Abstract
Primary immune thrombocytopenia (ITP) is an acquired autoimmune disorder characterized by an isolated decrease in platelets below 100 × 109/l after the exclusion of other conditions associated with thrombocytopenia. We investigated the role of different memory T-cell subsets, including T stem cell memory (TSCM), in children diagnosed with primary ITP and its association with therapeutic duration. This case–control study included 39 pediatric patients with acute ITP admitted to the Children's Hospital at Assiut University. Using a FACSCanto flow cytometer, CD8 + and CD4 + T-lymphocytes were gated. Five different subsets were characterized in each of these cells according to CD45RO and CD45RA expression. Afterward, gating was performed based on CCR7, CD95, and CD27. Examination of the CD8 + T cells subpopulation showed that Central memory T (TCM) and CD8+ Naïve T (TN) cells were significantly lower in ITP patients than in healthy children (p < 0.0001) and (p = 0.01), respectively. In addition, CD8 + TEMRA was significantly higher in ITP children than in controls (p = 0.001). CD4 + TCM cells were significantly lower in the ITP patient group (p = 0.04). However, CD4 + TEM was significantly higher in patients than controls (p = 0.04). Our research found that ITP patients had an imbalance in the ratio of CD4+ to CD8+ T cells in the peripheral blood and that TCM cells may be involved in the pathogenetic mechanism of ITP. TCMs could help in prediction of patients with higher risk of developing ITP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary immune thrombocytopenia (ITP) is an acquired autoimmune disorder characterized by an isolated decrease in platelets below 100 × 109/l after the exclusion of other conditions associated with thrombocytopenia. ITP often affects children between two and five years old at a frequency of 4.2 per 100,000 children [1,2,3]. Based on previous research, aberrant humoral immunity results from the body producing anti-platelet antibodies, causing platelet destruction through the mononuclear-macrophagocyte system [1,2,3]. Additionally, the loss of platelets is brought on by the damage to and malfunction of bone marrow megakaryocytes. However, only 50 to 70 percent of ITP patients have anti-platelet antibodies [4]. Therefore, the absence of such antibodies does not rule out the diagnosis. It is now proved that cellular immunity contributed significantly to the development of ITP [5]. However, the exact pathogenetic pathways are not fully understood.
After the immune response is triggered by the antigen and resolved, the naive T-cells can produce several subgroups of memory T-lymphocytes with variable genetic expression, phenotype, and anatomical distribution [6]. In addition, the concept of immunological memory is a fundamental characteristic of the adaptive immune system that recalls a particular antigenic exposure and subsequently mounts improved (immediate and effective) responses upon re-exposure [7].
Accordingly, Memory T-lymphocytes are generally classified as central memory (TCM), effector memory (TEM), and CD45RA + effector memory (TERA). These memory T-lymphocyte subsets have short half-lives and a high rate of turnover [8].
Another subset, T stem cell memory (TSCM) cells, possess self-renewal capability and act as a long-lived memory precursor that resembles stem cells [9], where they can differentiate into all memory subsets, including TCM cells [7]. Additionally, they share the same distribution recirculation patterns and keep genes expressed by TN cells [7]. TSCM cells show a CD45RA + CCR7 + CD95 + phenotype and significantly contribute to the development of autoimmune disorders by acting as a source of autoreactive effector T-lymphocytes that support the persistent damage of certain tissues [10].
The balance between CD8 + and CD4 + T lymphocyte frequency is crucial to maintain effective function in the immune system. An abnormal CD4 + / CD8 + ratio has been linked to a variety of illnesses, including malignancies, autoimmune, and infectious disorders [11]. Furthermore, a direct relationship was observed between the frequencies of CD4 + and CD8 + T stem cell memory (TSCM) cells in conditions such as systemic lupus erythematosus (SLE). Additionally, the initial frequency of CD8 + TSCM cells at the time of diagnosis was linked to the effectiveness of immunosuppressive therapy in these cases [11].
