Abstract
Spiranthinae orchids are known for being self-compatible and offering nectar as a reward. Although data on their pollinators are scarce, members of this tribe are mostly pollinated by bees, hummingbirds and moths. Some of them even reproduce through facultative self-pollination. Nothing is known about the pollinators and reproduction system in Pteroglossa. Based on records on flowering phenology, floral morphology, reward production, pollinators and breeding system, this paper aims to study the reproductive biology of two Pteroglossa spp. Both species offer nectar as a resource and are pollinated exclusively by diurnal Lepidoptera at the studied areas. Nectar is produced by two glandular nectaries, and is stored in a spur. Pollinaria possess a ventrally adhesive viscidium that is deposited on the basal portion of butterfly proboscides. Both species are self-compatible but pollinator-dependent. The reproductive success is low when compared to other Spiranthinae. Although no evident mechanical barrier to avoid self-pollination or geitonogamy was identified, the erratic behavior of the butterflies, with their infrequent visits to only one flower per inflorescence, contributes to an increased fruit set produced through cross-pollination. The presence of ventrally adhesive viscidia in Spiranthinae is responsible for greater pollinator diversity when compared to bee-pollinated Goodyerinae with dorsally adhesive viscidia, adapted to attach to bee mouthparts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the orchid family might be pollinated by abiotic and biotic vectors (see Aguiar et al. 2012; Neiland and Wilcock 1998; van der Pijl and Dodson 1966; Tremblay et al. 2005), the latter including birds (mainly hummingbirds), mammals (mice) and insects: Diptera, Hymenoptera, Orthoptera and Lepidoptera (e.g., Micheneau et al. 2010; van der Pijl and Dodson 1966; Wang et al. 2008). Within Orchidaceae, the most common and widespread group of pollinators is Hymenoptera, which exploit a variety of resources or are deceived through different floral strategies (e.g., van der Pijl and Dodson 1966). Butterflies are attracted to psychophilous orchids by floral nectar or food deception (see Fuhro et al. 2010; van der Pijl and Dodson 1966; Pansarin and Amaral 2008). They have also been recorded as pollinators of several unrelated orchid groups, as the epidendroid genera Epidendrum (Fuhro et al. 2010; Pansarin and Amaral 2008), Oeceoclades (Aguiar et al. 2012), and Comparettia (E.R. Pansarin and L.M. Pansarin, unpubl. data). Among Orchidoideae, butterfly pollination has been reported for several genera, as Disa (e.g., Johnson 1994; Johnson et al. 1998; van der Pijl and Dodson 1966), Platanthera (Robertson and Wyatt 1990), Habenaria (e.g., Pedron et al. 2012), Anacamptis (van der Cingel 1995), Orchis, and Ludisia (van der Pijl and Dodson 1966).
Subfamily Orchidoideae comprises about 3,630 species widespread in the temperate and tropical areas of both hemispheres (Pridgeon et al. 2001). It is divided into seven tribes, among which is Cranichidae, with 93 genera distributed in seven subtribes, including Spiranthinae. Spiranthinae is a Neotropical subtribe with approximately 40 genera, including Pteroglossa and its ten species distributed in Central to South America (Salazar 2003). In Brazil, this genus is represented by four species (de Barros et al. 2013). According to Salazar (2003), nothing is known about the reproductive biology, pollinators and pollination mechanisms of Pteroglossa.
Species of Spiranthinae offer nectar as a reward and are pollinated by social and solitary bees (Singer and Sazima 1999), hummingbirds (Singer and Sazima 2000), and moths (Singer 2002). Some species also reproduce through facultative self-pollination (e.g., Ackermann 1995), and self-compatibility has been recorded in several genera (Catling 1980, 1982; Singer and Sazima 1999, 2000; Singer 2002).
Based on records on flowering phenology, floral morphology, reward production, pollinator behavior and breeding system, this paper aims to investigate the reproductive biology of a Neotropical Spiranthinae, Pteroglossa, a rare genus with two species growing in the state of São Paulo, P. glazioviana (Cogn.) Garay (Pansarin and Pansarin 2008) and P. roseoalba (Rchb.f.) Salazar & M.W. Chase (Ferreira et al. 2010), never studied before.
Materials and methods
Study sites and plant material
The floral biology and reproduction of Pteroglossa glazioviana were recorded in a mesophytic, semi-deciduous forest in the natural reserve of Serra do Japi, within the boundaries of the city of Jundiaí (23º11′S, 46º52′W; 700–1,300 m a.s.l.). This region is primarily characterized by mesophytic, semi-deciduous forests of medium altitude and sparse rocky outcrops (Leitão-Filho 1992). The floral biology and reproduction of Pteroglossa roseoalba were recorded in a gallery forest adjacent to a pasture land, originally a “cerrado” vegetation (Brazilian savanna), within the boundaries of the town of Itirapina (approx. 22°20′S, 47°54′W; 870 m a.s.l.).
