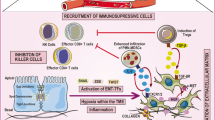
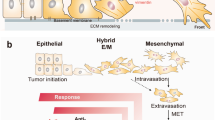
Abstract
Epithelial–mesenchymal transition (EMT) is a dynamic program crucial for organismal development and tissue regeneration. Unfortunately, this program is often hijacked by epithelial tumors to facilitate metastasis. Beyond its role in cancer spread, EMT increases cancer cell survival by activating stem cell programs and bypassing apoptotic programs. Importantly, the capacity of EMT to enforce tumor progression by altering the tumor cell phenotype without triggering immune responses opens the intriguing possibility of a mechanistic link between EMT-driven cancers and immune evasion. Indeed, EMT has been acknowledged as a of driver immune evasion, but the mechanisms are still evolving. Here, we review recent insights into the influence of EMT on tumor immune evasion. Specifically, we focus on the mechanistic roles of EMT in immune escape as the basis that may provide a platform for innovative therapeutic approaches in advanced tumors. We summarize promising therapeutic approaches currently in clinical trials and trending preclinical studies aimed at reinvigorating the tumor microenvironment to create immune-permissive conditions that facilitates immune-mediated tumor clearance. We anticipate that this will assist researchers and pharmaceutical companies in understanding how EMT compromises the immune response, potentially paving the way for effective cancer therapies.





Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- EMT:
-
Epithelial–mesenchymal transition
- MET:
-
Mesenchymal–epithelial transition
- TME:
-
Tumor microenvironment
- HLA:
-
Human leukocyte antigen
- CSCs:
-
Cancer stem cells
- EMP:
-
Epithelial–mesenchymal plasticity
- ECM:
-
Extracellular matrix
- EMT-TF:
-
Epithelial–mesenchymal transition transcription factors
- MHC:
-
Major histocompatibility class
- β2M:
-
β2-microglobulin
- CTLS:
-
Cytotoxic T lymphocytes
- TGF-β:
-
Transforming growth factor beta
- TAP:
-
Transporters associated with antigen processing
- IS:
-
Immunoproteasome
- PSMB :
-
Proteasome subunit beta type
- NSCLC:
-
Nonsmall cell lung cancer
- KLRG1:
-
Killer lectin-like receptor G1
- E-cadherin:
-
Epithelial cadherin
- KIRs:
-
Killer immunoglobin-like receptors
- IFN-γ:
-
Interferon gamma
- TCR:
-
T-cell receptor
- LC3:
-
Light chain 3
- ATG:
-
Autophagy-related
- ULK:
-
Unc-51-like autophagy-activating kinase
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- PD-1:
-
Programmed cell death protein 1
- TIM-3:
-
T-cell immunoglobulin domain and mucin domain-3
- LAG-3:
-
Lymphocyte activation gene 3
- TIGIT:
-
T-cell immunoreceptor with Ig and ITIM domains
- APCs:
-
Antigen presenting cells
- PD-L1:
-
Programmed death ligand
- MDSCs:
-
Myeloid-derived suppressor cells
- TRegs:
-
Regulatory T cells
- TAMs:
-
Tumor-associated macrophages
- DCreg:
-
Regulatory dendritic cells
- IDO:
-
Indoleamine 2,3-dioxygenase
- CCL:
-
Chemokine (CC motif) ligand
- CXCL:
-
Chemokine (CXC motif) ligand
- NF-κβ:
-
Nuclear factor kappa B
- CXCR:
-
CXC chemokine receptor
- HDACs:
-
Histone deacetylases
- LSD:
-
Lysine-specific histone demethylase
References
Adedokun KA, Imodoye SO, Bello IO, Lanihun A, Bello IO. Therapeutic potentials of medicinal plants for the treatment of cancer and significance of computational tools in anticancer drug discovery. In: Egbuna C, Rudrapal M, Tijjani H, editors. Phytochemistry, computational tools, and databases in drug discovery. Amsterdam: Elsevier; 2023.
Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201–18.
Imodoye SO, Adedokun KA, Muhammed AO, Bello IO, Muhibi MA, Oduola T, Oyenike MA. Understanding the complex milieu of epithelial-mesenchymal transition in cancer metastasis: new insight into the roles of transcription factors. Front Oncol. 2021;11:762817.
Nieto MA, Cano A. The epithelial–mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol. 2012;22:361–8.
Zhang L, Liao Y, Tang L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 2019;38:53.
Chang R, Zhang Y, Zhang P, Zhou Q. Snail acetylation by histone acetyltransferase p300 in lung cancer. Thorac Cancer. 2017;8:131–7.
Manfioletti G, Fedele M. Epithelial–mesenchymal transition (EMT) 2021. Int J Mol Sci. 2022;23:5848.
Galbraith M, Levine H, Onuchic JN, Jia D. Decoding the coupled decision-making of the epithelial-mesenchymal transition and metabolic reprogramming in cancer. iScience. 2023;26(1):105719.
Tan T, Shi P, Abbas MN, Wang Y, Xu J, Chen Y, Cui H. Epigenetic modification regulates tumor progression and metastasis through EMT (review). Int J Oncol. 2022;60:1–17.
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–26.
Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. 2022;15:129.
