Abstract
B cell-activating factor (BAFF) is an essential cytokine in primary Sjögren’s syndrome (pSS) physiopathology. It has been reported that pSS patients develop germinal center-like (GC-like) structures in their minor salivary glands (MSGs). BAFF, BAFF-R, TACI, and BCMA expression was analyzed in MSGs from 29 subjects (nonspecific chronic sialadenitis and focal lymphocytic sialadenitis with the presence [pSS-GC(+)] or absence [pSS-GC(−)] of GC-like structures). Twenty-four percent of patients showed ectopic GC-like structures and a high focus score [p < 0.001 vs pSS-GC(−)]. BAFF serum levels (sBAFF) were high in pSS patients (p = 0.025 vs healthy subjects). However, the pSS-GC(−) group showed higher sBAFF levels than pSS-GC(+) patients. BAFF and BAFF-R glandular expression levels were higher in pSS-GC(+) patients, without significant differences compared to pSS-GC(−) patients. Soluble levels of BAFF correlated with anti-La/SSB antibodies and disease duration. Our results showed that BAFF could contribute to focal lymphocytic infiltration. The role of BAFF-binding receptors in MSGs is proposed as a mechanism for the possible establishment of ectopic GC-like structures and disease progression in some patients. In conclusion, this study supports previous evidence that considers the active BAFF system role in the pathogenesis of pSS and the need for strong biomarkers in this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary Sjögren’s syndrome (pSS) is a chronic autoimmune disease characterized by salivary and lacrimal gland affection due to focal lymphocytic infiltration [1].
The most frequent histopathological feature for the classification of pSS patients is focal lymphocytic sialadenitis (FLS). In minor salivary glands, cell aggregates are adjacent to the gland acini and periductal ducts. However, it is also possible that some pSS patients develop nonspecific chronic sialadenitis (NSCS) characterized by a diffuse infiltrate, mainly located through normal acini close to the perilobular capsule [2].
Lymphocyte subpopulations in the target tissue are dependent on the lesion severity. T cells predominate in mild lesions, while B cells are abundant in more severe cases [3]; moreover, in glandular tissues, plasma cells have also been identified [4]. Once antigen-presenting cells start producing early cytokines, particularly type I and II interferons, they can promote the activation of T and B lymphocytes [5]. It has been previously reported that in approximately 15–40% of pSS cases with FLS, lymphocytic aggregates develop ectopic germinal center (GC)-like structures that resemble B cell germinal centers from secondary lymphoid tissues [6, 7].
One of the key molecules associated with pSS pathogenesis is B cell-activating factor (BAFF), which is essential for B cell survival, maturation, and differentiation [8]. BAFF belongs to the TNF superfamily together with another homologous molecule named a proliferation-inducing ligand (APRIL). The interaction between BAFF and APRIL with their receptors mediates all physiological functions. BAFF-receptor (BAFF-R), which binds exclusively to BAFF, is fundamental in the early stages of B cell maturation. The transmembrane activator and CAML interactor (TACI) has a higher affinity for BAFF than APRIL, which acts as a negative regulator in B cell homeostasis, isotype class-switching, and T-independent antigen responses. The B cell maturation antigen (BCMA), which may bind preferentially to APRIL instead of BAFF, is present mostly in plasmablasts and long-lived plasma cells; BCMA mediates the survival of the latter differentiated cells [9].
The role of BAFF has been studied in autoimmune diseases, including rheumatoid arthritis (RA) [10] and systemic lupus erythematosus (SLE) [11, 12], where it was associated with the presence of antibodies, severe clinical outcome of these diseases, and alterations in B cell population and signaling pathways.
Previous studies in pSS showed that a high concentration of soluble BAFF (sBAFF) was associated with the levels of anti-SSA/Ro, anti-SSB/La, and rheumatoid factor (RF) antibodies [13]. In addition, an increased disease activity evaluated by the ESSDAI index was associated with sBAFF levels [14]. Furthermore, high BAFF mRNA expression, as well as the presence of BAFF in epithelial cells from minor salivary glands in pSS patients, has been demonstrated [15, 16].
The clinical and immunological associations of BAFF and the presence of GC-like structures in pSS immunopathology are still under consideration. Some studies have already shown that disease severity in patients with GC-like structures is increased, showing high focus score [17] and a high prevalence of anti-Ro/SSA, anti-La/SSB [18], rheumatoid factor, C reactive protein (CRP) [19], antinuclear antibodies (ANAs) [20], and immunoglobulin G (IgG) titers [21]. Nevertheless, there is some other evidence that suggests that BAFF by itself is not associated with GC-like formation, but correlates positively with focus score and hypergammaglobulinemia in pSS patients [22].
Therefore, the BAFF system could be the key factor for understanding the formation and maintenance of germinal centers at the glandular level. In this context, it is also important to consider the interactions with BAFF-binding receptors, which may explain the effects of the immunological network involved in pSS. For this reason, the present study aimed to analyze BAFF, BAFF-R, TACI, and BCMA glandular expression in MSGs of patients with the presence or absence of GC-like structures, and their association with serum levels of BAFF and clinical severity in pSS.
Materials and methods
Study subjects
Minor salivary glands (MSGs) and blood samples were obtained from 29 patients suspected of having primary Sjögren’s syndrome, who underwent to a lip biopsy at the Rheumatology Service of Hospital General de Occidente, Jalisco, Mexico, and Hospital Civil de Guadalajara Fray Antonio Alcalde, Jalisco, Mexico. All studied subjects signed an informed consent form, which included confidentiality issues according to the Declaration of Helsinki (lastly reviewed in Fortaleza, Brazil, in 2013) and the actual national guidelines and normativity. The Ethics Committee of Hospital General de Occidente, Jalisco, México (No. 448/16), and Hospital Civil de Guadalajara Fray Antonio Alcalde, Jalisco, México (No. 212/16), approved this study.