To study their floral features and breeding system, adult plants of both studied species were collected in natural populations and cultivated at the Orchidarium LBMBP (Orquidário do Laboratório de Biologia Molecular e Biossistemática de Plantas), University of São Paulo (FFCLRP-USP), at Ribeirão Preto (approx. 21º10′S, 47º48′W; 546 m a.s.l.). Ribeirão Preto is located approx. 240 km NW from Serra do Japi and about 100 km N from Itirapina. Ribeirão Preto and both study areas (Jundiaí and Itirapina) are located in the state of São Paulo, southeastern Brazil, in an ecotone characterized as being between Atlantic Forest and Cerrado (Kronka et al. 1993; Pinto 1989; Soares et al. 2003). According to Köppen (1948), climate is classified as “Cwa” (i.e., mesothermic with a dry winter season).
For the experimental treatments, plants growing at least 20 m away from each other were collected during the 2011 flowering period and planted in plastic pots with coconut fiber and leaf litter. 11 plants of Pteroglossa glazioviana and 12 individuals of Pteroglossa roseoalba were collected and one to three were planted in each pot.
Flowering phenology and floral features
Features of the flowering phenology and flower duration of Pteroglossa glazioviana and Pteroglossa roseoalba were recorded by monitoring the collected individuals and visiting the study areas bi-monthly, from January 2012 to July 2013. The visits were intensified (weekly) during the flower period.
The morphological features of fresh flowers of Pteroglossa glazioviana (n = 5; five inflorescences; five plants) and Pteroglossa roseoalba (n = 5; five inflorescences; five plants) were observed under a binocular stereomicroscope. Measurements were made directly on the floral structures using a caliper. The morphological study considered the format, symmetry, layout and size of floral parts such as sepals, petals, labellum, column, anther and pollinarium, taking possible intra-specific variations into account (Faegri and van der Pijl 1979). The production of floral fragrance was verified daily (day and night), by directly smelling the flowers from blooming to withering. Furthermore, fresh flowers were immersed in 0.1 % (w/v) aqueous neutral red for 20 min in order to localize possible secretory tissues, as osmophores (Dafni 1992). Once stained, they were rinsed in tap water and examined.
To characterize the anatomical structure of the secretory areas, flowers were manually sectioned and tests with Fehling’s reagent and Lugol were performed to detect reducing sugars (Purvis et al. 1964) and starch grains (Johansen 1940), respectively. Appropriate controls were run simultaneously to these histochemical tests. The images of the histochemical tests were captured with a Leica DM500 optical microscope equipped with a camera Leica ICC50 connected to a PC running IM50 image analysis software. Plates were prepared using Microsoft Power Point.
Nectar volume and concentration were measured for both studied species with a microliter syringe Hamilton 10 µl and a Bellingham & Stanley (series Eclipse) hand-held refractometer, respectively (Sazima et al. 2003). Nectar measurements were made on 74 flowers (12 inflorescences; 12 plants) of Pteroglossa glazioviana, and 76 flowers (10 inflorescences; 10 plants) of Pteroglossa roseoalba. The number of measurements varied according to the number of flowers available on each inflorescence. Measurements were made once only on 3–4-days flowers.
Pollinators and pollination mechanisms
Field visits to the study site were intended to observe and record the pollination process, visitation frequency and to capture pollinators for later identification. The observations on flowers of Pteroglossa glazioviana were carried out from 21 to 25 January and from 18 to 21 February 2013. The daily period of observation was from 08:00 to 16:30 h, totalizing 76.5 h. Additionally 17 observation hours were carried out from 04–05 January 2012. The observations on flowers of Pteroglossa roseoalba were carried out from 15 to 19 April 2013. The daily period of observation was from 07:30 to 17:30 h, totalizing 50 h. Pollinators were photographed using a Nikon D-SLR D800 camera and a Micro Nikkor 105 mm f2.8 lens. In order to detect possible night pollination, flowers of Pteroglossa glazioviana (n = 20; eight inflorescences; eight plants) and Pteroglossa roseoalba (n = 20; eight inflorescences; eight plants) were tagged in the afternoon and examined in the early morning, at about 08:00 h.
Additional night observations, from 13 to 17 January 2013 for Pteroglossa glazioviana, and from 13 to 17 April 2013 for P. roseoalba, were performed using two infrared cameras attached to a DVR (Digital Video Recorder) Stand Alone Intelbras. Possible night pollinations were recorded from 18:00 to 07:00, totaling 65 h for each studied species, based on plants collected in their natural environment and exposed in a garden area adjacent to the Orchidarium LBMBP, in Ribeirão Preto.
Floral visitors were captured, identified and vouchers were deposited at the Museu de História Natural of the Universidade Estadual de Campinas (ZUEC).
Breeding system
The experimental treatments to investigate the breeding system of Pteroglossa glazioviana and Pteroglossa roseoalba included intact (bagged) flowers to test spontaneous self-pollination, manual self-pollination, manual cross-pollination, and emasculations to check the occurrence of apomixis. A total of 133 flowers from eight plants (eight inflorescences) and a total of 129 flowers (13 plants; 13 inflorescences) were used for P. glazioviana and for P. roseoalba, respectively. The four treatments were applied to each inflorescence, using 1–4 days flowers. An entire pollinarium was used in each experimental pollination event (manual self- and manual cross-pollinations). Manual cross-pollinations were performed on previously emasculated flowers with the pollinaria of a plant from a different pot. Manipulations were made by using a dissecting forceps. The fruit set under natural conditions of 275 flowers (30 plants; 30 inflorescences) of P. glazioviana and 303 flowers (30 plants; 30 inflorescences) of P. roseoalba was recorded on 19 April 2013 and 04 July 2013, respectively. Fruit sets (experimental treatments and natural conditions) were recorded when fruits were mature.