Brabletz S, Schuhwerk H, Brabletz T, Stemmler MP. Dynamic EMT: a multitool for tumor progression. EMBO J. 2021;40:e108647.
Coghlin C, Murray GI. Current and emerging concepts in tumor metastasis. J Pathol. 2010;222:1–15.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–91.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84.
Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206.
Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185–98.
Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol. 2016;27:1492–504.
Patel SA, Minn AJ. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity. 2018;48:417–33.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10.
Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33:1755–63.
Taki M, Abiko K, Ukita M, Murakami R, Yamanoi K, Yamaguchi K, Hamanishi J, Baba T, Matsumura N, Mandai M. Tumor immune microenvironment during epithelial-mesenchymal transition. Clin Cancer Res. 2021;27:4669–79.
Romeo E, Caserta CA, Rumio C, Marcucci F. The vicious cross-talk between tumor cells with an EMT phenotype and cells of the immune system. Cells. 2019;8:460.
Horn LA, Fousek K, Palena C. Tumor plasticity and resistance to immunotherapy. Trends Cancer. 2020;6:432–41.
Seliger B. Molecular mechanisms of HLA class I-mediated immune evasion of human tumors and their role in resistance to immunotherapies. HLA. 2016;88:213–20.
López-Soto A, Huergo-Zapico L, Galván JA, Rodrigo L, de Herreros AG, Astudillo A, Gonzalez S. Epithelial-mesenchymal transition induces an antitumor immune response mediated by NKG2D receptor. J Immunol. 2013;190:4408–19.
Lee JH, Shklovskaya E, Lim SY, Carlino MS, Menzies AM, Stewart A, Pedersen B, Irvine M, Alavi S, Yang JYH, Strbenac D, Saw RPM, Thompson JF, Wilmott JS, Scolyer RA, Long GV, Kefford RF, Rizos H. Transcriptional downregulation of MHC class I and melanoma de differentiation in resistance to PD-1 inhibition. Nat Commun. 2020;11:1897.
Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, Weinberg RA. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Can Res. 2017;77:3982–9.
Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn Y-H, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal JD, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Jones S, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Peng D, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FX-F. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumor cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241.
Dongre A, Rashidian M, Eaton EN, Reinhardt F, Thiru P, Zagorulya M, Nepal S, Banaz T, Martner A, Spranger S, Weinberg RA. Direct and indirect regulators of epithelial-mesenchymal transition–mediated immunosuppression in breast carcinomas, cancer. Discovery. 2021;11:1286–305.
Chen X-H, Liu Z-C, Zhang G, Wei W, Wang X-X, Wang H, Ke H-P, Zhang F, Wang H-S, Cai S-H, Du J. TGF-β and EGF induced HLA-I downregulation is associated with epithelial-mesenchymal transition (EMT) through upregulation of snail in prostate cancer cells. Mol Immunol. 2015;65:34–42.
Aguilera TA, Giaccia AJ. Molecular pathways: oncologic pathways and their role in T-cell exclusion and immune evasion—a new role for the AXL receptor tyrosine kinase. Clin Cancer Res. 2017;23:2928–33.
Maccalli C, Rasul KI, Elawad M, Ferrone S. The role of cancer stem cells in the modulation of antitumor immune responses. Semin Cancer Biol. 2018;53:189–200.
Tu J, Xu H, Ma L, Li C, Qin W, Chen X, Yi M, Sun L, Liu B, Yuan X. Nintedanib enhances the efficacy of PD-L1 blockade by upregulating MHC-I and PD-L1 expression in tumor cells. Theranostics. 2022;12:747–66.
Cornel AM, Mimpen IL, Nierkens S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers. 2020;12:1760.
Morozov AV, Karpov VL. Proteasomes and several aspects of their heterogeneity relevant to cancer. Front Oncol. 2019;9:761.
Tripathi SC, Peters HL, Taguchi A, Katayama H, Wang H, Momin A, Jolly MK, Celiktas M, Rodriguez-Canales J, Liu H, Behrens C, Wistuba II, Ben-Jacob E, Levine H, Molldrem JJ, Hanash SM, Ostrin EJ. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc Natl Acad Sci. 2016;113:E1555–64.
Kalaora S, Lee JS, Barnea E, Levy R, Greenberg P, Alon M, Yagel G, Bar Eli G, Oren R, Peri A, Patkar S, Bitton L, Rosenberg SA, Lotem M, Levin Y, Admon A, Ruppin E, Samuels Y. Immunoproteasome expression is associated with better prognosis and response to checkpoint therapies in melanoma. Nat Commun. 2020;11:896.
Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagné F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42.
Wolf NK, Kissiov DU, Raulet DH. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat Rev Immunol. 2023;23:90–105.
Novelli L, Barbati C, Capuano C, Recalchi S, Ceccarelli F, Vomero M, Alessandri C, Morrone S, Conti F. KLRG1 is reduced on NK cells in SLE patients, inversely correlates with disease activity and is modulated by hydroxychloroquine in vitro. Lupus. 2023;32:549–59.
Lasek W, Zagożdżon R, Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother. 2014;63:419–35.
He Y, Bunn PA, Zhou C, Chan D. KIR 2D (L1, L3, L4, S4) and KIR 3DL1 protein expression in non-small cell lung cancer. Oncotarget. 2016;7:82104–11.