Of the 29 subjects included, only 25 patients fulfilled the 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for pSS [23]. The Sjögren’s Syndrome Disease Activity Index (SSDAI), Sjögren’s Syndrome Disease Damage Index (SSDDI) [24], and EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) [25] were applied to pSS patients at the enrollment of the study. Clinical and sociodemographic features were collected from the medical records of all patients. Blood samples were also obtained from 29 healthy subjects as a control group, paired by age and gender, with non-inflammatory conditions such as infections, obesity, or autoimmune diseases. In all studied subjects, erythrocyte sedimentation rate (ESR, performed by Wintrobe’s method) and CRP (determined by turbidimetry, BS120, Mindray, Shenzhen, China) were measured.
Patients enrolled in the study were stratified according to their histopathological and clinical features as nonspecific chronic sialadenitis with sicca symptoms (NSCS-Sicca) (n = 4/29), nonspecific chronic sialadenitis with pSS (NSCS-pSS) (n = 2/29), focal lymphocytic sialadenitis with pSS and non-GC-like structures [pSS-GC(−)] (n = 16/29), and focal lymphocytic sialadenitis with pSS and GC-like structures [pSS-GC(+)] (n = 7/29).
Histopathological evaluation of MSG biopsies and ectopic GC characterization
MSG biopsies were fixed in 4% paraformaldehyde and embedded in paraffin with a tissue processor (Leica TP1020, Leica Biosystems, Wetzlar, Germany). Tissue sections 4 microns wide were mounted on electrocharged slides and stained with hematoxylin and eosin (H&E). Histological preparations were evaluated by two experienced pathologists who reported the infiltrating mononuclear cells according to the number of lymphocytic foci per total area in mm2, which was later adjusted to foci/4 mm2 (focus score). Some of the FLS lip biopsies displayed ectopic germinal center formation, identified by a well-circumscribed chronic inflammatory cell infiltrate consisting of a lymphoid follicle-like organization, presenting a densely packed dark zone and a light zone and surrounded by a mantle zone. The presence of ectopic GC-like structures was further confirmed by CD21 staining of follicular dendritic cell (FDC) networks by immunohistochemistry.
Immunohistochemistry assay
Tissue slides were deparaffinized using dry heat at 59 degrees and rehydrated by immersion in subsequently graded alcohol dilutions until distilled water. Slides were placed in Coplin cups with 10 mM sodium citrate buffer (pH = 6) for BAFF (ab16081, rat monoclonal antibody, dilution 1:100), TACI (ab79023, rabbit polyclonal antibody, dilution 1:200), BCMA (ab5972, rabbit polyclonal antibody, dilution 1:400), or 1 mM EDTA buffer (pH = 9) for CD21 (ab9492, mouse monoclonal antibody, dilution 1:10), and BAFF-R (ab16232, mouse monoclonal antibody, dilution 1:500) (ABCAM, Cambridge, UK), and moist heat was used as a method for antigen retrieval. Later, all slides were cooled, and a 3% hydrogen peroxide–10% methanol solution was added to achieve endogenous peroxidase blockade. Serum blocking was performed according to the secondary antibody source (horse serum for mouse antibodies, goat serum for rabbit antibodies, and rabbit serum for rat antibody) (Vector, California, USA), and incubation with primary antibodies was carried out overnight at 4 °C. Secondary biotinylated antibodies and streptavidin (ABC Vectastain ELITE kit, Vector, California, USA) were used, and diaminobenzidine (DAB) was employed for detection until the development of a red-brown color. Finally, slides were counterstained with Harris hematoxylin.
For CD3 (790-4341, rabbit monoclonal antibody 2GV6), CD20 (760-2531, mouse monoclonal antibody L26) and CD138 (760-4248, mouse monoclonal antibody B-A38) immunohistochemistry assays, primary antibodies were obtained from Ventana iVIEW PATHWAY. Detection was performed with the iVIEW DAB detection kit (Roche Diagnostics, Arizona, USA), in an automated Benchmark ULTRA device (Roche Diagnostics, Arizona, USA) operated according to the manufacturer’s protocols for paraffin-embedded tissues.
In all immunohistochemistry assays, validation for antibody specificity was performed by incubating slides with PBS buffer solution as a negative control, while sections of human tonsils were considered as the positive control.
Immunohistochemical analysis
Digital images from stained slides were obtained with an Axio Lab. A1 optical microscope (Zeiss, Oberkochen, Germany) and an Axiocam 305 digital camera (Zeiss, Oberkochen, Germany). Photomicrographs were taken at 50× magnification in triplicate, capturing the same surface areas from most of the tissues. The AxioVision V4.6 software (Zeiss, Oberkochen, Germany) displayed the light and camera adjustments when the images were recorded. For image analysis, ImageJ open-access software (National Institutes of Health, Maryland, USA, https://imagej.nih.gov/ij/) was used. First, digital images needed to be color deconvoluted into three layers: DAB, hematoxylin, and a complementary image. Only the DAB color layer representing staining positivity was analyzed considering the pixel intensity values in the range of 0–255 (wherein 0 represents the darkest shade of the color, and 255 represents the lightest shade of the color as standard) as previously described [26]. The mean intensity default threshold was set at the ‘Image' menu, and using the ‘Measure' tool from the ‘Analyze' menu, the percentage of positive pixels within the selected area was determined.
BAFF serum levels and antibodies
Soluble BAFF (DBLYS0B) levels were quantified using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minnesota, USA) according to the manufacturer’s recommendation. The mean minimum detectable level of soluble BAFF was 2.68 pg/mL.