Potentially viable seeds were counted on fruits obtained through artificial pollinations. A sample of 100 seeds per fruit was examined under a light microscope. Seeds with rudimentary or no embryos were considered not viable (Pansarin et al. 2008). For each studied species, the number of potentially viable seeds obtained in each treatment was compared using a t test for independent samples with the software Statistica 6.0 (StatSoft 2003).
The plant specimens used in the manual treatments and in the morpho-anatomical studies are: Pteroglossa glazioviana: Brazil, São Paulo, Jundiaí (23º11′S, 46º53′W), Col. E. R. Pansarin LBMBP 801, LBMBP 802, LBMBP 803, LBMBP 804, LBMBP 805; and Pteroglossa roseoalba: Brazil, São Paulo, Itirapina (22°20′S, 47°54′W), Col. E. R. Pansarin LBMBP 699, LBMBP 701, LBMBP 702, LBMBP 703, LBMBP 704, LBMBP 705, LBMBP 706, LBMBP 707, LBMBP 708, LBMBP 709, LBMBP 710, LBMBP 711; (http://splink.cria.org.br/manager/detail?setlang=pt&resource=LBMBP).
Results
Flowering phenology and flower features
Pteroglossa glazioviana and P. roseoalba are terrestrial herbs occurring in semi-deciduous mesophytic forests in the Serra do Japi and in gallery forests in Itirapina, respectively. Both species grow on litter fall, but some individuals of P. roseoalba can be lithophytes. In early October, they begin to produce rosulate leaves and from November to December (P. glazioviana) and from January to February (P. roseoalba), each adult plant yields a terminal inflorescence. Flowering period occurs in summer, from January to February in P. glazioviana, and in summer-early fall, from March to early May in P. roseoalba. In both studied species, each intact flower lasts 7–8 days. Fruits of P. glazioviana ripen in April, while those of P. roseoalba are dehiscent in July.
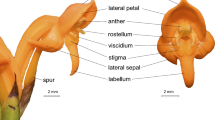
The inflorescences of Pteroglossa glazioviana produce up to 17 resupinate, predominantly whitish and waxy flowers (Fig. 1a). Ovary + pedicel (14–16 mm in length) is cylindrical, incurved, brown-greenish and puberulous. Sepals are elliptic to lanceolate, white-greenish, showy, and externally puberulous. Dorsal sepal (14–15 × 3.8–5 mm) is symmetrically elliptic and free. Lateral sepals (23–30 × 4.5–5 mm) are asymmetrically lanceolate, falcate and fused with the base of the labellum and the ovary forms a spur. Spur (12–14 mm in length) is incurved. Petals (12.5–14 × 4.5–5.5 mm) are asymmetrically elliptic, falcate, white, connivent with a dorsal sepal. Labellum (19–22.5 × 7.5–8.9 mm) is spatulate, white with green stripes, with nectar glands on both margins of its basal portion. Column (7.5–9 × 2.5–3 mm) is white, and widens as it approaches the apex, with a cordiform stigma. Anther (ca. 4.5 × 2 mm) is lanceolate to clavate and brown. Pollinarium (ca. 4.5 × 2 mm) is made up of two clavate, white friable pollinia and a triangular, gray viscidium.
a Flower of Pteroglossa glazioviana. b Flower of P. roseoalba. c Detail of the apical portion of the column of P. roseoalba. Note the longitudinal furrow between both stigmatic lobes (arrow). d Detached labellum of P. roseoalba showing the glandular nectaries on the margins of labellum base (arrows). e Transversal cut of the labellum base of P. roseoalba showing a glandular nectary stained with Fehling’s reagent. Note the brown precipitates. f Detail of a glandular nectary of P. roseoalba in transversal cut stained with lugol. Note the starch grains within the cells. Bars a, b 1 cm; c–e 1 mm; f 50 µm
The inflorescences of Pteroglossa roseoalba produce up to 21 resupinate flowers. As in P. glazioviana, their flowers are predominantly white, but opaque (Fig. 1b). Ovary + pedicel (30–34 mm in length) is cylindrical, straight, brown-greenish, glabrous, and parallel to inflorescence rachis. Sepals are white or white-pinkish, membranous, and glabrous. Dorsal sepal (24.4–26 × 4.5–5.5 mm) is symmetrically lanceolate to linear-lanceolate. Lateral sepals (24.5–26 × 7.5–8 mm) are asymmetrically lanceolate to elliptic-lanceolate, and fused with the base of the labellum and the ovary forming a spur. Spur (29.5–32 mm in length) is cylindrical, glabrous, brown-greenish, perpendicular to the labellum and parallel to the inflorescence rachis. Petals (22.5–25.5 × 6.5–7.2 mm) are asymmetrically spatulate, falcate, and white, with pinkish lines near the apex. Labellum (49–54 × 10–13 mm) is 3-lobate, spatulate, with pink oblique lines and serrulate margins. Lateral lobes are rounded. Apical lobe is triangular to deltoid. Column (9–10 × 4–4.5 mm) is straight, white, and perpendicular to the ovary, with a longitudinal furrow in the median portion, between both stigma lobes (Fig. 1c). Stigma (ca. 2.8–4.1 × 2.5 mm) is cordiform and reddish. Anther (ca. 4.9–5.4 × 2.5 mm) is brown. Pollinarium (ca. 5–5.5 × 2 mm) is made up of two white, clavate pollinia and a gray terminal viscidium.