Chockley PJ, Chen J, Chen G, Beer DG, Standiford TJ, Keshamouni VG. Epithelial-mesenchymal transition leads to NK cell–mediated metastasis-specific immunosurveillance in lung cancer. J Clin Investig. 2018;128:1384–96.
Exposito F, Redrado M, Houry M, Hastings K, Molero-Abraham M, Lozano T, Solorzano JL, Ortega JS, Andradas V, Amat R, Redin E, Leon S, Legarra N, García J, Serrano D, Valencia K, Robles-Oteiza C, Foggetti G, Felip E, Lasarte JJ, Paz-Ares L, Zugazagoitia J, Politi K, Montuenga LM, Calvo A. Abstract 1124: PTEN loss confers resistance to anti-PD-1 therapy in NSCLC by increasing tumor infiltration of T regulatory cells. Can Res. 2023;83:1124–1124.
Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery J-P, Chouaib S. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11:824–46.
Finetti F, Cassioli C, Baldari CT. Transcellular communication at the immunological synapse: a vesicular traffic-mediated mutual exchange. F1000Res. 2017;6:1880.
Lerner EC, Woroniecka KI, D’Anniballe VM, Wilkinson DS, Mohan AA, Lorrey SJ, Waibl-Polania J, Wachsmuth LP, Miggelbrink AM, Jackson JD, Cui X, Raj JA, Tomaszewski WH, Cook SL, Sampson JH, Patel AP, Khasraw M, Gunn MD, Fecci PE. CD8+ T cells maintain killing of MHC-I-negative tumor cells through the NKG2D–NKG2DL axis. Nat Cancer. 2023.
Mullins RDZ, Pal A, Barrett TF, Heft Neal ME, Puram SV. Epithelial-mesenchymal plasticity in tumor immune evasion. Cancer Res. 2022;82:2329–43.
Terry S, Buart S, Tan TZ, Gros G, Noman MZ, Lorens JB, Mami-Chouaib F, Thiery JP, Chouaib S. Acquisition of tumor cell phenotypic diversity along the EMT spectrum under hypoxic pressure: consequences on susceptibility to cell-mediated cytotoxicity. Oncoimmunology. 2017;6:e1271858.
Akalay I, Tan TZ, Kumar P, Janji B, Mami-Chouaib F, Charpy C, Vielh P, Larsen AK, Thiery JP, Sabbah M, Chouaib S. Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression. Oncogene. 2015;34:2261–71.
Akalay I, Janji B, Hasmim M, Noman MZ, André F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, Sabbah M, Tan TZ, Keira JH, Ying Hung NT, Thiery JP, Mami-Chouaib F, Chouaib S. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell–mediated lysis. Cancer Res. 2013;73:2418–27.
Izdebska M, Zielińska W, Hałas-Wiśniewska M, Grzanka A. Involvement of actin and actin-binding proteins in carcinogenesis. Cells. 2020;9:2245.
Corgnac S, Damei I, Gros G, Caidi A, Terry S, Chouaib S, Deloger M, Mami-Chouaib F. Cancer stem-like cells evade CD8(+)CD103(+) tumor-resident memory T (T(RM)) lymphocytes by initiating an epithelial-to-mesenchymal transition program in a human lung tumor model. J Immunother Cancer. 2022;10.
Abd Hamid M, Colin-York H, Khalid-Alham N, Browne M, Cerundolo L, Chen J-L, Yao X, Rosendo-Machado S, Waugh C, Maldonado-Perez D, Bowes E, Verrill C, Cerundolo V, Conlon CP, Fritzsche M, Peng Y, Dong T. Self-maintaining CD103+ cancer-specific T cells are highly energetic with rapid cytotoxic and effector responses. Cancer Immunol Res. 2020;8:203–16.
Akalay I, Janji B, Hasmim M, Noman MZ, Thiery JP, Mami-Chouaib F, Chouaib S. EMT impairs breast carcinoma cell susceptibility to CTL-mediated lysis through autophagy induction. Autophagy. 2013;9:1104–6.
Jachetti E, Caputo S, Mazzoleni S, Brambillasca CS, Parigi SM, Grioni M, Piras IS, Restuccia U, Calcinotto A, Freschi M, Bachi A, Galli R, Bellone M. Tenascin-C protects cancer stem–like cells from immune surveillance by arresting T-cell activation. Can Res. 2015;75:2095–108.
Klionsky KRPADJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–73.
Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol. 2021;22:733–50.
Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cadwell K, Cecconi F, Choi AMK, Choi ME, Chu CT, Codogno P, Colombo MI, Cuervo AM, Deretic V, Dikic I, Elazar Z, Eskelinen E-L, Fimia GM, Gewirtz DA, Green DR, Hansen M, Jäättelä M, Johansen T, Juhász G, Karantza V, Kraft C, Kroemer G, Ktistakis NT, Kumar S, Lopez-Otin C, Macleod KF, Madeo F, Martinez J, Meléndez A, Mizushima N, Münz C, Penninger JM, Perera RM, Piacentini M, Reggiori F, Rubinsztein DC, Ryan KM, Sadoshima J, Santambrogio L, Scorrano L, Simon H-U, Simon AK, Simonsen A, Stolz A, Tavernarakis N, Tooze SA, Yoshimori T, Yuan J, Yue Z, Zhong Q, Galluzzi L, Pietrocola F. Autophagy in major human diseases. EMBO J. 2021;40:e108863.
