Abstract
Establishing superior preclinical models is critical for translational cancer research owing to the high failure rates of novel therapeutics in clinical studies. Even though cell line-derived xenograft models are easy to create, they have numerous limitations since these models do not represent the distinctive features of each cancer patient adequately. To circumvent the discrepancies between xenograft models and tumors, patient-derived xenograft (PDX) models have been developed. These models are established through the engraftment of tissue from a patient’s tumor into an immune-deficient mouse, which preserves cell–cell interactions and tumor microenvironment. Since PDXs precisely replicate intratumoral heterogeneity, a range of chemotherapeutic agents can be tested on individual tumors. Colorectal cancer represents a unique case to demonstrate clinical perspectives revealed by PDX models since they surmount limitations of conventional ex vivo models. Even though PDX models have been associated with drawbacks with respect to prediction of clinical outcomes, they are currently the model of choice for preclinical investigations in colorectal cancer. In the current review, we provide an overview of the methodology and applications of PDX for colorectal cancer and discuss critical issues for the advancement of these models for preclinical research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is among the most common forms of cancer and is globally ranked fourth as the leading cause of mortality due to cancer. By 2030, CRC is projected to cause approximately 2.2 million new cases and 1.1 million deaths [1]. Treatment modalities mainly involve surgery, chemotherapy and radiotherapy. On the other hand, these modalities lack selectivity and may cause many adverse effects [2]. Hence, researchers are exploring new molecular-based agents with high potency, efficacy and safety outcomes. Like with other cancer types, detecting CRC cancer in early stages leads to better prognosis [3, 4]. There is a medical need that requires extensive exploration for more efficient therapeutic modalities, and emphasizes on the importance of preclinical CRC models. Cancer cell lines are commonly used for in vitro screening studies of new anticancer agents [5]; however, these cell lines do not imitate the complex tumor heterogeneity observed in clinical results and are not very effective at predicting clinical outcomes for specific cancers [6]. The cell lines undergo genetic, epigenetic and transcriptomic alterations ensuing from their adaptations to grow in artificial cultures [7]. These drawbacks limit the translation of the in vitro outcomes to the clinical response of the tumor in patients. On the other hand, in vivo murine models can be employed to investigate mechanisms of metastasis and to develop new therapeutic strategies [8].
In the last few years, it has become increasingly apparent that each patient’s cancer is unique, and thus, may have varying responses to conventional treatments like chemotherapy and radiation. At present, our cancer healthcare system is experiencing a shift from the traditional “one-size-fits-all” model to personalized medicine or precision medicine [9]. Personalized medicine aids the development of customized treatments for each cancer subtype, based on the patient genetic and omics data (transcriptomics, metabolomics, proteomics, etc.) [10]. This helps healthcare providers to discover more effective strategies for prevention, screening and treatment based on accurate estimations of individual’s risks. Personalized treatments may cause fewer adverse effects than conventional methods. Nevertheless, it has to be recognized that different omics approaches are still underdeveloped: After genomics, metabolomics and microbiome profiling are the closest to enter clinical practice [11].
Patient-derived xenograft models of CRC with high histological and heterogeneous fidelity
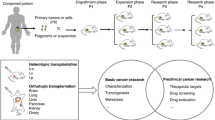
Patient-derived xenografts (PDXs) are models derived from the primary tumor of a patient with either primary or metastatic disease. The tissues are removed through resection surgery and implanted into an immune-deficient mouse via subcutaneous or orthotopic implantation (Table 1) [12]. Models that can overcome the limitations of cell lines and introduce an accurate prediction of the bioactivity of the tumor progression, and their response to some treatments will enable more accurate development of future therapies [13]. Several reports have confirmed high levels of concordance between the manifestation of primary tumor and its genetic variations across diverse genetic and stromal composition against the PDX models [14,15,16,17]. These include similarity in the molecular characterization of the CRC tabulating the key frequencies and genetic mutations modeled by PDX [18]. Additionally, PDX models which are created close to the time of procurement are ideal due to minimal changes to the cell lines.
Engrafting intact tumor fragments is more commonly used for PDX models of CRC, mainly because of the conservation of the stromal architectures of the tumor. In specific cases, Matrigel matrix (a gelatinous mixture consisting of collagen, laminin, proteoglycans and entactin, mimicking the extra-cellular matrix) is used in order to provide more support to tumor fragments during the implantation [19]. Furthermore, many Matrigel matrixes contain growth factors to enhance the proliferation and differentiation of the tissues [20]. It has been demonstrated that, when PDX models are created using isolated primary cells, tumor heterogeneity is enhanced compared to established cell lines. Even though strain variations do not interfere with the engraftment efficiency, they may affect the rate of tumor growth, especially in the case of the solid tumors [21].