Anti-Ro/SSA (ORG 208) and anti-La/SSB (ORG 209) antibodies were measured in pSS patients using ELISA kits (ORGENTEC, Mainz, Germany) according to the manufacturer’s instructions with an assay limit of detection of 1.0 U/mL and a cutoff point of 25.0 U/mL.
Immunoglobulin G (ELH-IGG1) levels were also measured with an ELISA kit (RayBiotech, Georgia, USA) following appropriate indications, and the assay minimum detectable level was 150 pg/mL.
Antinuclear antibody (ANA) (EKC31161) detection was performed with a qualitative ELISA kit (Biomatik, Ontario, Canada) comparing optical densities of samples with the negative control to establish the values for antibody positivity.
The absorbances were measured in a microplate reader (MultiSkan Go, Thermo Fisher Scientific, Massachusetts, USA). A four-parameter logistic regression was used to calculate BAFF, anti-SSA/Ro, anti-SSB/La, and IgG concentrations in all samples.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 23 (IBM Corporation, New York, USA) and GraphPad Prism 6 software (GraphPad Software, California, USA). Data were analyzed using the Shapiro–Wilk normality test, the Kruskal–Wallis test, Mann–Whitney’s U test with post hoc Dunn’s multiple comparisons test, and Chi-squared test for proportions. In addition, Spearman’s rank correlation test in nonparametric data was performed for the association between BAFF, clinical parameters, and disease activity. Values of p equal to or less than 0.05 were considered significant. Otherwise, nonsignificant p values are not shown.
Results
Demographic and clinical characteristics
All primary Sjögren’s syndrome patients were female with an average age of 53 years and two years of disease duration. In addition, 56% of the patients were positive for anti-Ro/SSA, and 28% were positive for anti-La/SSB. Likewise, 92% had a focus score of lymphocytic infiltration ≥ 1 (evaluated in 4 mm2), and all of them showed oral or ocular symptoms. The most common extraglandular manifestations were arthritis/arthralgia (60%), fatigue (56%), cytopenia (16%), parotid swelling (8%), and neuropathy/neurological damage (4%). The treatments included hydroxychloroquine (48%), azathioprine (28%), methotrexate (16%), indomethacin (16%), and prednisone (8%). Patients with biological therapy were not included in this study (Table 1).
Demographic and clinical characteristics, according to histopathological and clinical features, are shown in Table 2. Patients with pSS-GC(+) presented the highest focus score [3.77 foci/4 mm2 vs GC(−) patients = 2.41 foci/4 mm2, p = 0.001; Table 2].
BAFF serum levels in pSS patients and controls
BAFF soluble (sBAFF) levels in pSS patients were higher than those in healthy subjects (HS) (1104.0 pg/mL vs HS = 870.2 pg/mL, p = 0.025; Fig. 1a). Moreover, when patients were stratified according to histopathological features, the pSS-GC(−) patients presented the highest levels of sBAFF (1257.0 pg/mL vs HS = 870.2 pg/mL, p = 0.002; Fig. 1b).
Soluble levels of BAFF. a Soluble levels of BAFF in pSS and HS. Significant difference was observed between pSS patients and HS (1104.0 pg/mL vs 870.2 pg/mL, p = 0.025). Statistical analysis was performed using Mann–Whitney U test. b Soluble levels of BAFF in pSS patients and non-pSS subjects according to histopathological features. Statistical differences were observed between pSS-GC(−) and HS (1257.0 pg/mL vs 870.2 pg/mL, p = 0.002). Statistical analysis was performed using Kruskal–Wallis test and post hoc Dunn’s multiple comparisons test. HS healthy subjects, pSS primary Sjögren’s syndrome, NSCS nonspecific chronic sialadenitis, GC germinal center
Histopathological features of minor salivary glands and GC-like structure identification
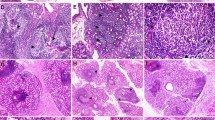
Minor salivary gland biopsies were analyzed according to their histopathological features (Fig. 2a–d). Patients with the presence or absence of germinal center-like structures were identified. Characterization of germinal center-like structures in minor salivary glands was carried out by the recognition of morphological features that resemble germinal centers in secondary lymphoid tissues and the detection of CD21 positivity as a cell surface marker for follicular dendritic cell networks (Fig. 2h). In addition, the CD21 staining positivity percentage showed increased expression in pSS-GC(+) patients (pSS-GC(+) = 41.91% vs NSCS-Sicca = 0.53%, p = 0.002; and NSCS-pSS = 0.71%, p = 0.024; Fig. 2i).
Minor salivary glands from pSS patients according to histopathological features. a–d Hematoxylin and eosin staining in minor salivary glands. Total magnification ×50 (Bar indicates approximately 400 µm). e–h CD21 positive staining in MSGs. Total magnification ×50 (Bar indicates approximately 400 µm). i Statistical differences in percentage of staining positivity were observed between NSCS-Sicca and pSS-GC(+) (0.53% vs 41.91%, p = 0.002); NSCS-Sicca and pSS-GC(−) (0.53% vs 5.78%, p < 0.001); NSCS-pSS and pSS-GC(+) (0.71% vs 41.91%, p = 0.024). Statistical analysis was performed using Kruskal–Wallis test and post hoc Dunn’s multiple comparisons test. pSS primary Sjögren’s syndrome, NSCS nonspecific chronic sialadenitis, GC germinal center
Immunophenotype of infiltrating cells in minor salivary glands
The proportion of CD3+ cells was high as the tissue damage increased. The percentage of CD3+ cells was less of 10% in NSCS-Sicca (Fig. 3a) and NSCS-pSS (Fig. 3b). In pSS-GC(−) patients, the CD3+ cell distribution was more dispersed (approximately 33% of the inflammatory infiltrate, Fig. 3c). In pSS-GC(+) patients, 53% of CD3+ cells were located mainly in the surrounding areas, and some other CD3 cells were inside the GC-like structures (pSS-GC(+) = 53.14% vs NSCS-Sicca = 5.87%, p = 0.006; NSCS-pSS = 9.66%, p = 0.017; and pSS-GC(−) = 33.36%, p < 0.001; Fig. 3d, e).
