Koi S, Kato M (2010a) Developmental anatomy of seedling of Indodalzellia gracilis (Podostemaceae). Plant Biol 12:794–799
Koi S, Kato M (2010b) Developmental morphology of shoot and seedling and phylogenetic relationship of Diplobryum koyamae (Podostemaceae). Am J Bot 97:373–387
Koi S, Kato M (2012) A taxonomic study of Podostemaceae subfamily Podostemoideae of Laos with phylogenetic analyses of Cladopus, Paracladopus and Polypleurum. Kew Bull 67:331–365
Koi S, Kato M (2015a) The taxonomy of Podostemaceae subfamily Tristichoideae in Laos, with descriptions of seven new species. Acta Phytotax Geobot 66:61–79
Koi S, Kato M (2015b) Additions to Podostemaceae subfamily Podostemoideae of Laos. Acta Phytotax Geobot 66:173–179
Koi S, Imaichi R, Kato M (2005) Endogenous leaf initiation in the apical-meristemless shoot of Cladopus queenslandicus (Podostemaceae) and implications for evolution of shoot morphology. Int J Plant Sci 166:199–206
Koi S, Tsukamoto I, Inagawa R, Kubo N, Fujinami R, Imaichi R, Kato M (2006) Comparative anatomy of root meristem and root cap in some species of Podostemaceae and the evolution of root dorsiventrality. Amer J Bot 93:682–692
Koi S, Kita Y, Kato M (2008) Paracladopus chanthaburiensis, a new species of Podostemaceae from Thailand, with notes on its morphology, phylogeny and distribution. Taxon 57:201–210
Koi S, Rutishauser R, Kato M (2009) Phylogenetic relationship and morphology of Dalzellia gracilis (Podostemaceae, subfamily Tristichoideae) with proposal of a new genus. Int J Plant Sci 170:237–246
Koi S, Kita Y, Hirayama Y, Rutishauser R, Huber KA, Kato M (2012a) Molecular phylogenetic analysis of Podostemaceae: implications for taxonomy of major groups. Bot J Linn Soc 169:461–492
Koi S, Werukamkul P, Amporpan L, Kato M (2012b) Seedling development of Hanseniella, Hydrobryum and Thawatchaia (Podostemaceae) and implications on body plan evolution in the Hydrobryum clade. Plant Syst Evol 298:1755–1766
Koi S, Ikeda H, Rutishauser R, Kato M (2015) Historical biogeography of river-weeds (Podostemaceae). Aquat Bot 127:62–69
Korotkova N, Schneider JV, Quandt D, Worberg A, Zizka G, Borsch T (2009) Phylogeny of the eudicot order Malpighiales: analysis of a recalcitrant clade with sequences of the petD group II intron. Plant Syst Evol 282:201–228
Landolt E, Jäger-Zürn I, Schnell RAA (eds) (1988) Extreme adaptations in angiospermous hydrophytes. Gebrüder Borntraeger, Berlin
Lansdown RV (2012) The conservation of aquatic and wetland plants in the Indo-Burma region. In: Allen DJ, Smith KG, Darwall WRT (eds) The status and distribution of freshwater biodiversity in Indo-Burma, Chap 7, pp 114‒133. https://cmsdata.iucn.org/downloads/ib_chapter_7__the_conservation_of_aquatic_and_wetland_plants_in_the_indo_burma_region.pdf
Lieberman BS, Eldredge N (1996) Trilobite biogeography in the Middle Devonian: geological processes and analytical methods. Paleobiology 22:66–79
Mathew CJ, Satheesh VK (1997) Taxonomy and distribution of the Podostemaceae in Kerala, India. Aquat Bot 57:243–274
Mohan Ram HY, Sehgal A (1997) In vitro studies on developmental morphology of Indian Podostemaceae. Aquat Bot 57:97–132
Moline P, Thiv M, Ameka GK, Ghogue J-P, Pfeifer E, Rutishauser R (2007) Comparative morphology and molecular systematics of African Podostemaceae-Podostemoideae, with emphasis on Dicraeanthus and Ledermanniella from Cameroon. Int J Plant Sci 168:159–180
Nei M (2013) Mutation-driven evolution. Oxford University Press, Oxford
Ota M, Imaichi R, Kato M (2001) Developmental morphology of the thalloid Hydrobryum japonicum (Podostemaceae). Am J Bot 88:382–390
Philbrick CT (1984) Aspects of floral biology, breeding system, and seed and seedling biology in Podostemum ceratophyllum (Podostemaceae). Syst Bot 9:166–174
Philbrick CT, Novelo RA, Irgang BE (2004) Two new genera of Podostemaceae from the state of Minas Gerais, Brazil. Syst Bot 29:109–117
Philbrick CT, Bove CP, Stevens HI (2010) Endemism in Neotropical Podostemaceae. Ann Missouri Bot Gard 97:425–456
Piazza P, Jasinski S, Tsiantis M (2005) Evolution of leaf developmental mechanisms. New Phytol 167:693–710
Ruhfel BR, Bittrich V, Bove CP, Gustafsson MHG, Philbrick CT, Rutishauser R, Xi Z, Davis CC (2011) Phylogeny of the clusioid clade (Malpighiales): evidence from the plastid and mitochondrial genomes. Am J Bot 98:306–325
Rutishauser R (1995) Developmental patterns of leaves in Podostemaceae compared with more typical flowering plants: saltational evolution and fuzzy morphology. Can J Bot 73:1305–1317
Rutishauser R (1997) Structural and developmental diversity in Podostemaceae (river-weeds). Aquat Bot 57:29–70