The flowers of both species are scentless as no fragrances were detected by day or by night. Furthermore, tests with neutral red were negative, indicating the absence of osmophores in the flowers of Pteroglossa glazioviana and P. roseoalba. The only secretory tissues in these two species are nectaries located on both margins of the labellum base (Fig. 1d). These nectariferous glands possess a homogeneous parenchyma covered with a simple epidermal surface with sparse trichomes (Fig. 1e). Parenchymal cells are isodiametric. The histochemical tests were positive for reducing sugars, which is evidenced by the dark brown precipitates (Fig. 1e), and for starch grains in both parenchyma and epidermal cells (Fig. 1f). Furthermore, nectar accumulates in the spur. The nectar volume of P. glazioviana is 2–20 μL (mean 8.22 ± 3.51) and average concentration is 17–33 % (mean 26.75 ± 3.87). That of P. roseoalba is 3–26 μL (mean 9.22 ± 4.88) and average concentration is 14–41 % (mean 27.98 ± 6.41).
Floral visitors and pollination process
At both study areas the flowers of Pteroglossa glazioviana and P. roseoalba were exclusively pollinated by butterflies. In the Serra do Japi, the pollinators of P. glazioviana were Heliconius (i.e., H. ethilla narcaea (Godart, 1819) and H. erato phyllis (Fabricius, 1775); Lepidoptera, Nymphalidae; Fig. 2a–c), while those of P. roseoalba (municipality of Itirapina) were Hesperiidae butterflies (i.e., Lychnuchoides ozias ozias (Hewitson, 1878); Lepidoptera, Hesperiidae; Fig. 2d), and H. ethilla narcaea. Pollinators visited one or two flowers per inflorescence from 10:00 to 16:30 h. Each visit lasted 2–11 s. During the observations of flowers of P. glazioviana, 23 visits by H. erato and two by H. ethilla were recorded. During the field observations of P. roseoalba, two visits by L. ozias ozias and a unique visitation by H. ethilla were recorded. The total number of pollinarium removals and pollinations could not be recorded unequivocally because Heliconius butterflies ingest the pollinia attached to their proboscis. However, in all the visits, individuals of H. erato and H. ethilla were seen carrying pollinaria or a mass of pollen of P. glazioviana at the base of their proboscis (Fig. 2c). Visits started with the butterfly landing on one flower of the inflorescence. The insect then uncoiled its proboscis and inserted it in the spur (Fig. 2a, b). Pollinarium removal occurred when butterflies contacted the viscidium with the base of their proboscis (Fig. 2c, d). Since pollinaria are made up of two friable pollinia, during each visit, the butterflies deposited pollen-pads on the stigmatic surface, effecting pollination (Fig. 2e).
a–c Pteroglossa glazioviana. a Heliconius erato phyllis inserting its proboscis inside the nectary. Note the pollen on proboscis base (arrow). b Heliconius erato phyllis inserting its proboscis inside the nectary. Note the pollen mass contacting the stigmatic surface (arrow). c A Heliconius erato phyllis with a pollen mass at proboscis base. The detail shows a butterfly with a fresh (not digested) pollinarium. d–f Pteroglossa roseoalba. d Lychnuchoides ozias ozias leaving a flower after probing for nectar in the spur. Note the pollinarium attached to proboscis base. e Flower in the natural habitat with pollen-pads deposited on the stigmatic surface (arrows). f Miltomiges cinnamomea probing for nectar in a flower. Bars a, b, d, f 1 cm; c, e 5 mm
During field observations on flowers of Pteroglossa glazioviana we recorded visits by Bombus brasiliensis (Lepeletier, 1836), Euglossa sp., and one unidentified skipper. However, as no pollinarium was removed, they were considered mere floral visitors. Furthermore, during the observations on P. roseoalba visits by skipper Miltomiges cinnamomea (Herrich-Schäffer, 1869; Fig. 2f) and an unidentified wasp were also recorded. Both acted solely as nectar robbers. A Meliponini bee and a hoverfly were observed collecting pollen directly from the anther. None of the previously marked flowers had its pollinarium removed by night. Additionally, no visitors were recorded by the infrared cameras between 18:00 and 07:00 h.
Breeding system and natural fruit set
Pteroglossa glazioviana and P. roseoalba are completely self-compatible. In both species, fruit set in cross- and self-pollinated flowers was 100 %. No intact (bagged) or emasculated flower yielded fruits. Thus, a biotic vector is needed for pollen transfer. Under natural conditions, the fruit set of P. glazioviana and P. roseoalba (2013 flowering period) was 35.3 and 27.4 %, respectively. The fruit set results of both species are summarized in Table 1.