CD3, CD20 and CD138 expression in minor salivary glands. a–d CD3 positive staining in minor salivary glands. Total magnification ×50 (Bar indicates approximately 400 µm). e Statistical differences in percentage of staining positivity were observed between NSCS-Sicca and pSS-GC(+) (5.87% vs 53.14%, p = 0.006); NSCS-pSS and pSS-GC(+) (9.66% vs 53.14%, p = 0.017); pSS-GC(−) and pSS-GC(+) (33.36% vs 53.14%, p < 0.001). f–i CD20 positive staining in minor salivary glands. Total magnification ×50 (Bar indicates approximately 400 µm). j Statistical differences in percentage of staining positivity were observed between NSCS-Sicca and pSS-GC(+) (2.88% vs 68.47%, p = 0.006); NSCS-pSS and pSS-GC(+) (9.18% vs 68.47%, p = 0.017); pSS-GC(−) and pSS-GC(+) (42.21% vs 68.47%, p < 0.001). k–n CD138 positive staining in minor salivary glands. Total magnification ×50 (Bar indicates approximately 400 µm). o Statistical differences in percentage of staining positivity were observed between NSCS-Sicca and pSS-GC(+) (2.53% vs 37.29%, p = 0.006); NSCS-pSS and pSS-GC(+) (3.97% vs 37.29%, p = 0.017); pSS-GC(−) and pSS-GC(+) (15.56% vs 37.29%, p < 0.001). Statistical analysis was performed using Kruskal–Wallis test and post hoc Dunn’s multiple comparisons test. pSS primary Sjögren’s syndrome, NSCS nonspecific chronic sialadenitis, GC germinal center
Furthermore, CD20+ cells also increased according to the lesion degree; the nonspecific chronic sialadenitis groups presented the least amount of CD20+ cells, 2% and 9%, respectively (Fig. 3f, g). CD20+ cells were found in well-defined areas in MSGs of focal lymphocytic sialadenitis groups, representing approximately 42% of infiltrating cells (Fig. 3h), which coincided with the GC-like structures observed in pSS-GC(+) patients, where CD20+ cells covered approximately 68% of the lymphocytic infiltrate (pSS-GC(+) = 68.47% vs NSCS-Sicca = 2.88%, p = 0.006; NSCS-pSS = 9.18%, p = 0.017; and pSS-GC(−) = 42.21%, p < 0.001; Fig. 3i, j).
Additionally, CD138+ plasma cells were identified near salivary ducts in NSCS-Sicca (2%, Fig. 3k), NSCS-pSS (4%, Fig. 3l) and pSS-GC(−) (15%, Fig. 3m). Nevertheless, in GC-like structures, the number of CD138+ cells was increased (37% of the infiltrating cells) and distributed in regions outside of the follicle (pSS-GC(+) = 37.29% vs NSCS-Sicca = 2.53%, p = 0.006; NSCS-pSS = 3.97%, p = 0.017; and pSS-GC(−) = 15.56%, p < 0.001; Fig. 3n, o).
BAFF and BAFF-binding receptors expression in minor salivary glands
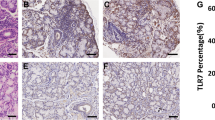
BAFF expression displayed a homogeneous distribution among minor salivary glands, where compared to the nonspecific chronic sialadenitis groups, the focal lymphocytic sialadenitis groups had the highest levels of BAFF (Fig. 4a, b). No significant differences were observed between pSS-GC(−) and pSS-GC(+) patients (Fig. 4c, d). The image analysis revealed differences in the BAFF percentage of staining positivity in pSS-GC(+) patients (pSS-GC(+) = 44.72% vs NSCS-Sicca = 6.43%, p = 0.009; and NSCS-pSS = 10.05%, p = 0.009; Fig. 4e).
BAFF and BAFF-binding receptors in minor salivary glands. a–d BAFF positive staining in minor salivary glands. Total magnification ×50 (Bar indicates approximately 400 µm). e Statistical differences in percentage of staining positivity were observed between NSCS-Sicca and pSS-GC(+) (6.43% vs 44.72%, p = 0.009); NSCS-pSS and pSS-GC(+) (10.05% vs 44.72%, p = 0.009). f–i BAFF-R positive staining in minor salivary glands. Total magnification ×50 (Bar indicates approximately 400 µm). j Statistical differences in percentage of staining positivity were observed between NSCS-pSS and pSS-GC(−) (1.04% vs 23.22%, p = 0.009); NSCS-pSS and pSS-GC(+) (1.04% vs 23.24%, p = 0.009). k–n TACI positive staining in minor salivary glands. Total magnification ×50 (Bar indicates approximately 400 µm). o Statistical differences in percentage of staining positivity were observed between NSCS-Sicca and pSS-GC(+) (4.37% vs 27.72%, p = 0.009); NSCS-pSS and pSS-GC(+) (4.36% vs 27.72%, p = 0.009). p–s BCMA positive staining in minor salivary glands. Total magnification ×50 (Bar indicates approximately 400 µm). t Statistical differences in percentage of staining positivity were observed between NSCS-Sicca and pSS-GC(+) (10.22% vs 24.64%, p = 0.009); NSCS-pSS and pSS-GC(+) (10.82% vs 24.64%, p = 0.009); pSS-GC(−) and pSS-GC(+) (14.17% vs 24.64%, p < 0.001). Statistical analysis was performed using Kruskal–Wallis test and post hoc Dunn’s multiple comparisons test. pSS primary Sjögren’s syndrome, NSCS nonspecific chronic sialadenitis, GC germinal center
BAFF-R staining positivity was absent in nonspecific chronic sialadenitis groups (Fig. 4f, g). Meanwhile, BAFF-R+ cells were located in specific areas distant from the lymphocytic infiltrate of the glandular tissue in pSS-GC(−) patients (pSS-GC(−) = 23.22% vs NSCS-pSS = 1.04%, p = 0.009; Fig. 4h, j). Similarly, pSS-GC(+) patients expressed intense BAFF-R staining in GC-like structures around follicles and some positive cells inside follicles (pSS-GC(+) = 23.24% vs NSCS-pSS = 1.04%, p = 0.009), which represented most CD3+ and some CD20+ infiltrating cells (Fig. 4i, j).
