Abstract
Breast cancer (BC) is the leading cause of cancer deaths in women. One of the reasons for the failure of BC treatment is reportedly the ineffectiveness of chemotherapeutic drugs against breast cancer stem-like cells (BCSCs). HER2 receptors have an important role in the self-renewal of BCSCs. Matrix metalloproteinase (MMP) and cytokine levels were found to be higher in BCSCs, which demonstrates their potential metastatic capacity. Therefore, the aim of this study was to evaluate the response of BCSCs to trastuzumab and to investigate the MMP levels in primary breast cancer cells and HER2+ BCSCs. Tumour tissue samples were obtained during surgical intervention from ten breast cancer patients, and primary culture cells were established from these tissues. Four major molecular subgroups were sorted from the primary culture: HER2+ BCSCs (CD44+CD24−HER2+), HER2− BCSCs (CD44+CD24−HER2−), HER2− primary culture cells (CD44+CD24+HER2−) and triple positive primary culture cells (CD44+CD24+HER2+). These cells were cultured and treated with trastuzumab, paclitaxel, carboplatin, and the combination of those three drugs for 96 h. Cellular responses to these drugs were determined by XTT cytotoxicity test. MMPs and cytokine array analysis showed that MMPs and TIMP-1, TIMP-2 proteins were expressed more in HER2+ BCSCs than in primary culture. HER2− BCSCs were more resistant to drugs than HER2+ BCSCs. Our findings suggest that the presence of HER2− BCSCs may be responsible for primary trastuzumab resistance in HER2+ BC cell population. Further studies investigating the function of MMPs are needed for drug targeting of BCSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the leading cause of deaths in women worldwide. Over the last decade, there has been a significant reduction in BC mortality, which is associated with early diagnosis and the use of effective adjuvant therapies. However, the ineffectiveness of chemotherapeutic drugs against breast cancer stem-like cells (BCSCs) has been indicated as one of the reasons for the failure of BC treatments [1]. Therefore, there is a need for new strategies in the treatment for breast cancer [2].
BCSCs were first identified by their expression of CD44+ and lack of CD24 (CD24−) cell surface markers [3]. Mesenchymal-like BCSCs characterised as CD44+CD24− are primarily quiescent and localised at the tumour invasive front, whereas epithelial-like BCSCs express aldehyde dehydrogenase (ALDH), are proliferative, and are located more centrally [4]. The plasticity of BCSCs that allows them to transition between mesenchymal-like and epithelial-like states may play a critical role in metastasis [4]. HER2 is an important regulator of BCSCs self-renewal in both HER2+ [5] and luminal [6] breast cancer.
Trastuzumab, an anti-HER2 antibody, targets the extracellular domain of the HER2 receptor and blocks the homodimerisation of the receptor in HER2-positive BC patients [7]. Paclitaxel binds to microtubules, inhibits mitotic division in tumour cells, and prevents the proliferation of tumour cells [8]. Carboplatin is an alkylating agent. These agents have important toxic effects during all phases of the cell cycle, inhibiting cell division, causing DNA strand damage such as abnormal base pairing, and eventually causing cell death [9].
Conventional chemotherapy has impressively controlled tumour growth; however, over time, many patients develop resistance [10]. Two hypotheses have been proposed to explain this resistance: (1) all the cancer cells become resistant to chemotherapy over time, which was called multidrug resistance [11], and (2) intrinsic resistance to chemotherapy is present at the beginning of tumorigenesis [12]. According to the second hypothesis, chemically sensitive cells are eliminated, but chemotherapy-resistant cells remain and increase after chemotherapy. When intrinsically resistant cells were examined by flow cytometry, it was found that they exhibited CD44 expression but not CD24 expression [13]. The most challenging obstacle encountered by patients with HER2-expressing tumours is overcoming drug resistance in these tumours. The BCSCs marker, CD44, is upregulated in trastuzumab-resistant BC cells [14]. Studies have shown the importance of HER2 receptors in the regulation of BC stem cells [15]. In one study, a tyrosine kinase inhibitor for the HER2 receptor was shown to be effective in primary BC cell cultures [12]. The epidermal growth factor receptor (EGFR) signalling pathway is required for cell proliferation [16]. HER2 receptors and cytokines (IL-6 and IL-8) have also been shown to be responsible for the regulation of cancer stem cell proliferation [5]. Interestingly, HER2 and IL-6 gene polymorphisms are not associated with clinicopathological characteristics in HER2-positive breast cancer [17].