The percentage of potentially viable seeds yielded by the fruits obtained through experimental treatments of Pteroglossa glazioviana varied between 27 and 83 % (mean 54.5 %) in self-pollinations, and from 25 to 69 % (mean 46.1 %) in cross-pollinations. The percentage of potentially viable seeds obtained through the experimental treatments in flowers of P. roseoalba varied between 21 and 83 % (mean 55.5 %) in manual self-pollinations and from 16 to 80 % (mean 58.7 %) in manual cross-pollinations. No polyembryonic seeds were recorded in the manually cross- and self-pollinated fruits of either Pteroglossa. There was no significant difference in seed viability among fruits obtained through manual self- and cross-pollinations treatments involving flowers of P. roseoalba (t test = −0.712, d.f. = 67, P = 0.487). However, a significant difference in potentially viable seeds between self- and cross-pollinated flowers was observed in treatments with P. glazioviana (t test = 2.125, d.f. = 70, P = 0.0142).
Discussion
The flower periods of both studied Pteroglossa are uncommon among the Spiranthinae occurring in the two studied region, whose species blossom during the dry season, from April to September (Ferreira et al. 2010; Pansarin and Pansarin 2008), although the blooming time of some species, such as Mesadenella cuspidata, overlaps that of both studied taxa (Pansarin and Pansarin 2008). The flower morphology of Pteroglossa is widespread among members of the subtribe Spiranthinae (see Salazar 2003). According to Singer and Sazima (1999), some morphological features of Spiranthinae, as divisible pollinia and broad stigmas, favor the occurrence of cross-pollination. In fact, members of the subtribe Spiranthinae are characterized by their friable pollinia, which may pollinate a higher number of flowers than the hard pollinia of species belonging to other groups (i.e., Epidendroideae). Furthermore, the presence of large stigmatic surfaces, as is the case with Pteroglossa, is considered an important factor to increase the possible deposition of multiple pollen loads and, at least presumably, the chances of cross-pollination (Singer and Sazima 2001). In Pteroglossa, the stigma of one-day flowers are not very adhesive and, in this phase, pollen loads do not adhere well. Thus, although pollen-pads deposition is facilitated in flowers from the second day on, the studied Pteroglossa did not show obvious protandry. Within Spiranthinae, the occurrence of protandry has been recorded in some genera, as Spiranthes (Catling and Catling 1991), Sauroglossum (Singer 2002) and Mesadenella (Singer 2002; Cabral and Pansarin, unpubl. data). All protandrous Spiranthinae are pollinated by bees, except Sauroglossum (S. elatum Lindl.), which is pollinated by Lepidoptera (i.e., Noctuidae moths; Singer 2002). In bee-pollinated Mesadenella and Spiranthes, the column of one-day flowers is directed toward the labellum. Thus, even though their stigma is adhesive, fresh flowers only act as pollen donors. After a few days, the labellum moves away from the column, exposing the stigmatic surface that can receive pollen-pads. The viscidia of old flowers are usually dry, which hinders pollinarium removal (Catling 1983). Yet, in Pteroglossa, pollinarium removal is possible from blooming to withering.
Pollinarium depositions on pollinator mouthparts appear to be the rule among the members of the subtribe Spiranthinae. As reported here for Pteroglossa, in Sauroglossum pollinaria are deposited on the proboscis of Noctuidae moths (Singer 2002). In fact, when orchid flowers are pollinated by Lepidoptera, the pollinaria get stuck either on the eyes (e.g., Pedron et al. 2012; Singer and Cocucci 1997), tongue (e.g., Pansarin and Amaral 2008; Pedron et al. 2012), or, more rarely, legs (Johnson and Bond 1994) and between the palpi (Pedron et al. 2012), which are the only areas not covered by scales, where viscidium can adhere (Johnson and Edwards 2000). Although Spiranthinae includes some genera with dorsally adhesive viscidium (e.g., Cyclopogon, Sarcoglottis and Pelexia), as recorded here for Pteroglossa, in many other members of this subtribe (e.g., Stenorrhynchos, Sacoila and Eltroplectris), the adhesive surface of the viscidium assumes a ventral position (Salazar 2003). The occurrence of dorsally adhesive viscidia is considered a synapomorphy of the clade, the so called “Pelexia alliance” (see Dressler 1993; Salazar 2003; Singer and Sazima 1999). Since they are adapted to stick to bee mouthparts, as the ventral surface of labrum, dorsally adhesive viscidia appear to have evolved as a consequence of pollinator pressures. They seem to be an ecological advantage because they diminish the chances that bees clean the pollinarium and both friable pollinia remain protected under the head (Singer and Sazima 1999). According to Salazar (2003), among Spiranthinae, Sacoila, Stenorrhynchos, Mesadella, Pteroglossa and Eltroplectris are closely related genera. Although they are pollinated by different pollen vectors, bees for Mesadenella (Singer 2002), hummingbirds for Sacoila and Stenorrhynchos (Singer and Sazima 2000), and butterflies for Pteroglossa (data presented here), they all share a ventrally adhesive viscidia. The fact that closely related genera possess ventral viscidia does not seem to be linked to ecological pressures exerted by pollinators, as is the case with the “Pelexia alliance” (Singer and Sazima 1999). Even though ventrally adhesive viscidia are perfectly adapted to pollination by Lepidoptera and birds, which pollinate the members of the “Stenorrhynchos alliance” (i.e., Sacoila, Stenorrhynchos and Pteroglossa; Salazar 2003), as far as we know, Mesadenella is pollinated by small solitary bees whose mouthparts remove their pollinarium. Now, dorsally adhesive viscidia are considered ecologically important to reduce pollen loss in the “Pelexia alliance” (Singer and Sazima 1999). In addition, in bee-pollinated Mesadenella, the fact that pollinarium fixes on mouthparts does not seem to be disadvantageous, since fructification rates are close to 100 % in natural conditions (open pollination; Cabral and Pansarin, unpubl. data).