TACI showed a decreased proportion of positive cells in the NSCS groups (Fig. 4k, l). Nevertheless, a wide distribution of stained cells with high intensity in pSS-GC(−) (Fig. 4m) and pSS-GC(+) were observed. However, TACI staining was more evident in the GC-like structures where CD20+ cells were present (pSS-GC(+) = 27.72% vs NSCS-Sicca = 4.37%, p = 0.009; and NSCS-pSS = 4.36%, p = 0.009; Fig. 4n, o).
BCMA glandular expression was higher in the NSCS groups than in the BAFF-R and TACI groups (Fig. 4p, q). BCMA expressing cells were identified in periacinar areas near salivary ducts, with a morphology resembling plasma cells, which coincided with CD138 positive cells in the pSS-GC(−) and pSS-GC(+) groups (Fig. 4r, s). Primary Sjögren’s syndrome patients with GC-like structures showed increased BCMA staining positivity (pSS-GC(+) = 24.64% vs NSCS-Sicca = 10.22%, p = 0.009; NSCS-pSS = 10.82%, p = 0.009; and pSS-GC(−) = 14.17%, p < 0.001; Fig. 4t).
Association between BAFF, clinical parameters, and disease severity
Primary Sjögren’s syndrome patients with increased levels of sBAFF showed a high disease duration (r = 0.439, p = 0.032; Fig. 5a) and the highest anti-La/SSB levels (r = 0.420, p = 0.037; Fig. 5b). In addition, the disease duration correlated with anti-Ro/SSA levels (r = 0.409, p = 0.042; Fig. 5c) and SSDAI score (r = 0.491, p = 0.013; Fig. 5d).
Correlations of sBAFF, autoantibodies and clinical activity in pSS patients. a Correlation between disease duration and sBAFF levels (r = 0.439, p = 0.031). b Correlation between sBAFF levels and anti-La/SSB levels (r = 0.420, p = 0.037). c Correlation between disease duration and anti-Ro/SSA levels (r = 0.409, p = 0.042). d Correlation between disease duration and SSDAI score (r = 0.491, p = 0.013). Statistical analysis was performed using Spearman’s correlation coefficient. Anti-Ro/SSA anti-Sjögren’s syndrome-related antigen A, Anti-La/SSB anti-Sjögren’s syndrome-related antigen B, SSDAI Sjögren’s syndrome disease activity index, RF rheumatoid factor
Furthermore, positive correlations were observed between anti-Ro/SSA and anti-La/SSB levels (r = 0.753, p < 0.0001), anti-Ro/SSA and RF levels (r = 0.558, p = 0.004), and anti-La/SSB and RF levels (r = 0.677, p < 0.001). Finally, the SSDAI score was associated with anti-Ro/SSA levels (r = 0.442, p = 0.027), anti-La/SSB levels (r = 0.419, p = 0.037), and RF levels (r = 0.571, p = 0.008) (data not shown).
Discussion
In primary Sjögren’s syndrome, the focus score and features of minor salivary gland histopathology have been evaluated and strengthened as potential clinical biomarkers, which increase the predictive value of minor salivary gland biopsy [27, 28].
In this context, the clinical course of patients with pSS could be directly associated with the subsets of cells that infiltrate the glandular tissue. For instance, B cell predominance has been correlated with systemic manifestations and antibody production [1] as well as the linkage between the presence of GC-like structures with elevated titers of rheumatoid factor, anti-Ro/SSA, anti-La/SSB, and IgG in pSS-GC(+) patients [22, 29].
The pSS-GC(−) patients in our study showed more extraglandular clinical manifestations (articular damage, cytopenia, and parotid swelling). Additionally, an increased proportion of CD3+ and CD20+ cells and the highest levels of sBAFF were observed, analogous to the findings of previous studies [14, 20, 22]. Although we hypothesize that an increasing BAFF serum levels is related to the development of systemic effects and the focal infiltrate formation, BAFF has been associated with the focus score but not with the presence of GC-like structures by some authors [22, 30]. Therefore, a higher BAFF concentration within the MSG could be the key for major lymphocytic recruitment and retention in well-located areas [31, 32].
Previous reports support that in vivo BAFF blockade with an anti-BAFF-R antibody drastically reduced the number of follicles in MSGs from a murine model of pSS [33], whereas BAFF overexpression promotes higher lymphocytic infiltration [30]. In addition, in some pSS patients who received rituximab, focal lymphocytic infiltration persists after biological treatment [34].
Moreover, BAFF may interact with other cytokines and chemokines, such as CXCL12, CXCL13, IL-6, IL-15, IL-17A, IL-22, and type I and II IFN, through specific signaling pathways, producing an appropriate microenvironment that promotes lymphocytic infiltration and an inflammatory process in glandular tissue [33, 35,36,37,38].
Regarding BAFF-binding receptors, even though nonsignificant differences were found, the balance between the BAFF-R, TACI, and BCMA distribution could also explain some relevant features in the pSS groups.