Matrix metalloproteinases (MMPs) are the enzyme group in charge of the destruction of extracellular matrix proteins during organogenesis, normal tissue regeneration, and growth. The activities of MMPs are important for cell processes and many essential functional events, including tissue remodelling, such as angiogenesis, bone progress, mammary involution, and wound healing [18]. Also, the importance of the functions of MMPs is due to their role in chronic inflammatory diseases or cancer [19]. MMPs and cytokine levels were found to be higher in BCSCs, which demonstrates their potential metastatic capacity. The secretion of MMPs plays a role in the degradation of extracellular matrix (ECM) during cancer metastasis. MMPs have proteinase activities in tumour tissues and extracellular matrix elements, especially epithelial basal lamina elements. MMPs also affect cell–matrix and cell–cell connections for the movement of cells along the extracellular matrix [20, 21]. The gelatinase class of MMPs forms MMP-2 and MMP-9, which are very effective in cancer invasion [22, 23]. MMP-9 has a role in tumour invasion and angiogenesis and mediates the tumour microenvironment and metastasis [24, 25]. Therefore, in this study, we assessed the levels of MMPs in BCSC-like cells.
The main aim of the study was to determine the response of BCSCs to trastuzumab and to investigate the levels of extracellular MMPs in the primary BC cell population and subgroups of BCSCs.
Materials and methods
Establishment of primary cultures from BC tissues
Patients (n = 10) who were admitted to Necmettin Erbakan University Meram Medical Faculty due to breast cancer were informed, and the tumour samples were obtained during surgical intervention by a pathologist. Informed consent was obtained from these patients before surgery. The project was carried out upon ethical approval by Ethical Committee of Meram Medical Faculty (2013/391). Primary breast cancer cells were established from the obtained tissues. The tissue sample was transferred to the laboratory in cold DMEM-F12 (Biochrom) medium supplemented with antibiotics (50–100 U/ml pen./50–100 µg/ml strep) and antimycotic agent (Amphotericin B 0.25–2.5 µg/ml). Tissue sample was minced and incubated at 37 °C shaker in DMEM-F12 supplemented with collagenase (300 U/mL) and antibiotics for 18 h. Cells were filtered through a 30-μm strainer after incubation and cultured in complete medium supplemented with mammary epithelium growth supplement (MEGS, Life Technologies) to enrich the epithelial cell population. The primary culture was established as the cells attached to the culture flasks within one week. The cells were sorted and subpopulations were cultured from one of the patients whose tumour tissue was confirmed by immunohistochemistry (IHC) to be HER-2 receptor positive (+ 3 level) and oestrogen and progesterone receptor negative. These cells were successfully cultured for further analyses (Fig. 1). Cytotoxicity tests, drug combination assays, and protein array analyses were conducted using this primary culture.
Flow diagram of the study protocol and gating strategy of CD44+CD24− and CD44+CD24+ cell populations sorting from primary breast culture cells. a The viable cells were gated as a 7-AAD-negative population. b Breast cancer (BC) cells were characterised using flow cytometry based on the CD44/CD24 expression. The CD44+CD24− and CD44+CD24+ cell populations were determined and sorted into different tubes. c HER2 expressions in CD44+CD24− cells, d CD44+CD24+ cells were analysed
Isolation of BCSCs from primary culture
Primary culture cells were trypsinised, counted, and washed in PBS before sorting. The cultured cells were then treated with anti-fluorescein isothiocyanate (FITC)-CD44, anti-phycoerythrin (PE)-CD24, anti-allophycocyanin (APC)-HER2, and 7-AAD antibodies (BD, San Diego, USA) at room temperature for 20 min. 7-AAD was used to evaluate the live cell population. The ALDEFLUOR kit (Stem Cell Technologies) was used to identify the ALDH+ population according to the kit instruction manual. Trypsinised and washed cells were resuspended in ALDEFLUOR assay buffer containing ALDH substrate and then incubated for 40 min at 37 °C. An aliquot of cells treated with 15 mmol/L diethylaminobenzaldehyde (DEAB, a specific ALDH inhibitor) was used as an ALDH negative control.