Russell RC, Guan K-L. The multifaceted role of autophagy in cancer. EMBO J. 2022;41:e110031.
Amaravadi RK, Kimmelman AC, Debnath J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 2019;9:1167–81.
Young TM, Reyes C, Pasnikowski E, Castanaro C, Wong C, Decker CE, Chiu J, Song H, Wei Y, Bai Y, Zambrowicz B, Thurston G, Daly C. Autophagy protects tumors from T-cell–mediated cytotoxicity via inhibition of TNFα-induced apoptosis. Sci Immunol. 2020;5:eabb9561.
Li Z-L, Zhang H-L, Huang Y, Huang J-H, Sun P, Zhou N-N, Chen Y-H, Mai J, Wang Y, Yu Y, Zhou L-H, Li X, Yang D, Peng X-D, Feng G-K, Tang J, Zhu X-F, Deng R. Autophagy deficiency promotes triple-negative breast cancer resistance to T-cell-mediated cytotoxicity by blocking tenascin-C degradation. Nat Commun. 2020;11:3806.
Lawson KA, Sousa CM, Zhang X, Kim E, Akthar R, Caumanns JJ, Yao Y, Mikolajewicz N, Ross C, Brown KR, Zid AA, Fan ZP, Hui S, Krall JA, Simons DM, Slater CJ, De Jesus V, Tang L, Singh R, Goldford JE, Martin S, Huang Q, Francis EA, Habsid A, Climie R, Tieu D, Wei J, Li R, Tong AHY, Aregger M, Chan KS, Han H, Wang X, Mero P, Brumell JH, Finelli A, Ailles L, Bader G, Smolen GA, Kingsbury GA, Hart T, Kung C, Moffat J. Functional genomic landscape of cancer-intrinsic evasion of killing by T cells. Nature. 2020;586:120–6.
Han LL, Jia L, Wu F, Huang C. Sirtuin6 (SIRT6) promotes the EMT of hepatocellular carcinoma by stimulating autophagic degradation of E-cadherin. Mol Cancer Res. 2019;17:2267–80.
Bao Y, Ding Z, Zhao P, Li J, Chen P, Zheng J, Qian Z. Autophagy inhibition potentiates the anti-EMT effects of alteronol through TGF-β/Smad3 signaling in melanoma cells. Cell Death Dis. 2020;11:223.
Fuhrman CA, Yeh W-I, Seay HR, Saikumar Lakshmi P, Chopra G, Zhang L, Perry DJ, McClymont SA, Yadav M, Lopez M-C, Baker HV, Zhang Y, Li Y, Whitley M, von Schack D, Atkinson MA, Bluestone JA, Brusko TM. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J Immunol. 2015;195:145–55.
Wei T, Zhang J, Qin Y, Wu Y, Zhu L, Lu L, Tang G, Shen Q. Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small cell lung cancer patients. Am J Cancer Res. 2015;5:2190–201.
Derakhshani A, Hashemzadeh S, Asadzadeh Z, Shadbad MA, Rasibonab F, Safarpour H, Jafarlou V, Solimando AG, Racanelli V, Singh PK, Najafi S, Javadrashid D, Brunetti O, Silvestris N, Baradaran B. Cytotoxic T-lymphocyte antigen-4 in colorectal cancer: another therapeutic side of capecitabine. Cancers. 2021;13:2414.
Noman MZ, Van Moer K, Marani V, Gemmill RM, Tranchevent L-C, Azuaje F, Muller A, Chouaib S, Thiery JP, Berchem G, Janji B. CD47 is a direct target of SNAI1 and ZEB1 and its blockade activates the phagocytosis of breast cancer cells undergoing EMT. OncoImmunology. 2018;7:e1345415.
Jiang B, Zhang T, Liu F, Sun Z, Shi H, Hua D, Yang C. The costimulatory molecule B7–H3 promotes the epithelial-mesenchymal transition in colorectal cancer. Oncotarget. 2016;7:31755–71.
Wang Y, Du J, Gao Z, Sun H, Mei M, Wang Y, Ren Y, Zhou X. Evolving landscape of PD-L2: bring new light to checkpoint immunotherapy. Br J Cancer. 2023;128:1196–207.
Raimondi C, Carpino G, Nicolazzo C, Gradilone A, Gianni W, Gelibter A, Gaudio E, Cortesi E, Gazzaniga P. PD-L1 and epithelial-mesenchymal transition in circulating tumor cells from non-small cell lung cancer patients: a molecular shield to evade immune system? OncoImmunology. 2017;6:e1315488.
Nunes-Xavier CE, Angulo JC, Pulido R, López JI. A critical insight into the clinical translation of PD-1/PD-L1 blockade therapy in clear cell renal cell carcinoma. Curr Urol Rep. 2019;20:1.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29.
Hou Y-C, Chao Y-J, Hsieh M-H, Tung H-L, Wang H-C, Shan Y-S. Low CD8+ T-cell infiltration and high PD-L1 expression are associated with level of CD44+/CD133+ cancer stem cells and predict an unfavorable prognosis in pancreatic cancer. Cancers. 2019;11:541.
Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, Reibel JB, Walton Z, Ji H, Watanabe H, Jänne PA, Castrillon DH, Rustgi AK, Bass AJ, Freeman GJ, Padera RF, Dranoff G, Hammerman PS, Kim CF, Wong KK. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25:590–604.
Yang Z, Huo Y, Zhou S, Guo J, Ma X, Li T, Fan C, Wang L. Cancer cell-intrinsic XBP1 drives immunosuppressive reprogramming of intratumoral myeloid cells by promoting cholesterol production. Cell Metab. 2022;34:2018-2035.e2018.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37.
O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T-cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67.
Ye L-Y, Chen W, Bai X-L, Xu X-Y, Zhang Q, Xia X-F, Sun X, Li G-G, Hu Q-D, Fu Q-H, Liang T-B. Hypoxia-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Can Res. 2016;76:818–30.
Hsu DS, Wang HJ, Tai SK, Chou CH, Hsieh CH, Chiu PH, Chen NJ, Yang MH. Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell. 2014;26:534–48.
Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Can Res. 2013;73:662–71.
Taki M, Abiko K, Baba T, Hamanishi J, Yamaguchi K, Murakami R, Yamanoi K, Horikawa N, Hosoe Y, Nakamura E, Sugiyama A, Mandai M, Konishi I, Matsumura N. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat Commun. 2018;9:1685.
Xiang Z, Jiang DP, Xia GG, Wei ZW, Chen W, He Y, Zhang CH. CXCL1 expression is correlated with Snail expression and affects the prognosis of patients with gastric cancer. Oncol Lett. 2015;10:2458–64.
Faget J, Groeneveld S, Boivin G, Sankar M, Zangger N, Garcia M, Guex N, Zlobec I, Steiner L, Piersigilli A, Xenarios I, Meylan E. Neutrophils and snail orchestrate the establishment of a pro-tumor microenvironment in lung cancer. Cell Rep. 2017;21:3190–204.
Yanagawa J, Walser TC, Zhu LX, Hong L, Fishbein MC, Mah V, Chia D, Goodglick L, Elashoff DA, Luo J, Magyar CE, Dohadwala M, Lee JM, St. John MA, Strieter RM, Sharma S, Dubinett SM. Snail promotes CXCR2 ligand dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res. 2009;15:6820–9.
Katsura A, Tamura Y, Hokari S, Harada M, Morikawa M, Sakurai T, Takahashi K, Mizutani A, Nishida J, Yokoyama Y, Morishita Y, Murakami T, Ehata S, Miyazono K, Koinuma D. ZEB1-regulated inflammatory phenotype in breast cancer cells. Mol Oncol. 2017;11:1241–62.
Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, Shu P, Li D, Wang Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6:362.
Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Can Res. 2005;65:3044–8.
Grzywa TM, Sosnowska A, Matryba P, Rydzynska Z, Jasinski M, Nowis D, Golab J. Myeloid cell-derived arginase in cancer immune response. Front Immunol. 2020;11:938.
Zhang J, Zhang Y, Yang Z, Cheng D, Zhang H, Wei L, Liu C, Yan F, Li C, Dong G, Wang C, Shi D, Xiong H. Inducible nitric oxide synthase-expressing myeloid-derived suppressor cells regulated by interleukin 35 contribute to the pathogenesis of psoriasis. Front Immunol. 2023;14:1091541.
Tu E, Chia PZC, Chen W. TGFβ in T-cell biology and tumor immunity: Angel or devil? Cytokine Growth Factor Rev. 2014;25:423–35.
Razavi AS, Mohtashami M, Razi S, Rezaei N. TGF-β signaling and the interaction between platelets and T cells in tumor microenvironment: Foes or friends? Cytokine. 2022;150:155772.
Joffroy CM, Buck MB, Stope MB, Popp SL, Pfizenmaier K, Knabbe C. Antiestrogens induce transforming growth factor β–mediated immunosuppression in breast cancer. Can Res. 2010;70:1314–22.
Wu X, Xiao Y, Guo D, Zhang Z, Liu M. Reduced NK cell cytotoxicity by papillomatosis-derived TGF-β contributing to low-risk HPV persistence in JORRP patients. Front Immunol. 2022;13:849493.
Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, Cui X, Zheng F, Li H, Yao H, Su F, Song E. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–20.
Sangaletti S, Tripodo C, Santangelo A, Castioni N, Portararo P, Gulino A, Botti L, Parenza M, Cappetti B, Orlandi R, Tagliabue E, Chiodoni C, Colombo MP. Mesenchymal transition of high-grade breast carcinomas depends on extracellular matrix control of myeloid suppressor cell activity. Cell Rep. 2016;17:233–48.
Feng Y-L, Chen D-Q, Vaziri ND, Guo Y, Zhao Y-Y. Small molecule inhibitors of epithelial-mesenchymal transition for the treatment of cancer and fibrosis. Med Res Rev. 2020;40:54–78.
Patrik-Iwuanyanwu K, Egbuna C, Onyeike EN, Uche CZ, Ogoke UP, Riaz M, Ibezim EN, Khan J, Adedokun KA, Imodoye SO. Food Sci Nutr. 2023.