The main function described for BAFF-R is related to the survival and maintenance of B cells [12], as well as mediation of BAFF-dependent costimulation in T cells [39]. A recent pilot study proved that abatacept affected the formation of germinal center-like structures in pSS, suggesting a relationship between BAFF and activated TFH cells [40]. BAFF could also act by an indirect mechanism through the PI3K-Akt signaling pathway [41] and the later differentiation of TFH cells. In addition, it is crucial to analyze the presence of BAFF/BAFF-R mutations, the expression of genetic variants and their heterogeneity in the context of susceptibility factors associated with pSS pathogenesis [42,43,44].
The interaction of the BAFF/BAFF-R system is being explored as a promising pharmacological target. For instance, treatment with belimumab in pSS patients contributes to normalizing elevated circulating B cell subsets and restoring BAFF-R expression, which is downregulated in peripheral B cells [45]. Moreover, the use of ianalumab (VAY736) has also displayed good results in B cell depletion [46] and a reduction of clinical outcomes, evaluated by ESSDAI and ESSPRI, in pSS patients [47].
Concerning TACI, an increase in the TFH and B cell subpopulations, but a reduction in the number of antibody-secreting cells has been demonstrated in Taci -/- murine lupus model [48]. Moreover, TACI deletion confers protection against clinical manifestations of systemic lupus erythematosus [49], and mutations in the TACI gene have been linked to antibody deficiencies [50]. Altogether, these findings may offer a specific therapeutic alternative for pathologies where B cell responses and autoantibody production are major components of disease severity.
Some previous studies in murine models have shown the regulatory function of BCMA [51] and its fundamental role in B cell homeostasis and self-tolerance in systemic autoimmunity [52]. The absence of BCMA favors TFH cell expansion, germinal center formation, autoantibody production, and IFN-y production by TFH cells through BAFF-R in a lupus model; therefore, BCMA and BAFF-R balance could be essential to maintain tolerance [53]. The homeostatic function of BCMA could also be altered in autoimmune diseases, as seen in Behcet’s disease [54] and peripheric B cell subsets from patients with systemic lupus erythematosus, in whom decreased BCMA expression has been found to be associated with disease activity [11].
Our experimental design has some limitations that allow us only to describe the relationship between the clinical outcomes, the infiltrating degrees in MSGs and the expression of local and systemic BAFF and BAFF-binding receptors. Additionally, it is important to take into consideration that some of the observed effects could also be attributed to APRIL, as supported by the positive correlations found between sBAFF and sAPRIL in pSS patients [22] and the implication of APRIL in rheumatoid arthritis [55] and systemic lupus erythematosus [56]. However, a recent analysis in MSGs from pSS showed that local expression of APRIL is decreased and that the glandular tissues may not depend on APRIL for their survival [57, 58], which could also be linked to the low levels of BCMA compared to TACI or BAFF-R observed in our study.
The correlation between sBAFF levels and disease duration strongly suggests that the progression of pSS promotes a more extensive BAFF production by local infiltrating cells and glandular epithelia. Consequently, this may be a risk factor for disease activity and some clinical manifestations [59], as well as the development of secondary Sjögren’s syndrome in other autoimmune diseases, such as systemic lupus erythematosus [60].
In summary, pSS displayed a wide variety of clinical conditions where elevated BAFF levels are associated with focal lymphocytic sialadenitis, disease duration and anti-La/SSB antibody titers in our studied group. Moreover, BAFF and BAFF-binding receptors expression in glandular tissue differs from distinct features between the GC(−) and GC(+) groups.
The balance between BAFF-binding receptors expression and the interactions of BAFF with other cytokines in the local microenvironment could be the key mechanism for the establishment of GC-like structures, while disease progression occurs. In conclusion, this study supports previous evidence that considers the active BAFF system role in the pathogenesis of pSS and the need for strong biomarkers in this disease.
References
Goules AV, Kapsogeorgou EK, Tzioufas AG. Insight into pathogenesis of Sjögren’s syndrome: dissection on autoimmune infiltrates and epithelial cells. Clin Immunol Orlando Fla. 2017;182:30–40.
Brito-Zerón P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, et al. Sjögren syndrome. Nat Rev Dis Primer. 2016;2:16047.
Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. J Autoimmun. 2010;34:400–7.
Mingueneau M, Boudaoud S, Haskett S, Reynolds TL, Nocturne G, Norton E, et al. Cytometry by time-of-flight immunophenotyping identifies a blood Sjögren’s signature correlating with disease activity and glandular inflammation. J Allergy Clin Immunol. 2016;137(1809–1821):e12.
Muskardin TLW, Niewold TB. Type I interferon in rheumatic diseases. Nat Rev Rheumatol. 2018;14:214–28.
Johnsen SJ, Berget E, Jonsson MV, Helgeland L, Omdal R, Jonsson R. Evaluation of germinal center-like structures and B cell clonality in patients with primary Sjögren syndrome with and without lymphoma. J Rheumatol. 2014;41:2214–22.
Sène D, Ismael S, Forien M, Charlotte F, Kaci R, Cacoub P, et al. Ectopic germinal centre-like structures in minor salivary gland biopsy predict lymphoma occurrence in patients with primary Sjögren syndrome. Arthritis Rheumatol. 2018;70:1481–88.
Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013;24:203–15.
Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263–75.
Wei F, Chang Y, Wei W. The role of BAFF in the progression of rheumatoid arthritis. Cytokine. 2015;76:537–44.
Salazar-Camarena DC, Ortiz-Lazareno PC, Cruz A, Oregon-Romero E, Machado-Contreras JR, Muñoz-Valle JF, et al. Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus. 2016;25:582–92.
Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. 2014;10:365–73.
Mariette X, Roux S, Zhang J, Bengoufa D, Lavie F, Zhou T, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren’s syndrome. Ann Rheum Dis. 2003;62:168–71.