Flow cytometry analysis was performed using a multiparametric cell sorter (BD FACS Aria III) with FACS Diva version 6.1.3 software. First, viable cells were gated as 7-AAD-negative populations in flow cytometry analysis. Subsequently, CD44+CD24− BCSCs, CD44+CD24+ BC cell populations, and the HER2 receptor expression profiles of these populations were identified (Fig. 1). Also, the ALDH profile of the primary culture cells was determined. The BC cell subpopulations were sorted and cultured in complete medium for further analyses (Fig. 2).
Cytotoxicity tests and drug combination assays
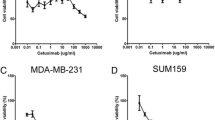
Four subgroup populations (CD44+CD24−HER2+, CD44+CD24−HER2−, CD44+CD24+HER2+, and CD44+CD24+HER2−) were treated with trastuzumab, paclitaxel, carboplatin, and with the combination of these drugs for 96 h. Responses of the cells to the chemotherapy were determined. The effects of the chemotherapeutic agents on the proliferation of the cells were tested in 96-well microtiter plates by XTT cell proliferation assay. The responses of cell proliferation to drug inhibition and IC50 values were designated for each subpopulation [26, 27].
A checkerboard microplate procedure was performed to study the drug interactions. The dilutions of anticancer drug (Drug-1) were prepared in a horizontal direction and the dilutions of other drug (Drug-2) vertically in a microplate. The cells were seeded in each well (1 × 104 cells). The cells were incubated for 96 h at 37 °C in a CO2 incubator. The cell growth was specified after XTT staining, and the optical density of the orange product was measured on an ELISA reader. Fractional inhibitory concentration (FIC) was calculated by the following formulas:
Fractional inhibition index demonstrates the effect of combination of drugs (FIX = FICdrug-1 + FICdrug-2). If the FIX value is in the 0.51–1.00 range, the effect of the drug combination is additive on the cells. When the FIX value is less than 0.50, the effect is synergistic. A FIX value between 1.00 and 2.00 is considered to indicate an indifferent effect, whereas values greater than 2.00 indicate antagonism.
Analysis of expression levels of MMPs and cytokines by protein array
The expression levels of MMP-related proteins and cytokines were determined in the total primary breast cancer cell population and CD44+CD24−HER2+ (HER2+ BCSCs) subpopulation. Total cell lysate was extracted from cells using RIPA lysis buffer (Thermo Scientific), and human antibody array (Abcam) analysis was performed for ten human MMP-related proteins and 23 human cytokines in compliance with the manufacturer’s instructions. Chemiluminescent protein spots were analysed using the Vilber Lourmat Gel Documentation System. The target proteins of the MMP panel were MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-13, TIMP-1, TIMP-2, and TIMP-4. The target proteins of the cytokine panel were G-CSF, GM-CSF, GRO, GRO-alpha, IL-1alpha, IL-2, IL-3, IL-5, IL-6, IL-7, IL-8, IL-10, IL-13, IL-15, IFN-gamma, MCP-1, MCP-2, MCP-3, MIG, RANTES, TGF-beta1, TNF-alpha, and TNF-beta. Both array membranes consisted of positive control spots for densitometric normalisations and negative controls. Each protein was represented by two spots on the membranes.
Statistical analysis
SPSS for Windows 13.0 version (SPSS Inc., Illinois, USA) was utilised for statistical analysis. A two-tailed t test was used to compare the results between groups (p < 0.05). In all analyses, p < 0.05 was considered statistically significant. Based on the dose groups of trastuzumab, the IC50 value of primary culture cells was calculated as 1.79 µM (R2 = 0.99), p < 0.05. The IC50 value at the 96th hour was 10 µM for paclitaxel, 1 μM for trastuzumab, and 0.3 μM for carboplatin (p < 0.05; p < 0.001).