Although studies on the pollination biology of members of Spiranthinae are scarce, as far as we know, most of them are melittophilous. However, within subtribe Spiranthinae, hummingbirds have been recorded as pollinators of Sacoila (as Stenorrhynchos; Singer and Sazima 2000). Furthermore, besides Pteroglossa, Lepidoptera have been reported as pollinators of the moth-pollinated genus Sauroglossum (Singer 2002). Based on flower morphology, Eltroplectris, a genus closely related to Pteroglossa, is assumed to be pollinated by moths (Salazar 2003). Nevertheless, at least one unidentified Brazilian species (probably a new taxon) produced fruits through spontaneous self-pollination (E.R. Pansarin and A.W.C. Ferreira, unpubl. data). Based on the architecture of the flowers of Pteroglossa, only long-tongued Lepidoptera are able to access the bottom of the spur. Additionally, the flowers of both Pteroglossa are whitish, a characteristic shared by many phalenophilous Orchidoideae, including Spiranthinae (Singer 2002), but scentless. Thus, butterflies are their exclusive pollinators. In fact, no night visits were recorded. Furthermore, since the spur is parallel to the inflorescence rachis and perpendicular to both the labellum and the column, a successful visit by a hummingbird is quite unlikely. Although the study regions are rich in hummingbird species, including the long-billed hermit hummingbird Phaethornis eurynome (Lesson, 1832), our orchids, which are exclusively pollinated by butterflies, were visited by Hymenoptera and diurnal Lepidoptera. In southeastern Brazil, flowers of Aspidogyne longicornu (Cogn.) Garay (Goodyerinae) are visited by Phaethornis ruber (Linnaeus, 1758), which acts solely as a nectar robber, since the effective pollinators are euglossine bees (Singer and Sazima 2001). In South America, visits by butterflies have been recorded in the Goodyerinae genus Aspidogyne (Singer and Sazima 2001), and by skippers and Heliconius in Habenaria (Orchidinae; Moreira et al. 1996; Pedron et al. 2012). Yet, as far as we know, psychophilous pollination is documented for the first time in a member of the subtribe Spiranthinae. Pollination by Nymphalidae butterflies (i.e., Heliconius), as occurs in Pteroglossa, was also recorded in other southeastern Brazilian orchids, as nectar-producing Oeceoclades maculata (Lindl.) Lindl. (Aguiar et al. 2012) and Comparettia coccinea Lindl. (E.R. Pansarin and L.M. Pansarin, unpubl. data), and nectar-deceptive Epidendrum secundum Jacq. (Pansarin and Amaral 2008).
In Pteroglossa, floral nectar is produced by the two glandular margins of the labellum base and accumulates in the spur. Within the orchid family, floral nectar is produced by a great variety of structures (Dressler 1993). Nectar secretion by nectariferous glands located at the lip base has also been recorded in vanilloid Cleistes (Pansarin et al. 2012). It is considered a synapomorphic character, because it supports the monophyly of this South-Central American genus (Pansarin et al. 2012). Although the nectar volume accumulated in the spur of both Pteroglossa is considerably higher than in other nectar-producing orchids occurring in Southeastern Brazil, its concentration is similar to what has previously been recorded for Oeceoclades maculata and Comparettia coccinea (Aguiar et al. 2012; E.R. Pansarin and L.M. Pansarin, unpubl. data). Some authors argue that nectar can be energetically expensive and resources for reward production could be more usefully allocated to other functions able to increase fitness in species pollinated through deception (Ackerman 1986; Boyden 1982). The main problem with this hypothesis is that in many orchids fitness is pollination-limited rather than resource-limited (Calvo and Horvitz 1990; Calvo 1993). Among Spiranthinae, nectar production seems to be the rule, at least among the non-obligatorily autogamous species. In addition, the reproductive success of members of this group has been assumed to be high because of nectar production. In fact, many Spiranthinae pollinated by bees, hummingbirds and moths have a high fructification rate under natural conditions (see Singer and Sazima 1999, 2000, 2001). Yet, in the case of Pteroglossa, the reproductive success is low (35.3 % for P. glazioviana and 27.4 % for P. roseoalba), when compared to other bee-pollinated Spiranthinae. Although comprehensive data is scarce, the visitation rates to southeastern Brazilian orchids pollinated by butterflies is usually low, which results in a poor fruit set (Aguiar et al. 2012; Pansarin and Amaral 2008).
