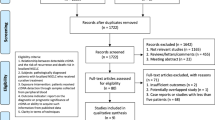
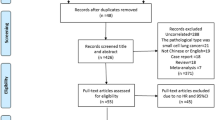
Abstract
A high level of circulating tumor DNA (ctDNA) has been linked to poor survival in patients with certain solid tumors. In spite of this, it is still unclear whether ctDNA is associated with poor survival in small cell lung cancer (SCLC). To investigate the above association, we conducted a systematic review and meta-analysis. PubMed, Web of Science, Cochrane’s Library, and Embase were searched for relevant cohort studies from the inception of the databases to November 28, 2022. Data collection, literature search, and statistical analysis were carried out independently by two authors. To account for heterogeneity, we used a random-effects model. In this meta-analysis, 391 patients with SCLC were identified, and the data were pooled from nine observational studies and followed for 11.4 to 25.0 months. A high ctDNA was associated with worse overall survival (OS, risk ratio [RR] 2.50, 95% confidence interval [CI]1.85 to 3.38, p < 0.001; I2 = 25%) and progression-free survival (PFS, RR 2.33, 95% CI 1.48 to 3.64, p < 0.001, I2 = 42%). Subgroup analyses retrieved consistent results in prospective and retrospective studies, in studies with ctDNA measured with polymerase chain reaction or next-generation sequencing, and in studies analyzed with univariate or multivariate regression models. Studies suggest that ctDNA may be an important factor in predicting poor OS and PFS in SCLC patients.





Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Abbreviations
- SCLC:
-
Small cell lung cancer
- NSCLC:
-
Non-small cell lung cancer
- ctDNA:
-
Circulating tumor DNA
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- NOS:
-
Newcastle–Ottawa Scale
- RRs:
-
Risk ratios
- Cis:
-
Confidence intervals
- SEs:
-
Standard errors
- PCR:
-
Polymerase chain reaction
- NGS:
-
Next-generation sequencing
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Wood DE, Kazerooni EA, Aberle D, et al. NCCN guidelines(R) insights: lung cancer screening, version 1.2022. J Natl Compr Canc Netw. 2022;20:754–64.
Ganti AKP, Loo BW, Bassetti M, et al. Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:1441–64.
Zugazagoitia J, Paz-Ares L. Extensive-stage small-cell lung cancer: first-line and second-line treatment options. J Clin Oncol. 2022;40:671–80.
Wang Y, Zou S, Zhao Z, Liu P, Ke C, Xu S. New insights into small-cell lung cancer development and therapy. Cell Biol Int. 2020;44:1564–76.
Sanchez-Herrero E, Serna-Blasco R, Robado de Lope L, Gonzalez-Rumayor V, Romero A, Provencio M. Circulating tumor DNA as a cancer biomarker: an overview of biological features and factors that may impact on ctDNA analysis. Front Oncol. 2022;12:943253.
Stadler JC, Belloum Y, Deitert B, et al. Current and future clinical applications of ctDNA in immuno-oncology. Cancer Res. 2022;82:349–58.
Pessoa LS, Heringer M, Ferrer VP. ctDNA as a cancer biomarker: a broad overview. Crit Rev Oncol Hematol. 2020;155:103109.
Guo RQ, Peng JZ, Sun J, Li YM. Clinical significance of circulating tumor DNA in localized non-small cell lung cancer: a systematic review and meta-analysis. Clin Exp Med. 2022;31:1–1.
Mi J, Han X, Wang R, Ma R, Zhao D. Circulation tumour DNA in predicting recurrence and prognosis in operable colorectal cancer patients: a meta-analysis. Eur J Clin Invest. 2022;52:e13842.
Liu H, Yang H, Chen X. Prognostic value of circulating tumour DNA in asian patients with hepatocellular carcinoma: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2022;2022:8019652.
Bunduc S, Gede N, Vancsa S, et al. Prognostic role of cell-free DNA biomarkers in pancreatic adenocarcinoma: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2022;169:103548.
Lu Y, Li L. The prognostic value of circulating tumor DNA in ovarian cancer: a meta-analysis. Technol Cancer Res Treat. 2021;20:15330338211043784.