Lee SJ, Oh HJ, Choi BY, Jang YJ, Lee JY, Park JK, et al. Serum 25-hydroxyvitamin D3 and BAFF levels are associated with disease activity in primary Sjogren’s syndrome. J Immunol Res. 2016;2016:5781070.
Lahiri A, Varin M-M, Le Pottier L, Pochard P, Bendaoud B, Youinou P, et al. Specific forms of BAFF favor BAFF receptor-mediated epithelial cell survival. J Autoimmun. 2014;51:30–7.
Lavie F, Miceli-Richard C, Quillard J, Roux S, Leclerc P, Mariette X. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjögren’s syndrome. J Pathol. 2004;202:496–502.
He J, Jin Y, Zhang X, Zhou Y, Li R, Dai Y, et al. Characteristics of germinal center-like structures in patients with Sjögren’s syndrome. Int J Rheum Dis. 2017;20:245–51.
Fayyaz A, Kurien BT, Scofield RH. Autoantibodies in Sjögren’s syndrome. Rheum Dis Clin North Am. 2016;42:419–34.
Lee K-E, Kang J-H, Yim Y-R, Kim J-E, Lee J-W, Wen L, et al. The significance of ectopic germinal centers in the minor salivary gland of patients with Sjögren’s syndrome. J Korean Med Sci. 2016;31:190–5.
Maślińska M, Kontny E, Kwiatkowska B. The relationship between the presence of autoantibodies, indicators of local and systemic inflammation, the serum concentration of B-cell activating factor (BAFF) and the intensity of salivary gland infiltration in patients with primary Sjögren’s syndrome—a preliminary study. Reumatologia. 2015;53:321–7.
Nocturne G, Mariette X. B cells in the pathogenesis of primary Sjögren syndrome. Nat Rev Rheumatol. 2018;14:133–45.
Jonsson MV, Szodoray P, Jellestad S, Jonsson R, Skarstein K. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjögren’s syndrome. J Clin Immunol. 2005;25:189–201.
Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76:9–16.
Vitali C, Palombi G, Baldini C, Benucci M, Bombardieri S, Covelli M, et al. Sjögren’s syndrome disease damage index and disease activity index: scoring systems for the assessment of disease damage and disease activity in Sjögren’s syndrome, derived from an analysis of a cohort of Italian patients. Arthritis Rheum. 2007;56:2223–31.
Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69:1103–9.
Chatterjee S, Malhotra R, Varghese F, Bukhari AB, Patil A, Budrukkar A, et al. Quantitative immunohistochemical analysis reveals association between sodium iodide symporter and estrogen receptor expression in breast cancer. PLoS ONE. 2013;8:e54055.
Fisher BA, Brown RM, Bowman SJ, Barone F. A review of salivary gland histopathology in primary Sjögren’s syndrome with a focus on its potential as a clinical trials biomarker. Ann Rheum Dis. 2015;74:1645–50.
Wicheta S, Van der Groen T, Faquin WC, August M. Minor salivary gland biopsy—an important contributor to the diagnosis of Sjögren syndrome. J Oral Maxillofac Surg. 2017;75:2573–8.
Jonsson MV, Skarstein K, Jonsson R, Brun JG. Serological implications of germinal center-like structures in primary Sjögren’s syndrome. J Rheumatol. 2007;34:2044–9.
Ding J, Zhang W, Haskett S, Pellerin A, Xu S, Petersen B, et al. BAFF overexpression increases lymphocytic infiltration in Sjögren’s target tissue, but only inefficiently promotes ectopic B-cell differentiation. Clin Immunol Orlando Fla. 2016;169:69–79.
Naradikian MS, Perate AR, Cancro MP. BAFF receptors and ligands create independent homeostatic niches for B cell subsets. Curr Opin Immunol. 2015;34:126–9.
Szodoray P, Alex P, Jonsson MV, Knowlton N, Dozmorov I, Nakken B, et al. Distinct profiles of Sjögren’s syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin Immunol Orlando Fla. 2005;117:168–76.
Sharma A, Kiripolsky J, Klimatcheva E, Howell A, Fereidouni F, Levenson R, et al. Early BAFF receptor blockade mitigates murine Sjögren’s syndrome: concomitant targeting of CXCL13 and the BAFF receptor prevents salivary hypofunction. Clin Immunol. 2016;164:85–94.
Carubbi F, Cipriani P, Di Benedetto P, Ruscitti P, Alunno A, Gerli R, et al. Persistence of focal lymphocytic sialadenitis in patients with primary Sjögren’s syndrome treated with rituximab: a possible role for glandular BAFF. Clin Exp Rheumatol. 2016;34:1123–4.
Navarro-Mendoza EP, Aguirre-Valencia D, Posso-Osorio I, Correa-Forero SV, Torres-Cutiva D-F, Loaiza D, et al. Cytokine markers of B lymphocytes in minor salivary gland infiltrates in Sjögren’s syndrome. Autoimmun Rev. 2018;17:709–14.
Baldini C, Ferro F, Elefante E, Bombardieri S. Biomarkers for Sjögren’s syndrome. Biomark Med. 2018;12:275–86.
Sisto M, Lorusso L, Lisi S. TLR2 signals via NF-κB to drive IL-15 production in salivary gland epithelial cells derived from patients with primary Sjögren’s syndrome. Clin Exp Med. 2017;17:341–50.
Sisto M, Lisi S, D’Amore M, Lofrumento DD. The metalloproteinase ADAM17 and the epidermal growth factor receptor (EGFR) signaling drive the inflammatory epithelial response in Sjögren’s syndrome. Clin Exp Med. 2015;15:215–25.
Ng LG, Sutherland APR, Newton R, Qian F, Cachero TG, Scott ML, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173:807–17.