Regarding ITP, an increased percentage of CD8 + T cells was associated with increased platelet destruction [12]. We previously reported that The CD4 + /CD8 + ratio was significantly decreased in ITP pediatric patients and that CD8 + cells could be a prognostic marker in these patients [13]. However, the connection between TSCM and ITP in pediatric patients has not yet been documented. Understanding how TSCMs are generated and maintained in ITP patients is essential to advance their therapeutic potential and reduce their adverse effects. In the present study, we investigated the role of different memory T cell subsets, including TSCMs, in children diagnosed with ITP and its association with the therapeutic response.
Materials and methods
Study design
This is a case–control study that included 39 children with acute ITP admitted to the Pediatric Clinical Hematology Unit of Children's Hospital in Assiut University. Patients were recruited from January 2021 to the end of November 2021. Twenty healthy children were included in the study as a control group. The control group consisted of children of the same age and sex with good general health status, including the absence of chronic or acute medical conditions and normal platelet count.
The diagnosis was made by evaluating the platelet count (below 100 × 109 cells/L) for less than one year. Newly diagnosed ITP is defined as a disease diagnosed within three months; however, persistent ITP is a disease with a duration between 3 and 12 months. Chronic ITP is defined as thrombocytopenia continuing beyond one year [14] No other hematological abnormalities or organomegaly were seen. The exclusion criteria were secondary thrombocytopenia, including infections, pediatric immunodeficiency disorders, and connective tissue diseases such as SLE, malignancies, drug-induced and congenital thrombocytopenia.
Patients underwent a thorough evaluation that included a detailed medical history, with particular emphasis on prior medications, bleeding symptoms and signs, ITP grading, comprehensive physical examinations, and standard laboratory assessments.
Management was performed according to the ITP grade of severity [15]. Patients were classified based on their response to treatment into two groups: patients with short duration, who recovered before three months, and patients with long duration, who did not recover before three months [16]. Complete remission or complete response to treatment was assessed based on platelet count ≥ 100 × 109/L with no clinically relevant bleeding [17]. Patients received treatment according to the American Society of Hematology 2019 guidelines for immune thrombocytopenia [18].
Samples collection
Two ml of peripheral venous blood was withdrawn from ITP patients before therapy and controls in ethylenediaminetetraacetic acid (EDTA) vacutainer blood collection tubes. Peripheral blood samples were obtained for routine and flow cytometry studies.
Flow cytometry
One hundred microliters (µl) of whole blood underwent incubation with specific antibodies under the following conditions: 10 µl of fluoroisothiocyanate (FITC)-conjugated anti-CD27, 10 µl of phycoerythrin (PE)-conjugated anti-CD8, 10 µl of PE-cyanine 7 (PE-CY7)-conjugated anti-CD45RO, 10 µl of allophycocyanin (APC)-conjugated anti-CD45RA, 10 µl of APC-H7-conjugated anti-CD4, 10 µl of PerCP-Cy5.5-conjugated anti-CCR7, and 10 µl of V500-conjugated anti-CD95. This incubation occurred for 15 min at a temperature of 4 ℃ in a light-protected environment. All the monoclonal antibodies used in this process were procured from Becton Dickinson (BD, CA). Subsequently, red blood cell (RBC) lysis buffer was introduced, followed by centrifugation at 2500 revolutions per minute. The resulting pellet was washed with phosphate-buffered saline (PBS). An isotype-matched negative control was utilized to identify any background staining signals present in each sample.
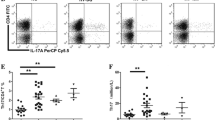
Approximately 100,000 events were recorded for each sample, and data analysis was carried out using the FACSCanto flow cytometer, with analysis facilitated by the FACS DIVA 7.0 software (BD). Lymphocytes were selectively chosen based on their light scatter properties. CD4 + and CD8 + T cells were subsequently gated, and within each of these cell populations, five distinct subsets were delineated based on the expression of CD45RA and CD45RO. Further gating was performed based on CD27, CCR7, and CD95 expression. These identified subsets encompass the following categories: TN (naive T cells, CD45RO − CD45RA + CCR7 + CD27 + CD95 −), TSCM (T memory stem cells, CD45RO − CD45RA + CCR7 + CD27 + CD95 +), TCM (central memory T cells, CD45RO + CD45RA-CCR7 +), TEM (effector memory T cells, CD45RO + CD45RA-CCR7 −), and TEMRA (effector memory T cells re-expressing CD45RA, CD45RO − CD45RA + CCR7 − CD27 −) Fig 1 .