Results
In the primary culture, 42.7% of the cells were BCSCs (CD44+CD24−) and HER2 expression was found to be positive in 75.9% of the cells. The percentage of CD44+CD24+ BC cell population was 49.1% in the primary culture cell populations, and the HER2 expression of this population was 89.7% (Fig. 1).
Another marker for the detection and isolation of cancer stem cells is ALDH activity. Flow cytometric analysis results show that primary culture cell and HER2+ BCSCs populations included 22.3% and 6.9% ALDH positive cells, respectively (Fig. 3).
Evaluation of the effect of chemotherapy on BCSC populations
The antiproliferative effects of the chemotherapeutic drugs on BCSC populations are presented in Table 1. The BCSCs responded to chemotherapy in a manner different from the primary culture. The primary culture cells were completely destroyed by 100 μM paclitaxel and 10 μM trastuzumab administered singly. Proliferation and IC50 values of trastuzumab, paclitaxel, and carboplatin primary culture cell showed that trastuzumab was the most effective drug for these cells. We combined two drugs at doses that inhibited 50% of that cell population, which were 1 μM for trastuzumab (p < 0.05), 0.3 μM for carboplatin (p < 0.05), and 10 μM for paclitaxel (p < 0.05). Interestingly, paclitaxel and trastuzumab combinations showed antagonistic effects on the cells (Table 1). A combination of trastuzumab, paclitaxel, and carboplatin exerted more antiproliferative effects on the BCSCs. HER2− BCSCs were observed to be more resistant to these drugs than HER2+ BCSCs (p < 0.05). Trastuzumab was the most effective drug on the BCSCs, compared to the primary culture cells and CD44+CD24+HER2+ cells. By contrast, there was also a visible reduction in CD44+CD24−HER2+ BCSCs with trastuzumab (p < 0.05). We found that trastuzumab-–carboplatin binary combination treatment was more effective in subcultures obtained from CD44+CD24+HER2+ cells.
Expression profiles of MMPs and cytokines in HER2+ BCSCs
Protein array results revealed that MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-13, TIMP-1, and TIMP-2 proteins were expressed in HER2+ BCSCs about 1.25–2.42 fold more than in primary culture cell (p < 0.05), (Table 2, Fig. 4). Except for G-CSF, IL-6, IL-10, MCP-1, and TGF beta-1, other analysed cytokines were also expressed more in HER2-positive BCSCs than in primary culture cells (p < 0.05) (Fig. 4).
Human MMP and cytokine arrays of primary breast cancer cells (PBCC) and HER2+ BCSCs. a Human MMP protein array of established PCC. b Human MMP protein array of CD44+CD24−HER2+ BCSCs population. c Human cytokine protein array of established PBCC. d Human cytokine protein array of CD44+CD24−HER2+ BCSCs population. e Expression levels of MMPs in CD44+CD24−HER2+ cells with respect to PBCC population. f Expression levels of cytokines in CD44+CD24−HER2+ cells with respect to the PBCC population. Some representative protein spots are indicated on the protein array membrane figure. All the proteins spotted on the array membranes except (TIMP-4, G-CSP, GRO, IL-6, IL-10, MCP, TGF-beta-1) were expressed higher in HER2+ BCSCs when compared to the PBCCs (p < 0.05)
Discussion
This study demonstrated that HER2−BCSCs are more resistant to drugs than HER2+ BCSCs in primary breast cancer cell cultures. Trastuzumab had an antiproliferative effect on HER2-positive BCSCs and primary culture cells. Carboplatin has equal effects on primary culture cells and its subcultures. When we evaluated the overall effects of the drugs, the most effective drug for HER2-positive cells and stem cells was trastuzumab. MMP and cytokine levels were found to be higher in BCSCs. These results illustrated that HER2− BCSCs present in the total population may be responsible for the drug resistance of HER2+ BC population against trastuzumab.