Egbuna C, Patrick-Iwuanyanwu KC, Onyeike EN, Khan J, Palai S, Patel SB, Parmar VK, Kushwaha G, Singh O, Jeevanandam J, Kumarasamy S. Phytochemicals and bioactive compounds effective against acute myeloid leukemia: A systematic review. Food Sci Nutr. 2023.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Kuburich NA, Sabapathy T, Demestichas BR, Maddela JJ, den Hollander P, Mani SA. Proactive and reactive roles of TGF-β in cancer. Semin Cancer Biol. 2023;95:120–39.
Trelford CB, Dagnino L, Di Guglielmo GM. Transforming growth factor-β in tumor development. Front Mol Biosci. 2022;9:991612.
Hata A, Chen YG. TGF-β signaling from receptors to smads. Cold Spring Harb Perspect Biol. 2016;8:a022061.
Jonckheere S, Adams J, De Groote D, Campbell K, Berx G, Goossens S. Epithelial-mesenchymal transition (EMT) as a therapeutic target. Cells Tissues Organs. 2021;211:157–82.
Turati M, Mousset A, Issa N, Turtoi A, Ronca R. TGF-β mediated drug resistance in solid cancer. Cytokine Growth Factor Rev. 2023;71–72:54–65.
Yingling JM, McMillen WT, Yan L, Huang H, Sawyer JS, Graff J, Clawson DK, Britt KS, Anderson BD, Beight DW, Desaiah D, Lahn MM, Benhadji KA, Lallena MJ, Holmgaard RB, Xu X, Zhang F, Manro JR, Iversen PW, Iyer CV, Brekken RA, Kalos MD, Driscoll KE. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-β receptor type I inhibitor. Oncotarget. 2018;9:6659–77.
Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, Inigo I, Dobkin J, Manro JR, Iversen PW, Surguladze D, Hall GE, Novosiadly RD, Benhadji KA, Plowman GD, Kalos M, Driscoll KE. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes antitumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer. 2018;6:47.
Son JY, Park S-Y, Kim S-J, Lee SJ, Park S-A, Kim M-J, Kim SW, Kim D-K, Nam J-S, Sheen YY. EW-7197, a Novel ALK-5 kinase inhibitor, potently inhibits breast to lung metastasis. Mol Cancer Ther. 2014;13:1704–16.
Hamilton DH, Griner LM, Keller JM, Hu X, Southall N, Marugan J, David JM, Ferrer M, Palena C. Targeting estrogen receptor signaling with fulvestrant enhances immune and chemotherapy-mediated cytotoxicity of human lung cancer. Clin Cancer Res. 2016;22:6204–16.
Basu S, Cheriyamundath S, Ben-Ze’ev A. Cell-cell adhesion: linking Wnt/β-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Res. 2018;7:100.
Hu W, Wang Z, Zhang S, Lu X, Wu J, Yu K, Ji A, Lu W, Wang Z, Wu J, Jiang C. IQGAP1 promotes pancreatic cancer progression and epithelial-mesenchymal transition (EMT) through Wnt/β-catenin signaling. Sci Rep. 2019;9:7539.
Yang W, Li Y, Gao R, Xiu Z, Sun T. MHC class I dysfunction of glioma stem cells escapes from CTL-mediated immune response via activation of Wnt/β-catenin signaling pathway. Oncogene. 2020;39:1098–111.
Yang S, Liu Y, Li M-Y, Ng CSH, Yang S-L, Wang S, Zou C, Dong Y, Du J, Long X, Liu L-Z, Wan IYP, Mok T, Underwood MJ, Chen GG. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. 2017;16:124.
Sim WJ, Iyengar PV, Lama D, Lui SKL, Ng HC, Haviv-Shapira L, Domany E, Kappei D, Tan TZ, Saei A, Jaynes PW, Verma CS, Kumar AP, Rouanne M, Ha HK, Radulescu C, ten Dijke P, Eichhorn PJA, Thiery JP. c-Met activation leads to the establishment of a TGFβ-receptor regulatory network in bladder cancer progression. Nat Commun. 2019;10:4349.
Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-β targeted cancer therapy. Int J Biol Sci. 2012;8:964–78.
Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, Brunton VG, Pilarsky C, Winkler TH, Brabletz S, Stemmler MP, Brabletz T. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19:518–29.
Addison JB, Voronkova MA, Fugett JH, Lin C-C, Linville NC, Trinh B, Livengood RH, Smolkin MB, Schaller MD, Ruppert JM, Pugacheva EN, Creighton CJ, Ivanov AV. Functional hierarchy and cooperation of EMT master transcription factors in breast cancer metastasis. Mol Cancer Res. 2021;19:784–98.
Lin Y, Wu Y, Li J, Dong C, Ye X, Chi Y-I, Evers BM, Zhou BP. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J. 2010;29:1803–16.
Egolf S, Aubert Y, Doepner M, Anderson A, Maldonado-Lopez A, Pacella G, Lee J, Ko EK, Zou J, Lan Y, Simpson CL, Ridky T, Capell BC. LSD1 inhibition promotes epithelial differentiation through derepression of fate-determining transcription factors. Cell Rep. 2019;28:1981-1992.e1987.