Pizzutilo EG, Pedrani M, Amatu A, et al. Liquid biopsy for small cell lung cancer either de novo or transformed: systematic review of different applications and meta-analysis. Cancers. 2021;13:2265.
Mondelo-Macia P, Garcia-Gonzalez J, Leon-Mateos L, et al. Current status and future perspectives of liquid biopsy in small cell lung cancer. Biomedicines. 2021;9:48.
Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.2. The Cochrane Collaboration. 2021;www.training.cochrane.org/handbook.
Wells GA, Shea B, O’Connell D, et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010;http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–57.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Gonzalez R, Silva JM, Sanchez A, et al. Microsatellite alterations and TP53 mutations in plasma DNA of small-cell lung cancer patients: follow-up study and prognostic significance. Ann Oncol. 2000;11:1097–104.
Almodovar K, Iams WT, Meador CB, et al. Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J Thorac Oncol. 2018;13:112–23.
Du M, Thompson J, Fisher H, Zhang P, Huang CC, Wang L. Genomic alterations of plasma cell-free DNAs in small cell lung cancer and their clinical relevance. Lung Cancer. 2018;120:113–21.
Nong J, Gong Y, Guan Y, et al. Circulating tumor DNA analysis depicts subclonal architecture and genomic evolution of small cell lung cancer. Nat Commun. 2018;9:3114.
Herbreteau G, Langlais A, Greillier L, et al. Circulating tumor DNA as a prognostic determinant in small cell lung cancer patients receiving Atezolizumab. J Clin Med. 2020;9:3861.
Iams WT, Kopparapu PR, Yan Y, et al. Blood-based surveillance monitoring of circulating tumor DNA from patients with SCLC detects disease relapse and predicts death in patients with limited-stage disease. JTO Clin Res Rep. 2020;1:100024.
Jin Y, Chen YM, Hu X, et al. [Analysis of the feasibility and prognostic value of circulating tumor DNA in detecting gene mutations in small cell lung cancer]. Zhonghua Yi Xue Za Zhi. 2020;100:3614–21.
Mohan S, Foy V, Ayub M, et al. Profiling of circulating free DNA using targeted and genome-wide sequencing in patients with SCLC. J Thorac Oncol. 2020;15:216–30.
Mondelo-Macia P, Garcia-Gonzalez J, Abalo A, et al. Plasma cell-free DNA and circulating tumor cells as prognostic biomarkers in small cell lung cancer patients. Transl Lung Cancer Res. 2022;11:1995–2009.
Fernandez-Cuesta L, Perdomo S, Avogbe PH, et al. Identification of circulating tumor DNA for the early detection of small-cell lung cancer. EBioMedicine. 2016;10:117–23.
Thomas A, Vilimas R, Trindade C, et al. Durvalumab in combination with olaparib in patients with relapsed SCLC: results from a phase II study. J Thorac Oncol. 2019;14:1447–57.
Carter L, Rothwell DG, Mesquita B, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med. 2017;23:114–9.
Zhang J, Tian C, Lv F, et al. Molecular analysis of cell-free DNA identifies distinct molecular features in patients with chemosensitive and chemorefractory small cell lung cancer. Cancer Commun. 2019;39:20.
Gilson P. Enrichment and analysis of ctDNA. Recent Results Cancer Res. 2020;215:181–211.
Acknowledgements
None
Funding
None.
Author information
Authors and Affiliations
Contributions
JL contributed to conceptualization; data curation; formal analysis; and writing-original draft. LW contributed to data curation and formal analysis. ZD contributed to validation; visualization; writing-original draft. QS contributed to data curation and formal analysis. ZW contributed to methodology; supervision; and writing-review and editing. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Institutional Review Board approval was not required because this is a meta-analysis.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10238_2023_1052_MOESM1_ESM.pdf
Fig. S1 Sensitivity analysis for the association between ctDNA and PFS of SCLC limited to studies with ctDNA measured with NGS (PDF 152 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Wang, L., Dong, Z. et al. A meta-analysis of circulating tumor DNA as a survival indicator in small cell lung cancer patients. Clin Exp Med 23, 3935–3945 (2023). https://doi.org/10.1007/s10238-023-01052-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01052-x