Statistical analysis
The statistical package for social sciences (SPSS), version 16, was employed for statistical analysis. Descriptive statistics were computed for the variables, including mean values and standard errors of the mean. Due to the small sample size and the potential presence of outliers in some variables, group differences were assessed for statistical significance using the Mann–Whitney analysis. A p-value less than 0.05 was deemed statistically significant. Pearson’s correlation analysis was used to investigate the relationships between variables. Simple regression analysis was performed using JMP Pro 16 software (JMP, SAS Institute, North Carolina). Figures were generated using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
Basic demographic and laboratory characteristics of the studied groups
The present study was conducted on 39 ITP patients, 18 males and 21 females, and 26 controls. The mean age of ITP patients and controls was 6.6 ± 0.7 and 6.96 + 0.53, respectively. Cases and controls were age and sex-matched. Demographic and laboratory data of patients and controls were shown in Table 1.
CD8 and CD4 imbalance in ITP patients
Our results showed that the mean percentage of CD8+ T cells in ITP children was significantly higher than in healthy control (28 ± 1 vs. 24.2 ± 0.9; p = 0.01). However, the mean percentage of CD4 + T cells was signifying lower in patients than in controls (29.8 ± 1 vs. 37.6 ± 5, P < 0.0001). In addition, CD4/CD8 ratios were significantly lower in ITP children when compared to controls (1 ± 0.06 vs.1.6 ± 0.07, p < 0.0001).
CD8 + population in ITP
Analysis of the CD8+ T cells subpopulation showed that the mean percentages of CD8+ Naïve T (TN) and Central memory T (TCM) were significantly lower in ITP patients than in controls (20 ± 2 vs. 31.4 ± 2, p < 0.0001) and (13 ± 0.5 vs. 16.8 ± 1, p = 0.01), respectively. In addition, the mean percentage of highly differentiated CD8 + TEMRA was significantly higher in ITP children than in healthy control (19.9 ± 1 vs. 14 ± 1, p = 0.001). However, there was no significant difference between patients and controls in the mean percentages of CD8+CD45RA+, CD8+CD45RO+, CD8+ TSCM, and CD8+ TEM T-cell subpopulations. (Table 2) (Figure S1 in supplementary file).
CD4 + population in ITP
Our results showed that the mean percentage of CD4+CD45RA+, CD4+CD45RO+, CD4+ TN, CD4+ TEMRA, and CD4 + TSCM T-cell subpopulations was comparable between patients and controls (Table 3). CD4 + Central memory T (TCM) cells were significantly lower in the ITP patient group (25 ± 1 vs. 29.8 ± 2, p = 0.04). However, CD4+ TEM was significantly higher in patients than in healthy controls (14.7 ± 1 vs. 11.6 ± 0.8, p = 0.04) (Figure S2 in supplementary file).
Changes in T cell subsets regarding ITP duration
Comparing T cell subsets among patients with ITP duration less than and more than three months revealed a significant increase in CD8+ T cells and CD8+ TSCM subsets in patients with shorter duration. In addition, patients with longer ITP duration had a significant increase in CD4+ TCM cells. There was no significant difference regarding other T cell subsets (Table 4) (Figure S3 in supplementary file).
Correlation of T cell subsets with laboratory parameters and ITP duration
Correlation analysis showed a significant negative correlation between CD8, CD8 TSCMs, and CD4 TNs with platelet count. Moreover, the percentage of CD8 TSCMs was inversely correlated to ITP duration. (Table 5).
In addition, simple regression analysis showed that CD8+ and CD8+TSCM cells significantly affect the Patient's platelet count (p = 0.002 and p < 0.0001), respectively (Figure S4 in supplementary file).
Discussion
The present study thoroughly investigated T cells and their subpopulation in the periphery of the ITP pediatric patient cohort and analyzed the connection between these immunological indicators and therapeutic outcomes [18]. T cells are still in the central stages of anti-platelet autoimmunity, playing a crucial role in its genesis, transmission, and maintenance [19].