Nagasawa S, Sedukhina AS, Nakagawa Y, Maeda I, Kubota M, Ohnuma S, Tsugawa K, Ohta T, Roche-Molina M, Bernal JA, Narváez AJ, Jeyasekharan AD, Sato K. LSD1 overexpression is associated with poor prognosis in basal-like breast cancer, and sensitivity to PARP inhibition. PLoS ONE. 2015;10:e0118002.
Fang Y, Liao G, Yu B. LSD1/KDM1A inhibitors in clinical trials: advances and prospects. J Hematol Oncol. 2019;12:129.
Ferrari-Amorotti G, Fragliasso V, Esteki R, Prudente Z, Soliera AR, Cattelani S, Manzotti G, Grisendi G, Dominici M, Pieraccioli M, Raschellà G, Chiodoni C, Colombo MP, Calabretta B. Inhibiting interactions of lysine demethylase LSD1 with snail/slug blocks cancer cell invasion. Can Res. 2013;73:235–45.
Wang M, Liu X, Guo J, Weng X, Jiang G, Wang Z, He L. Inhibition of LSD1 by Pargyline inhibited process of EMT and delayed progression of prostate cancer in vivo. Biochem Biophys Res Commun. 2015;467:310–5.
Pantelaiou-Prokaki G, Mieczkowska I, Schmidt GE, Fritzsche S, Prokakis E, Gallwas J, Wegwitz F. HDAC8 suppresses the epithelial phenotype and promotes EMT in chemotherapy-treated basal-like breast cancer. Clin Epigenet. 2022;14:7.
Li Y, Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med. 2016;6.
Ji M, Lee EJ, Kim KB, Kim Y, Sung R, Lee S-J, Kim DS, Park SM. HDAC inhibitors induce epithelial-mesenchymal transition in colon carcinoma cells. Oncol Rep. 2015;33:2299–308.
Duan Y-C, Ma Y-C, Qin W-P, Ding L-N, Zheng Y-C, Zhu Y-L, Zhai X-Y, Yang J, Ma C-Y, Guan Y-Y. Design and synthesis of tranylcypromine derivatives as novel LSD1/HDACs dual inhibitors for cancer treatment. Eur J Med Chem. 2017;140:392–402.
Xu Y, Guo B, Liu X, Tao K. miR-34a inhibits melanoma growth by targeting ZEB1. Aging (Albany NY). 2021;13:15538–47.
Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y, Araujo L, Carbone DP, Shilo K, Giri DK, Kelnar K, Martin D, Komaki R, Gomez DR, Krishnan S, Calin GA, Bader AG, Welsh JW. PDL1 regulation by p53 via miR-34. JNCI J Natl Cancer Inst. 2015;108:djv303.
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu C-C, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–30.
Salt MB, Bandyopadhyay S, McCormick F. Epithelial-to-mesenchymal transition rewires the molecular path to PI3K-dependent proliferation. Cancer Discov. 2014;4:186–99.
Pasquier J, Abu-Kaoud N, Al Thani H, Rafii A. Epithelial to mesenchymal transition in a clinical perspective. J Oncol. 2015; 792182.
Pattabiraman DR, Bierie B, Kober KI, Thiru P, Krall JA, Zill C, Reinhardt F, Tam WL, Weinberg RA. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science. 2016;351:aad3680.
Verma A, Mathur R, Farooque A, Kaul V, Gupta S, Dwarakanath BS. T-Regulatory cells in tumor progression and therapy. Cancer Manag Res. 2019;11:10731–47.
Yang Y, Li C, Liu T, Dai X, Bazhin AV. Myeloid-derived suppressor cells in tumors: from mechanisms to antigen specificity and microenvironmental regulation. Front Immunol. 2020;11:1371.
De Cicco P, Ercolano G, Ianaro A. The new era of cancer immunotherapy: targeting myeloid-derived suppressor cells to overcome immune evasion. Front Immunol. 2020;11:550783.
Gu Y, Zhang Z, ten Dijke P. Harnessing epithelial-mesenchymal plasticity to boost cancer immunotherapy. Cell Mol Immunol. 2023;20:318–40.
Tang T, Huang X, Zhang G, Hong Z, Bai X, Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. 2021;6:72.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18.
Yumimoto K, Sugiyama S, Mimori K, Nakayama KI. Potentials of C-C motif chemokine 2-C–C chemokine receptor type 2 blockers including propagermanium as anticancer agents. Cancer Sci. 2019;110:2090–9.
Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6:a021857.
Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massagué J. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–78.
Lüönd F, Sugiyama N, Bill R, Bornes L, Hager C, Tang F, Santacroce N, Beisel C, Ivanek R, Bürglin T, Tiede S, van Rheenen J, Christofori G. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev Cell. 2021;56:3203-3221.e3211.
Brown MS, Abdollahi B, Wilkins OM, Lu H, Chakraborty P, Ognjenovic NB, Muller KE, Jolly MK, Christensen BC, Hassanpour S, Pattabiraman DR. Phenotypic heterogeneity driven by plasticity of the intermediate EMT state governs disease progression and metastasis in breast cancer. Sci Adv. 2022;8:eabj8002.
Ran X, Xiao J, Zhang Y, Teng H, Cheng F, Chen H, Zhang K, Sun Z. Low intratumor heterogeneity correlates with increased response to PD-1 blockade in renal cell carcinoma. Therap Adv Med Oncol. 2020;12:1758835920977117.