Rutishauser R, Huber KA (1991) The developmental morphology of Indotristicha ramosissima (Podostemaceae, Tristichoideae). Plant Syst Evol 178:195–223
Rutishauser R, Pfeifer E, Grob V, Bernhard A (2007) Podostemaceae of African and Madagascar: keys to genera and species, including genera descriptions, illustrations to all species known, synonyms, and literature list. http://.systbot.uzh.ch/podostemaceae
Saitou N (2009) From selectionism to neutralism: paradigm shift of evolutionary studies. NTT Publishing, Tokyo (in Japanese)
Savolainen V, Fay MF, Albach DC, Backlund A, van der Bank M, Cameron KM, Johnson SA, Lledó MD, Pintaud J-C, Powell M, Sheahan MC, Soltis DE, Soltis PS, Weston P, Whitten WM, Wurdack KJ, Chase MW (2000) Phylogeny of the eudicots: a nearly complete familial analysis based on rbcL gene sequences. Kew Bull 55:257–309
Sculthorpe CD (1967) The biology of aquatic vascular plants. Edward Arnold, London (reprinted 1985 by Koeltz Scientific Books, Königstein)
Sehgal A, Khurana JP, Sethi M, Ara H (2011) Occurrence of unique three-celled megagametophyte and single fertilization in an aquatic angiosperm–Dalzellia zeylanica (Podostemaceae–Tristichoideae). Sexual Plant Repr 24:199–210
Seno J, Hattori M (2013) The northernmost locality of Cladopus japonicus Imamura (Podostemaceae). J Jap Bot 88:61–63
Sharma BD, Karthikeya D, Shetty BV (1974) Indotristicha tirunelveliana Sharma, Karthik. & Shetty—a new species of Podostemaceae from South India. Bull Bot Surv India 16:157–161
Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, Savolainen V, Hahn WH, Hoot SB, Fay MF, Axtell M, Swensen SM, Price LM, Kress WJ, Nixon KC, Farris JS (2000) Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Bot J Linn Soc 133:381–461
Soltis DE, Smith SA, Cellinese N, Wurdack KJ, Tank DC, Brockington SF, Refulio-Rodriguez NF, Walker JB, Moore MJ, Carlsward BS, Bell CD, Latvis M, Crawley S, Black C, Diouf D, Xi Z, Rushworth CA, Gitzendanner MA, Sytsma KJ, Qiu Y-L, Hilu KW, Davis CC, Sanderson MJ, Beaman RS, Olmstead RG, Judd WS, Donoghue MJ, Soltis PS (2011) Angiosperm phylogeny: 17 genes, 640 taxa. Am J Bot 98:704–730
Steeves TA, Sussex IM (1989) Patterns in plant development, 2nd edn. Cambridge University Press, Cambridge
Stuessy TF, Crawford DJ, Marticorena C (1990) Patterns of phylogeny in the endemic vascular flora of the Juan Fernandez Islands, Chile. Syst Bot 15:338–346
Suzuki K, Kita Y, Kato M (2002) Comparative developmental anatomy of seedlings in nine species of Podostemaceae (subfamily Podostemoideae). Ann Bot 89:755–765
Thiv M, Ghogue J-P, Grob V, Huber K, Pfeifer E, Rutishauser R (2009) How to get off the mismatch at the generic rank in African Podostemaceae? Plant Syst Evol 283:57–77
Tokuoka T, Tobe H (2006) Phylogenetic analyses of Malpighiales using plastid and unclear DNA sequences, with particular reference to the embryology of Euphorbiaceae sens. str. J Plant Res 119:599–616
Werukamkul P, Amporpan L, Koi S, Kato M (2012) Taxonomic study of Podostemaceae in Loei Province, northeastern Thailand. Acta Phytotax Geobot 63:11–28
Whittaker RJ, Fernández-Palacios JM (2007) Island biogeography: ecology, evolution, and conservation, 2nd edn. Oxford University Press, Oxford
Willis JC (1902) Studies in the morphology and ecology of the Podostemaceae of Ceylon and India. Ann R Bot Gard Peradeniya 1:268–465 (pl. IV‒XXXVIII)
Willis JC (1914) On the lack of adaptation in the Tristichaceae and Podostemaceae. Proc R Soc Lond B 87:532–550
Wurdack KJ, Davis CC (2009) Malpighiales phylogenetics: gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. Am J Bot 90:1151–1570
Xi Z, Ruhfel BR, Schaefer H, Amorim AM, Sugumaran M, Wurdack KJ, Endress PK, Matthews ML, Stevens PF, Mathews S, Davis CC (2012) Phylogenomics and a posteriori data partitioning resolve the Cretaceous angiosperm radiation Malpighiales. Proc Natl Acad Sci USA 109:17519–17524
Acknowledgments
I thank many colleagues in Japan and abroad for collaboration on the studies of Podostemaceae, in particular, S. Koi, N. Katayama, and Y. Hirayama, my coworkers in field and lab. This paper is a product of those collaborative studies. Thanks are also due to N. Katayama, S. Koi and H. Okada for their useful comments on the manuscript, to N. Katayama for her help with drawing Fig. 6, and D. E. Boufford for his linguistic check of the manuscript. This study was supported by JSPS KAKENHI Grant Number 25291091.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kato, M. Multidisciplinary studies of the diversity and evolution in river-weeds. J Plant Res 129, 397–410 (2016). https://doi.org/10.1007/s10265-016-0801-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0801-8