Our results demonstrated that ITP patients had an imbalance in T lymphocyte subsets, including a significant decrease in CD4+ Th cells and CD4/CD8 ratio with excessive CD8+ Tc cells in patients than controls (Fig. 1). Previous studies revealed that ITP’s pathogenesis involves aberrant immunocyte subsets [13, 20, and 21]. Moreover, studies showed that CD8+ Tc cell-mediated autoimmunity can occur independently of autoantibody-mediated autoimmunity, where they directly attack platelets and megakaryocytes [22].
Flow cytometric detection of T lymphocyte subsets. A: Lymphocytes were gated based on their characteristics on forward and side scatter histogram. B: Then CD4 + cells and CD8+ cells were assessed on lymphocytes and then gated for further analysis C–F: CD8+ T cells were subdivided based on characteristic expression patterns of CD45RA, CD45RO, CD27, CCR7 and CD95 into: TCM; CD8+ CD45RO+CCR7+. TEM;CD8+ CD45RO+ CCR7−. TEMRA;CD8+ CD45RO− CD45RA+ CCR7− CD27−. TN;CD8+ CD45RO− CD45RA+ CCR7+ CD27+ CD95−. TSCM;CD8+ CD45RO−CD45RA+ CCR7+ CD27+ CD95+ G–L:The same was done for CD4 + T cells
A significant increase in CD8+ Tc could be due to a prior viral infection, in which the production of platelets displaying viral antigens mounts CD8+ Tc cells' cytotoxic response, causing immune thrombocytopenia [23]. Also, T-helper 1 (TH1) bias was demonstrated in ITP, which in turn stimulates CD8+ Tc function and IgG production [24]. Aslam et al. [19] and Johnsen [25] found that the total CD4 + /CD8 + ratio is reduced in ITP and improves with illness remission, supporting our results. In addition, A significantly higher percentage of CD8+ Tc cells was detected in patients with thrombocytopenia for a brief duration (< 3 months) than in those with prolonged thrombocytopenia > 3 months. This is consistent with the preceding outcomes and may be related to previous viral infections [20, 26]. CD45 isoforms have long been used to distinguish between naive (CD45RA+CD45RO) and memory (CD45RACD45RO+) T cells [27]. Other surface markers, such as CCR7 [28], CD27 [29], and CD95 [30], when used along with CD45RA, provide a far more in-depth view of the T cell maturation process [2].
Memory T cells are classified as CD45RACCR7+ central memory T (TCM) cells that migrate to lymphoid tissues and CD45RACCR7 effector memory T (TEM) cells that travel to various peripheral tissue sites. TCM can efficiently differentiate into effector cells during proliferation. Both TCM and TEM subsets release effector cytokines in response to viruses, antigens, and other stimuli, whereas TCM cells have a higher proliferation capability [31]. Several identified populations, such as CD27CD45RA+CD197+CD95+ (now known as TEMRA) and CD27+CD45RA+ CD95+ (now known as TSCM), were first discovered in CD8+ T cells and afterward in CD4+ T lymphocytes. Both have Variable effector capacities and considerable changes in response based on the stimulating antigen [32]. In the current study, the percentage of naive T lymphocytes in ITP patients was significantly lower than in healthy controls regarding CD8+ cells, given the previously documented lower expression of CD45RA in autoimmune disorders [11]. However, no significant difference was noticed between patients with brief or long ITP duration.
Furthermore, a significant decrease in the percentage of CD4+ and CD8+ TCM cells with a corresponding high percentage of CD4+ and CD8+ TEM cells was observed among ITP patients compared to the control group. We suggest that TCM homing may be affected differently by changes in T cell subtypes in ITP patients, together with reduced T cell response. Elevated TEM cells with potent effector capacity have been identified as a potential indicator of abnormal immunity in aplastic anemia [33]. In addition, previous studies showed that exposure to chronic autoantigen seems to favor the development of CD4 + TEM while hindering CD4 + TCM cell formation, such as in chronic infection and different autoimmune diseases [34, 35]. Parallel results were specified by Xu et al. [36], who reported a decrease in TCM cells and excessive aggregation of TEM and TEF cells in patients with acute myeloid leukemia.