Haacke EA, van der Vegt B, Meiners PM, Vissink A, Spijkervet FKL, Bootsma H, et al. Abatacept treatment of patients with primary Sjögren’s syndrome results in a decrease of germinal centres in salivary gland tissue. Clin Exp Rheumatol. 2017;35:317–20.
Hu S, Wang R, Zhang M, Liu K, Tao J, Tai Y, et al. BAFF promotes T cell activation through the BAFF-BAFF-R-PI3K-Akt signaling pathway. Biomed Pharmacother. 2019;114:108796.
Nezos A, Papageorgiou A, Fragoulis G, Ioakeimidis D, Koutsilieris M, Tzioufas AG, et al. B-cell activating factor genetic variants in lymphomagenesis associated with primary Sjogren’s syndrome. J Autoimmun. 2014;51:89–988.
Papageorgiou A, Mavragani CP, Nezos A, Zintzaras E, Quartuccio L, De Vita S, et al. A BAFF receptor His159Tyr mutation in Sjögren’s syndrome-related lymphoproliferation. Arthritis Rheumatol Hoboken NJ. 2015;67:2732–41.
Thompson N, Isenberg DA, Jury EC, Ciurtin C. Exploring BAFF: its expression, receptors and contribution to the immunopathogenesis of Sjögren’s syndrome. Rheumatol Oxf Engl. 2016;55:1548–55.
Pontarini E, Fabris M, Quartuccio L, Cappeletti M, Calcaterra F, Roberto A, et al. Treatment with belimumab restores B cell subsets and their expression of B cell activating factor receptor in patients with primary Sjogren’s syndrome. Rheumatol Oxf Engl. 2015;54:1429–34.
McWilliams EM, Lucas CR, Chen T, Harrington BK, Wasmuth R, Campbell A, et al. Anti–BAFF-R antibody VAY-736 demonstrates promising preclinical activity in CLL and enhances effectiveness of ibrutinib. Blood Adv. 2019;3:447–60.
Dörner T, Posch MG, Li Y, Petricoul O, Cabanski M, Milojevic JM, et al. Treatment of primary Sjögren’s syndrome with ianalumab (VAY736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann Rheum Dis. 2019;78:641–7.
Ou X, Xu S, Lam K-P. Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proc Natl Acad Sci. 2012;109:15401–6.
Arkatkar T, Jacobs HM, Du SW, Li Q-Z, Hudkins KL, Alpers CE, et al. TACI deletion protects against progressive murine lupus nephritis induced by BAFF overexpression. Kidney Int. 2018;94:728–40.
Romberg N, Chamberlain N, Saadoun D, Gentile M, Kinnunen T, Ng YS, et al. CVID-associated TACI mutations affect autoreactive B cell selection and activation. J Clin Invest. 2013;123:4283–93.
Coquery CM, Erickson LD. Regulatory roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol. 2012;32:287–305.
Jiang C, Loo WM, Greenley EJ, Tung KS, Erickson LD. B cell maturation antigen deficiency exacerbates lymphoproliferation and autoimmunity in murine lupus. J Immunol Baltim Md. 1950;2011(186):6136–47.
Coquery CM, Loo WM, Wade NS, Bederman AG, Tung KS, Lewis JE, et al. BAFF regulates follicular helper T cells and affects their accumulation and interferon-γ production in autoimmunity: BAFF regulates follicular helper T cells. Arthritis Rheumatol. 2015;67:773–84.
Shaker OG, Tawfic SO, El-Tawdy AM, El-Komy MHM, El Menyawi M, Heikal AA. Expression of TNF-α, APRIL and BCMA in Behcet’s disease. J Immunol Res. 2014;2014:380405.
Chang Y, Jia X, Sun X, Xu S, Wu Y, Zhang L, et al. APRIL promotes proliferation, secretion and invasion of fibroblast-like synoviocyte from rats with adjuvant induced arthritis. Mol Immunol. 2015;64:90–8.
Eilertsen GØ, Nossent JC. APRIL levels strongly correlate with IL-17 in systemic lupus erythematosus. Lupus. 2014;23:1383–91.
Lombardi T, Moll S, Youinou P, Pers J-O, Tzankov A, Gabay C, et al. Absence of up-regulation for a proliferation-inducing ligand in Sjögren’s sialadenitis lesions. Rheumatol Oxf Engl. 2011;50:1211–5.
Vosters JL, Roescher N, Polling EJ, Illei GG, Tak PP. The expression of APRIL in Sjogren’s syndrome: aberrant expression of APRIL in the salivary gland. Rheumatol Oxf Engl. 2012;51:1557–622.
Fauchais AL, Martel C, Gondran G, Lambert M, Launay D, Jauberteau MO, et al. Immunological profile in primary Sjögren syndrome: clinical significance, prognosis and long-term evolution to other auto-immune disease. Autoimmun Rev. 2010;9:595–9.
Friebus-Kardash J, Branco L, Ribi C, Chizzolini C, Huynh-Do U, Dubler D, et al. Immune complexes containing serum B-cell activating factor and immunoglobulin G correlate with disease activity in systemic lupus erythematosus. Nephrol Dial Transplant. 2018;33:54–64.
Acknowledgements
This work was supported by the CONACYT (Fondo Sectorial SSA/IMSS/ISSSTE-CONACYT, México-Universidad de Guadalajara) under Grant no. 273324 to EOR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carrillo-Ballesteros, F.J., Palafox-Sánchez, C.A., Franco-Topete, R.A. et al. Expression of BAFF and BAFF receptors in primary Sjögren’s syndrome patients with ectopic germinal center-like structures. Clin Exp Med 20, 615–626 (2020). https://doi.org/10.1007/s10238-020-00637-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-020-00637-0