Nguyen PHD, Ma S, Phua CZJ, Kaya NA, Lai HLH, Lim CJ, Lim JQ, Wasser M, Lai L, Tam WL, Lim TKH, Wan WK, Loh T, Leow WQ, Pang YH, Chan CY, Lee SY, Cheow PC, Toh HC, Ginhoux F, Iyer S, Kow AWC, Young Dan Y, Chung A, Bonney GK, Goh BKP, Albani S, Chow PKH, Zhai W, Chew V. Intratumoural immune heterogeneity as a hallmark of tumor evolution and progression in hepatocellular carcinoma. Nat Commun. 2021;12:227.
Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, Lund T, Tanić M, Reading JL, Joshi K, Henry JY, Ghorani E, Wilson GA, Birkbak NJ, Jamal-Hanjani M, Veeriah S, Szallasi Z, Loi S, Hellmann MD, Feber A, Chain B, Herrero J, Quezada SA, Demeulemeester J, Van Loo P, Beck S, McGranahan N, Swanton C, Swanton C, Jamal-Hanjani M, Veeriah S, Czyzewska-Khan J, Johnson D, Laycock J, Rosenthal R, Gorman P, Hynds RE, Wilson G, Birkbak NJ, Watkins TBK, McGranahan N, Escudero M, Stewart A, Van Loo P, Rowan A, Hiley C, Abbosh C, Goldman J, Stone RK, Denner T, Ward S, Nye E, Joshi K, Ben Aissa A, Wong YNS, Georgiou A, Quezada S, Hartley JA, Lowe HL, Herrero J, Lawrence D, Hayward M, Panagiotopoulos N, Falzon M, Borg E, Marafioti T, Janes SM, Forster M, Ahmad T, Lee SM, Papadatos-Pastos D, Carnell D, Mendes R, George J, Ahmed A, Taylor M, Choudhary J, Summers Y, Califano R, Taylor P, Shah R, Krysiak P, Rammohan K, Fontaine E, Booton R, Evison M, Crosbie P, Moss S, Joseph L, Bishop P, Quinn AM, Doran H, Leek A, Harrison P, Moore K, Waddington R, Novasio J, Blackhall F, Rogan J, Smith E, Dive C, Tugwood J, Brady G, Rothwell DG, Pierce J, Gulati S, Naidu B, Langman G, Trotter S, Bancroft H, Kerr A, Kadiri S, Middleton G, Djearaman M, Fennell D, Shaw JA, Le Quesne J, Moore DA, Nakas A, Rathinam S, Monteiro W, Marshall H, Nelson L, Riley J, Primrose L, Martinson L, Anand G, Khan S, Nicolson M, Kerr K, Palmer S, Remmen H, Miller J, Buchan K, Chetty M, Gomersall L, Lester J, Morgan F, Adams H, Davies H, Kornaszewska M, Attanoos R, Lock S, MacKenzie M, Wilcox M, Bell H, Hackshaw A, Ngai Y, Smith S, Gower N, Ottensmeier C, Chee S, Johnson B, Alzetani A, Shaw E, Lim E, De Sousa P, Barbosa MT, Bowman A, Jordan S, Rice A, Raubenheimer H, Bhayani H, Hamilton M, Mensah N, Ambrose L, Devaraj A, Chavan H, Nicholson AG, Lau K, Sheaff M, Schmid P, Conibear J, Ezhil V, Prakash V, Russell P, Light T, Horey T, Danson S, Bury J, Edwards J, Hill J, Matthews S, Kitsanta Y, Suvarna K, Fisher P, Shackcloth M, Gosney J, Feeney S, Asante-Siaw J, Ryanna K, Dawson A, Tuffail M, Bajaj A, Brozik J, Walter H, Carey N, Price G, Gilbert K, Webb J, Patel A, Chaturvedi A, Granato F, Baker K, Carter M, Priest L, Krebs MG, Lindsay C, Gomes F, Chemie F, George R, Patrini D, Khiroya R, Shaw P, Skrzypski M, Sunderland MW, Reading JL, Beastall C, Mangal N, Peggs K, Lim E, Al-Bakir M, Navani N, Scarci M, Ensell L, Biswas D, Salgado R, Razaq M, Nicod J, Beck S, Lopez S, Huebner A, Dietzen M, Mourikis T, Adefila-Ideozu T, Begum S, Klein H, Mani A, Carvalho S, Kaniu D, Realingo C, Malima M, Booth S, Lim L, Rao J, Tenconi S, Socci L, Kibutu F, Agyemang M, Young R, Blyth KG, Dick C, Kirk A, Kidd A. The, Neoantigen-directed immune escape in lung cancer evolution. Nature. 2019;567:479–85.
Acknowledgements
All figures were created with Biorender.com.
Funding
This research did not receive any specific support or funding.
Author information
Authors and Affiliations
Contributions
SIO conceived the project and designed the figures. SOI and KAA wrote and edited the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Imodoye, S.O., Adedokun, K.A. EMT-induced immune evasion: connecting the dots from mechanisms to therapy. Clin Exp Med 23, 4265–4287 (2023). https://doi.org/10.1007/s10238-023-01229-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01229-4