Nonetheless, in patients with longer thrombocytopenic duration, we found a significant increase in the percentage of CD4+ TCM. Moreover, the percentage of CD8+ TEMRA was significantly higher in ITP patients than control. TEMRA cells were initially characterized in the context of viral infections and vaccination responses [37, 38]. These cells have expanded cytotoxic characteristics supporting the hypothesis of platelet lysis by CD8+ T cells [39]. A prior investigation demonstrated that these cells have the ability to attach to platelets, leading to TCR-mediated activation and subsequent death of the platelets. This mechanism operates independently of antibodies and offers an alternative means of causing platelet destruction. Besides, it was revealed that these cells exhibit multiple functions, including the production of IFN-gamma, TNF-alpha, and granzyme B. Notably, there were no indications of physiological depletion, and these findings were found to be correlated with the activity of immune thrombocytopenia (ITP) disease [40].
Stem cell memory T cells (TSCM cells) were shown to possess the ability to self-renew and exhibit multipotency. This characteristic suggests that TSCM cells could serve as a potential long-term reservoir for T-cell memory throughout an individual's life [41]. At this firm, there was no significant difference in the percentages of CD8+ and CD4+ TSCM between patients and controls. However, CD8 TSCM was significantly higher in patients with shorter ITP duration and inversely correlated with the platelet count. Since TSCM cells may produce all memory and effector T cell subsets, it was thought that their rising prevalence might contribute to the emergence of autoimmune diseases [11]. In aplastic anemia, higher CD8+ TSCM frequency at the time of diagnosis was discovered to be related to greater response. However, increased CD8 + TSCM cells following immunosuppressive therapy was linked to treatment failure and relapse [11], suggesting CD8 + TSCMs might be a potential biomarker.
Conclusion
Our research found that ITP patients had an imbalance in the ratio of CD4+ to CD8+ T cells in the peripheral blood and that TCM cells may be involved in the pathogenetic mechanism of ITP. TCMs could help in prediction of patients with higher risk of developing ITP.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Muroyama Y, Wherry EJ. Memory T-cell heterogeneity and terminology. Cold Spring Harb Perspect Biol. 2021;13(10):a037929.
Mahnke YD, et al. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43(11):2797–809.
Raphael I, Joern RR, Forsthuber TG. Memory CD4+ T cells in immunity and autoimmune diseases. Cells. 2020;9(3):531.
Vignali D, et al. Detection and characterization of CD8+ autoreactive memory stem T cells in patients with type 1 diabetes. Diabetes. 2018;67(5):936–45.
Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med. 2017;23(1):18–27.
Wang Y, et al. Stem cell-like memory T cells: the generation and application. J Leukoc Biol. 2021;110(6):1209–23.
Swinkels M, et al. Emerging concepts in immune thrombocytopenia. Front Immunol. 2018;9:880.
Elsayh KI, et al. Regulatory T-lymphocyte subsets in children with chronic immune thrombocytopenia after high-dose of dexamethasone. Pediatr Res. 2022;92(5):1432–6.
Kiyomizu K, et al. Recognition of highly restricted regions in the β-propeller domain of αIIb by platelet-associated anti-αIIbβ3 autoantibodies in primary immune thrombocytopenia. Blood J Am Soc Hematol. 2012;120(7):1499–509.
Ji X, Zhang L, Peng J, Hou M. T cell immune abnormalities in immune thrombocytopenia. J Hematol Oncol. 2014;7(1):72.
Hosokawa K, et al. Memory stem T cells in autoimmune disease: high frequency of circulating CD8+ memory stem cells in acquired aplastic anemia. J Immunol. 2016;196(4):1568–78.
McKenzie CG, Guo L, Freedman J, Semple JW. Cellular immune dysfunction in immune thrombocytopenia (ITP). Br J Haematol. 2013;163(1):10–23.
Zahran AM, Elsayh KI. CD4+CD25+high Foxp3+ regulatory T cells, B lymphocytes, and T lymphocytes in patients with acute ITP in assiut children hospital. Clin Appl Thromb Hemost. 2012;20(1):61–7.
Rodeghiero F, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–93.
Provan D, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood J Am Soc Hematol. 2010;115(2):168–86.
Brito HSH, et al. Helicobacter pylori infection & immune thrombocytopenic purpura in children and adolescents: a randomized controlled trial. Platelets. 2015;26(4):336–41.
Neunert C, et al. American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–66.
Zufferey A, et al. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16.