Self-compatibility and biotic pollinator-dependence, as recorded here for Pteroglossa glazioviana and P. roseoalba, seems to be the most widespread reproduction system among Spiranthinae (Catling 1987; Catling and Catling 1991; Singer and Sazima 1999, 2000; Singer 2002). However, autogamy and facultative spontaneous self-pollination have been recorded in Cyclopogon, Hapalorchis, Spiranthes and Stenorrhynchos (see Ackermann 1995; Catling 1983, 1987) and an unidentified species of Eltroplectris (Pansarin and Ferreira, unpubl. data). Within Spiranthinae, self-pollination tends to be avoided as a consequence of protandry (Singer and Sazima 2001). While Pteroglossa showed no evident mechanical barrier to avoid self-pollination, the erratic behavior of the butterflies, with infrequent visits to only one flower per inflorescence, contributes to increase the number of fruits produced by cross-pollination. In other butterfly-pollinated orchids, this infrequent behavior has been considered as favoring cross-pollinations in self-compatible (Pansarin and Amaral 2008; Pansarin and Pansarin, unpubl. data) and in predominantly rain-pollinated Brazilian orchids (Aguiar et al. 2012).
References
Ackerman JD (1986) Mechanisms and evolution of fooddeceptive pollination systems in orchids. Lindleyana 1:108–113
Ackermann JD (1995) An orchid flora of Puerto Rico and the Virgin Islands, vol 73. Memoirs of The New York Botanical Garden, New York
Aguiar JMRBV, Pansarin LM, Ackerman JD, Pansarin ER (2012) Biotic versus abiotic pollination in Oeceoclades maculata (Lindl.) Lindl. (Orchidaceae). Plant Species Biol 27:86–95
Boyden TC (1982) The pollination biology of Calypso bulbosa var. americana (Orchidaceae): initial deception of bumblebee visitors. Oecologia 55:178–184
Calvo RN (1993) Evolutionary demography of orchids: intensity and frequency of pollination and the cost of fruiting. Ecology 74:1033–1042
Calvo RN, Horvitz CC (1990) Pollinator limitation, cost of reproduction, and fitness in plants, a transition matrix demographic approach. Am Nat 136:499–516
Catling PM (1980) Rain-assisted autogamy in Liparis loeselii (L.) L. C. Rich. (Orchidaceae). B Torrey Bot Club 107:525–529
Catling PM (1982) Breeding systems of northeastern North American Spiranthes. Can J Bot 60:3017–3034
Catling PM (1983) Spiranthes ovalis erostellata (Orchidaceae), a new autogamous variety from the eastern United States. Brittonia 35:120–125
Catling PM (1987) Notes on the breeding systems of Saccoila lanceolata (Aublet) Garay (Orchidaceae). Ann Missouri Bot Gard 74:58–68
Catling PM, Catling VR (1991) A synopsis of breeding systems and pollination in North American orchids. Lindleyana 6:187–210
Dafni A (1992) Pollination ecology: a practical approach. Oxford University Press, Oxford
de Barros F, Vinhos F, Rodrigues VT, Barberena FFVA, Fraga CN, Pessoa EM, Forster W (2013) Orchidaceae. In: Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB179 Accessed 1 Nov 2013
Dressler RL (1993) Phylogeny and classification of the orchid family. Dioscorides Press, Oregon
Faegri K, van der Pijl L (1979) The principles of pollination ecology. Pergamon Press, Oxford
Ferreira AWC, Lima MIS, Pansarin ER (2010) Orchidaceae na região central de São Paulo, Brasil. Rodriguésia 61:243–259
Fuhro D, de Araújo AM, Irgang BE (2010) Are there evidences of a complex mimicry system among Asclepias curassavica (Apocynaceae), Epidendrum fulgens (Orchidaceae), and Lantana camara (Verbenaceae) in Southern Brazil? Rev Braz Bot 33:589–598
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Co, New York
Johnson SD (1994) Evidence for Batesian mimicry in a butterfly pollinated orchid. Biol J Linn Soc 53:91–104
Johnson SD, Bond WJ (1994) Red flowers and butterfly pollination in the fynbos of South Africa. In: Arianoutsou M, Groves RH (eds) Plant–animal interactions in Mediterranean-type ecosystems. Kluwer Academic Publisher, Dordrecht, pp 137–148
Johnson SD, Edwards TJ (2000) The structure and function of orchid pollinia. Plant Syst Evol 222:243–269
Johnson SD, Linder HP, Steiner KE (1998) Phylogeny and adaptative radiation of pollination systems in Disa (Orchidaceae). Am J Bot 85:402–411
Köppen W (1948) Climatologia: com um estúdio de los climas de la tierra. Fondo de Cultura Econômica, México
Kronka FJM, Matsukuma CK, Nalon MA, Cali IHD, Rossi M, Mattos IFA, Shinike MS, Pontinhas AAS (1993) Inventário florestal do Estado de São Paulo. Instituto Florestal de São Paulo, São Paulo
Leitão-Filho HF (1992) A flora arbórea da Serra do Japi. In: Morellato LPC (ed) História natural da Serra do Japi. Editora da Unicamp/Fapesp, Campinas, pp 40–62