The concept of intra-tumoural heterogeneity is considered important for the biological actions of cancer. Although the tumours of the patients appear to be histopathologically similar, they have different features in terms of molecular subtypes and potential to metastasise [28]. Cancer stem cells may be an important factor for intra-tumoural heterogeneity. According to the cancer stem cell hypothesis, tumours are composed of cells that exhibit the features of differentiation and self-renewal. BCSCs can be distinguished by their exhibition of surface indicators, CD24 and CD44. CD44, a hyaluronic acid receptor, interacts with osteopontin, which acts in tumour progression, regulating its cellular functions [29]. CD24 is a surface protein associated with extensive types of cancer cells and serves as a versatile ligand based on the different glycosylation profiles of these cells. Several studies have demonstrated that the expressions of CD24− and CD44+ are important markers of the BCSCs population in HER2-positive BCs [30]. Thus, we used these markers to determine BCSCs isolated in our study.
In a study carried out on breast cancer cells, CD44+CD24− cells were identified as tumorigenic [31]. In solid tumours, such as BC, the distribution of stem cells is considered to be 1–2% of the tumour [32]. In a study by Honeth et al., 31% of the tumour samples were CD44+CD24− cells [33]. In our study, CD44+CD24− cells were 42.7% of the primary culture cell population, with 49.1% of the population being CD44+CD24+ cells. These differences in the BCSCs population ratios may originate from the differential expression levels of these receptors in the tumour tissues due to individual features. Further, the expression of ALDH in the primary breast cancer population also confirms that HER2-positive breast cancer tissue contains BCSCs, which may hinder the success of chemotherapy. In breast cancer, ALDH1A1 expression is a good CSC marker and an important predictor of metastasis and drug resistance. Ginestier et al. and Morimoto et al. reported that high expression of ALDH1A1 expression correlated with poorer overall survival in breast cancer patients, implicating ALDH1A1 as the only ALDH1 isozyme capable of serving as a biomarker for predicting poor survival in breast cancer patients [34, 35]. Vassalli also declared that the presence of ALDH activity is both a marker for cancer stem cells and a functional regulator in cancer tissues [36].
The antiproliferative effects of paclitaxel on primary culture cells and subcultures were similar. Trastuzumab had an antiproliferative effect on HER2-positive BCSCs and HER2-positive primary culture cells. Carboplatin had equal effects on primary culture cells and its subcultures. When we evaluated the overall effects of the drugs, the most effective drug for HER2-positive cells and stem cells was trastuzumab. Carboplatin treatment in combination with paclitaxel and trastuzumab in BCSCs worked synergistically and additively, respectively. Carboplatin treatment was found to be more effective in combination with trastuzumab than with paclitaxel on subcultures. When the results of paclitaxel and trastuzumab combinations were examined, it was observed that the drugs exerted antagonistic activity on the cells, in contrast to the single-drug applications. In a study by Phillips and McBride, anticancer drugs were more effective on cell lines than on primary culture cells [20]. Magnifico et al. provided evidence of the efficacy of trastuzumab in inhibiting tumour-initiating cells of HER2-positive tumours. Our study confirmed the efficacy of trastuzumab on BCSCs in primary breast cell culture [37].
According to the results obtained from the primary cultures in this study, TIMP1 and TIMP2 metallopeptidase inhibitors were expressed in high amounts. MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-13, TIMP-1, and TIMP-2 proteins in HER2+ BCSCs were expressed 1.25–2.42 times more than in primary culture. Variations in MMP expression in cancer may include regulation at the microRNA level. A positive correlation has been observed between high MMP-9 levels and high histological grade BC. Mohammadian et al. recently reported that MMP-9 has a role in the initiation and proliferation of breast cancer cells by digesting collagen and interacting with tumour suppressor genes [38]. Further, a high plasma concentration of MMP-9 is related to reduced survival in breast cancer. Here, one of our objectives was to investigate MMP expression levels. Therefore, we considered increased MMP-9 expression levels an indicator of metastatic behaviour in BCSCs located in the primary breast cancer cell population. The expression level of MMP-9 could demonstrate the aggressive phenotype of the BC tissue. We found higher cytokine expression levels, except of IL-6, IL-10, MCP-1, and TGF beta-1, in HER2+ cancer stem cell-like cells when compared to the original primary culture population. Together, these findings indicate that the CD44+CD24−HER2+subpopulation has a different protein expression profile with respect to the primary culture population.