Aslam R, et al. Thymic retention of CD4+CD25+FoxP3+ T regulatory cells is associated with their peripheral deficiency and thrombocytopenia in a murine model of immune thrombocytopenia. Blood. 2012;120(10):2127–32.
Wang HC, Li WQ, Feng JM. The immunocyte subsets and their clinical significance in the peripheral blood of 35 patients with immune thrombocytopenic purpura. Zhonghua Nei Ke Za Zhi. 2011;50(9):763–5.
Li W, et al. A study of immunocyte subsets and serum cytokine profiles before and after immunal suppression treatment in patients with immune thrombocytopenia. Zhonghua Nei Ke Za Zhi. 2016;55(2):111–5.
Zhao C, et al. Increased cytotoxic T-lymphocyte-mediated cytotoxicity predominant in patients with idiopathic thrombocytopenic purpura without platelet autoantibodies. Haematologica. 2008;93(9):1428–30.
Laurent C, et al. Distribution, function, and prognostic value of cytotoxic T lymphocytes in follicular lymphoma: a 3-D tissue-imaging study. Blood. 2011;118(20):5371–9.
Liu H, et al. Involvement of levels of Toll-like receptor-4 in monocytes, CD4+ T-lymphocyte subsets, and cytokines in patients with immune thrombocytopenic purpura. Thromb Res. 2013;132(2):196–201.
Johnsen J. Pathogenesis in immune thrombocytopenia: new insights. Hematology. 2012;2012(1):306–12.
El-Rashedi FH, et al. Study of CD4+, CD8+, and natural killer cells (CD16+, CD56+) in children with immune thrombocytopenic purpura. Hematol Oncol Stem Cell Ther. 2017;10(1):8–14.
Leitenberg D, et al. Biochemical association of CD45 with the T cell receptor complex: regulation by CD45 isoform and during T cell activation. Immunity. 1999;10(6):701–11.
Campbell JJ, et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166(2):877–84.
Hamann D, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186(9):1407–18.
Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–7.
Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14(1):24–35.
Piatosa B, et al. T lymphocytes in patients with nijmegen breakage syndrome demonstrate features of exhaustion and senescence in flow cytometric evaluation of maturation pathway. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.01319.
Hu X, et al. Increased CD4+ and CD8+ effector memory T cells in patients with aplastic anemia. Haematologica. 2009;94(3):428–9.
Kryczek I, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011. https://doi.org/10.1126/scitranslmed.3002949.
Fritsch RD, et al. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum. 2006;54(7):2184–97.
Xu L, et al. Memory T cells skew toward terminal differentiation in the CD8+ T cell population in patients with acute myeloid leukemia. J Hematol Oncol. 2018;11(1):93.
Precopio ML, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med. 2007;204(6):1405–16.
Krug LM, et al. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1467–79.
Escorcio-Correia M, Provan A, Pennington DJ. Immune profiling of immune thrombocytopenia (ITP) patients: evidence for CD8 T cell involvement in the disease. Blood. 2015;126(23):2259.
Malik A, et al. CD8+ TEMRA clones cause platelet lysis in immune thrombocytopenia. Blood. 2022;140(Supplement 1):2209–10.
Lugli E, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123(2):594–9.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
KIE, KS, HM, EF, AE, SGM, and HGD collectively undertook the study's conception, design, patient management, data analysis, and initial manuscript composition. AMZ, OHE, AM, and ZAMZ conducted the laboratory investigations integral to the study. Subsequently, all authors participated in the rigorous evaluation of the final manuscript version. Furthermore, all authors granted their approval for the submitted manuscript and assumed responsibility for all facets of the study's execution.
Corresponding author
Ethics declarations
Conflicts of interest
No competing interest.
Ethical approval and consent to publication
The present study received approval from Assiut University Ethical Committee (IRB NO. 17300668–2021), adhering to the principles outlined in the Declaration of Helsinki. Informed consent was obtained from the parents of all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zahran, A.M., El-Badawy, O.H., Mahran, H. et al. Detection and characterization of autoreactive memory stem T-cells in children with acute immune thrombocytopenia. Clin Exp Med 24, 158 (2024). https://doi.org/10.1007/s10238-024-01386-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-024-01386-0