Micheneau C, Fournel J, Warren BH, Hugel S, Gauvin-Bialecki A, Pailler T, Strasberg D, Chase MW (2010) Orthoptera, a new order of pollinator. Ann Bot 105:355–364
Moreira GRP, Corrêa CA, Mugrabi-Oliveira E (1996) Pollination of Habenaria pleiophylla Hoehne & Schlechter (Orchidaceae) by Heliconius erato Phyllis Fabricius (Lepidoptera; Nymphalidae). Rev Braz Zool 13:791–798
Neiland MRM, Wilcock C (1998) Fruit set, nectar reward, and rarity in the Orchidaceae. Am J Bot 85:1657–1671
Pansarin ER, Amaral MCE (2008) Reproductive biology and pollination mechanisms of Epidendrum secundum (Orchidaceae). Floral variation: a consequence of natural hybridization? Plant Biol 10:211–219
Pansarin ER, Pansarin LM (2008) A família Orchidaceae na Serra do Japi, São Paulo, Brasil. Rodriguésia 59:99–111
Pansarin LM, Pansarin ER, Sazima M (2008) Reproductive biology of Cyrtopodium polyphyllum (Orchidaceae): a Cyrtopodiinae pollinated by deceit. Plant Biol 10:650–659
Pansarin ER, Salatino A, Pansarin LM, Sazima M (2012) Pollination systems in Pogonieae (Orchidaceae: Vanilloideae): a hypothesis of evolution among reward and rewardless flowers. Flora (Jena) 207:849–861
Pedron M, Buzatto CR, Singer RB, Batista JAN, Moser A (2012) Pollination biology of four sympatric species of Habenaria (Orchidaceae: Orchidinae) from southern Brazil. Bot J Linn Soc 170:141–156
Pinto MM (1989) Levantamento fitossociológico de uma mata residual: Campus de Jaboticabal da UNESP. Dissertação de mestrado, Universidade Estadual Paulista, Jaboticabal
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (2001) Genera Orchidacearum, Orchidoideae (part 1), vol 2. Oxford University Press, Oxford
Purvis MJ, Collier DC, Walls D (1964) Laboratory techniques in botany. Butterwoths, London
Robertson JL, Wyatt R (1990) Evidence for pollination ecotypes in the yellow-fringed orchid, Platanthera ciliaris. Evolution 44:121–133
Salazar GA (2003) Subtribe Spiranthinae. In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds) Genera Orchidacearum, vol 3., Orchidoideae part 2, VanilloideaeOxford University Press, Oxford, pp 164–278
Sazima M, Buzato S, Sazima I (2003) Dyssochroma viridiflorum (Solanaceae): a reproductively bat-dependent epiphyte from the Atlantic Rainforest in Brazil. Ann Bot 92:725–730
Singer RB (2002) The pollination biology of Sauroglossum elatum Lindl. (Orchidaceae: Spiranthinae): moth-pollination and protandry in Neotropical Spiranthinae. Bot J Linn Soc 138:9–16
Singer RB, Cocucci AA (1997) Eye attached hemipollinaria in the hawkmoth and settling moth-pollination of Habenaria (Orchidaceae): a study on functional morphology in five species from subtropical South America. Bot Acta 110:328–337
Singer RB, Sazima M (1999) The pollination mechanism in the ‘Pelexia alliance’ (Orchidaceae: Spiranthinae). Bot J Linn Soc 131:249–262
Singer RB, Sazima M (2000) The pollination of Stenorrhynchos lanceolatus (Aublet) L. C. Rich. (Orchidaceae: Spiranthinae) by hummingbirds in southeastern Brazil. Plant Syst Evol 223:221–227
Singer RB, Sazima M (2001) Flower morphology and pollination mechanism in three sympatric Goodyerinae orchids from southeastern Brazil. Ann Bot 88:989–997
Soares JJ, da Silva DW, Lima MIS (2003) Current state and projection of the probable original vegetation of the São Carlos region of São Paulo State, Brazil. Brazil J Biol 63:527–536
StatSoft Inc (2003) STATISTICA (data analysis software system), version 6. http://www.statsoft.com. Accessed 13 Sept 2003
Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN (2005) Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversifi cation. Biol J Linn Soc 84:1–54
van der Cingel NA (1995) An atlas of orchid pollination: European orchids. A. A. Balkema, Rotterdam
van der Pijl L, Dodson CH (1966) Orchid flowers, their pollination and evolution. University of Miami Press, Florida
Wang Y, Zhang Y, Ma X-K, Dong L (2008) The unique mouse pollination in an orchid species. Nature Precedings. http://precedings.nature.com/documents/1824/version/1. Accessed 2 Mar 2015
Acknowledgments
We thank the "Base Ecológica da Serra do Japi", the "Guarda Municipal de Jundiaí" and "Fazenda Pinheirinho" for granting permission for the field work, André Victor Lucci Freitas (Unicamp) for butterfly identification, and Alain François for English improvements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pansarin, E.R., Ferreira, A.W.C. Butterfly pollination in Pteroglossa (Orchidaceae, Orchidoideae): a comparative study on the reproductive biology of two species of a Neotropical genus of Spiranthinae. J Plant Res 128, 459–468 (2015). https://doi.org/10.1007/s10265-015-0707-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-015-0707-x