MMP-13 expression was significantly higher in the CD44+CD24− BCSCs in comparison with the primary BC cells. Furthermore, a high level of MMP-13 expression also appeared in HER2-positive BC cell cultures. According to this study, MMP-13 was expressed more in HER2-positive BCSCs. MMP-13 can play a potentially meaningful role in BC stem cells and their metastasis and invasion. This finding highly confirms the results revealed by the study demonstrating the significant role of MMPs and the microenvironment [39]. These results have important value for determining the role of MMPs as drug targets and strategies for targeting MMP function in therapeutic applications.
There are some limitations to this study. All cytotoxicity tests and drug combination assays were analysed in the primary cultures from a HER-2-positive patient. This is a correlation study with a small sample size; therefore, we could not draw generalised conclusions and assertions about stem cell-like subpopulations based on flow cytometry analysis of the expression of CD44, CD24, and HER2 in cells from primary culture. We need to conduct in vitro and in vivo studies to show these subpopulations’ highly metastatic potential. Future studies should include cytotoxicity tests on different primary cultures obtained from different HER-2 subgroups of patients. This pilot translational research and its results will guide in planning and directing multipatient studies to determine individual target proteins that cause resistance to chemotherapy in breast cancer.
Conclusion
The findings of this study showed that the presence of HER2− BCSCs may be responsible for primary trastuzumab resistance in histologically HER2-positive BC cell population. HER2− BCSCs were found to be more resistant to drugs than HER2+ BCSCs. Higher MMP levels were found in BCSCs. These results will allow revisiting of MMPs as drug targets in cancer. Studies investigating the function of MMPs for new directions towards our understanding of BCSCs are needed.
References
Cojoc M, Mäbert K, Muders MH, Dubrovska A. A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Semin Cancer Biol. 2015;31:16–27.
Sheng Y, Hu R, Zhang Y, Luo W. MicroRNA-4317 predicts the prognosis of breast cancer and inhibits tumor cell proliferation, migration, and invasion. Clin Exp Med. 2020;20:417–25.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8.
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2:78–91.
Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncog NIH Public Access. 2008;27:6120–30.
Trastuzumab A, Ithimakin S, Day KC, Malik F, Zen Q, Dawsey SJ, et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of. Cancer Res. 2013;73:1635–46.
Li Y, Chu J, Feng W, Yang M, Zhang Y, Zhang Y, et al. EPHA5 mediates trastuzumab resistance in HER2-positive breast cancers through regulating cancer stem cell-like properties. FASEB J. 2019;33:4851–65.
Wall ME, Wani MC. Camptothecin and taxol: from discovery to clinic. J Ethnopharmacol. 1996;51:239–54.
Conklin KA. Cancer chemotherapy and antioxidants. J Nutr. 2004;134:3201S-3204S.
Jiang H, Rugo HS. Human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer: how the latest results are improving therapeutic options. Ther Adv Med Oncol. 2015;7:321–39.
Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Tham Y-L, et al. Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients. J Clin Oncol. 2005;23:1169–77.
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu M-F, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–9.
Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11.
Dhillon J, Astanehe A, Lee C, Fotovati A, Hu K, Dunn SE. The expression of activated Y-box binding protein-1 serine 102 mediates trastuzumab resistance in breast cancer cells by increasing CD44+ cells. Oncogene. 2010;29:6294–300.
Chen W, Qin Y, Liu S. Cytokines, breast cancer stem cells (BCSCs) and chemoresistance. Clin Transl Med. 2018;7:27.
Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel). 2017;9:52.
Bouhniz OE, Zaied S, Naija L, Bettaieb I, Rahal K, Driss M, et al. Association between HER2 and IL-6 genes polymorphisms and clinicopathological characteristics of breast cancer: significant role of genetic variability in specific breast cancer subtype. Clin Exp Med. 2020;20:427–36.
Yousef EM, Tahir MR, St-Pierre Y, Gaboury LA. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer. 2014;14:609.
Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33.
Phillips TM, McBride WH, Pajonk F. The response of CD24-/low/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–85.
Leinonen T, Pirinen R, Böhm J, Johansson R, Ropponen K, Kosma V-M. Expression of matrix metalloproteinases 7 and 9 in non-small cell lung cancer: relation to clinicopathological factors, β-catenin and prognosis. Lung Cancer. 2006;51:313–21.
Cheng M, De B, Pikul S, Almstead NG, Natchus MG, Anastasio MV, et al. Design and synthesis of piperazine-based matrix metalloproteinase inhibitors. J Med Chem. 2000;43:369–80.
Birkedal-Hansen H, Moore WGI, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250.
Yokoyama M, Ochi K, Ichimura M, Mizushima T, Shinji T, Koide N, et al. Matrix metalloproteinase-2 in pancreatic juice for diagnosis of pancreatic cancer. Pancreas. 2002;24:344–7.
Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget. 2014;5:2736–49.
Pham P V, Vu BT, Chinh Phan NL, Duong TT, Vuong TG, Thuy Nguyen GD, et al. Isolation of breast cancer stem cells by single-cell sorting. In: Ceccherini-Nelli L, Matteoli B, editors. Biomedical tissue culture. IntechOpen; 2012. p. 59–72.
Tirino V, Desiderio V, Paino F, Papaccio G, De Rosa M. Methods for cancer stem cell detection and isolation. Methods Mol Biol. 2012;879:513–29.
Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, et al. CD44+/CD24-breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27.
Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–7.
Oliveras-Ferraros C, Vazquez-Martin A, Martin-Castillo B, Cufí S, Barco SD, Lopez-Bonet E, et al. Dynamic emergence of the mesenchymal CD44posCD24neg/low phenotype in HER2-gene amplified breast cancer cells with de novo resistance to trastuzumab (Herceptin). Biochem Biophys Res Commun. 2010;397:27–33.
Greve B, Kelsch R, Spaniol K, Eich HT, Götte M. Flow cytometry in cancer stem cell analysis and separation. Cytom Part A. 2012;81(4):284–93.
Honeth G, Bendahl P, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53.
Ginestier C, Korkaya H, Dontu G, Birnbaum D, Wicha MS, Charafe-Jauffret E. The cancer stem cell: the breast cancer driver. Medecine/Sciences. 2007;23:1133–9.
Morimoto K, Kim SJ, Tanei T, Shimazu K, Tanji Y, Taguchi T, et al. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci. 2009;100:1062–8.
Vassalli G. Aldehyde dehydrogenases: not just markers, but functional regulators of stem cells. Stem Cells Int Stem Cells Int. 2019;2019:3904645.
Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin cancer Res. 2009;15:2010–21.
Mohammadian H, Sharifi R, Rezanezhad Amirdehi S, Taheri E, Babazadeh BA. Matrix metalloproteinase MMP1 and MMP9 genes expression in breast cancer tissue. Gene Rep. 2020;21:100906.
Olivares-Urbano MA, Griñán-Lisón C, Ríos-Arrabal S, Artacho-Cordón F, Torralbo AI, López-Ruiz E, et al. Radiation and stemness phenotype may influence individual breast cancer outcomes: the crucial role of MMPs and microenvironment. Cancers (Basel). 2019;11:1781
Acknowledgments
This study was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) with the project number 113S559 and by Selcuk University Research Fund with the project number 15201017 and DPT_2009K121080.
Funding
This study was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) with project number 113S559 and by Selcuk University Research Fund with project number 15201017 and DPT_2009K121080.
Author information
Authors and Affiliations
Contributions
M.A., M.D.K., G.K.K., and H.A. conceived and planned the experiments. G.K.K, M.D.K., H.A., A.E., and M.A. carried out the experiments. M.A., G.K.K, A.E., F.A., M.C., and L.T. contributed to sample preparation. M.A., M.D.K., H.A., G.K.K., and A.E. contributed to the interpretation of the results. G.K.K. and M.A. took the lead in writing the manuscript. All authors reviewed and approved the submitted article.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Ethics approval
This study was conducted in accordance with the ethical standards described in the Declaration of Helsinki. The study protocol was approved by the Ethical Committee of Meram Medical Faculty, Konya (2013/391).
Informed consent
All the patients provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koygun, G.K., Kars, M.D., Emsen, A. et al. Response to trastuzumab and investigation of expression profiles of matrix metalloproteinase-related proteins in primary breast cancer stem cells. Clin Exp Med 21, 447–456 (2021). https://doi.org/10.1007/s10238-021-00685-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-021-00685-0