Typically, direct implantation of the tumor fragments conserves the 3D structures and preserves the vital cell–cell interactions. Determining the most appropriate method for PDX (direct implantations or cell suspensions) mainly depends on the aim of the research project [3]. Even though direct implantation of the tumor specimens preserves the biopsy architecture, the overall tumor structure may vary, particularly when some specimens are surgically resected after the treatment. On the other hand, the tumor dissociation introduces a proportionate implementation method that emphasizes on transferring an appropriate amount of viable tissues. Furthermore, the various parts of the tumor of the same patient may differ in the genetic features, which will interfere with the disease course. So, the use of the isolated cells can solve this situation, in which the loss of tissue architecture will largely interfere with the drugs research studies [3].
Many papers have also demonstrated that the engraftment efficiency may be linked to the severity of the primary disease, as the cases with advanced disease show superior engraftment rates [20]. As far as the implantation sites are concerned, PDX models of CRC have been both heterotopic and orthotopic; however, the most common site has been the heterotopic subcutaneous flank due to ease of access. Although more difficult, orthotopic engraftment studies allow the analysis of the metastatic lesions [22]. Mainly, the transplantation near the colon will result in growth in the primary site and the metastatic sites, namely to the lungs, lymph nodes and liver [23,24,25]. Successful engraftments of orthotopic models require a high level of technical modalities, as the tumor tissues should be carefully implanted to the outer wall of the colon [20]. After the resection of the xenografts, specimens are mainly frozen in liquid nitrogen and will be stored in order to be used later, or it may be chemically fixed in formalin for further histological tests. The next essential step during establishment of PDX models is to confirm the presence of clinical CRC hallmarks. Mostly, this is achieved by histopathological testing, in addition to mutational analysis in order to compare the patient’s biopsies to the xenograft tumors.
Clinical applications: CRC classification for targeted treatment via PDX
The importance of the PDX models lies in their accurate representativeness of the disease phases and progression mechanisms of tumors. Most of the published papers have focused on the importance of molecular and genetic markers in the assessment of the cytotoxic therapies [26]. So, exploration of treatment modalities needs the total uncovering of the profiles of these genetic mutations. Creating individual-specific PDX models for the clinical decision making for these patients is not practical due to the prolonged engraftment times, but it is feasible to compare the clinical outcomes with these individualized models in order to assess each patient’s treatment strategy. Moreover, these models could then be treated with newer therapeutic agents, thereby allowing researchers to test novel treatment modalities.
CRC can manifest itself in quite a heterogeneous manner, depending on proteome, clonal, genetic and other factors [27]. Targeted treatment of the disease can be performed if different variations and their response to treatments are characterized. Since a study can be carried out much faster and more easily with PDX models, several variations based on histopathology, proteome, immuno response and microbiome have been reported [28, 29].
Recently, five CRC intrinsic subtypes (CRIS) have been reported, namely: (1) CRIS-A: mucinous, glycolytic, enriched for MSI or KRAS mutations; (2) CRIS-B: transforming growth factor β (TGF-β) pathway activity, epithelial–mesenchymal transition (EMT), (3) CRIS-C: elevated EGFR signaling, sensitivity to EGFR inhibitors; (4) CRIS-D: WNT activation, IGF2 gene overexpression and amplification; and (5) CRIS-E: Paneth cell-like phenotype, TP53 mutations. These specifically focused on the genetic expression of the cancer cells using human-specific assays and filtering the stromal variations using murine components in the PDX model [30].
Recent advances in the molecular targets and methodologies for patient-derived xenograft models
PDX models are useful in the therapeutic assessment of tumors with high molecular aggression and variation between patients. Essentially, they need to accommodate the tumor heterogeneity that is observed in different cancer patients. Preferably, PDX models should also be complementary in nature to preclinical models, especially for the assessment of therapeutic efficiency [31]. High concordance found in results of PDX models and clinical responses in CRC has allowed the usage of these models to study newer therapeutic targets [15, 32].
Moreover, an evaluation method used the primary tumor cells that were xenografted in nude mice subcutaneously (patient-derived spheroid xenografts) while also preparing PDX from the same tumors. This approach compared the dosing and outcome on tumor elimination [33]. It was shown that the method that used patient-derived spheroid xenografts was more accurate in predicting tumor growth with less variability with the conventional PDX method, also in drug dosing tests, while the former had also stronger statistical power [34].
Another in vivo PDX study with tumor tissue on chorioallontoic membrane showed that there was a resemblance to the patient tumor with increased vascularity following engraftment with micrometastasis in the chick mesenchyme. This approach allows RNA- and DNA-based sequencing of patient tumors, with efficient (5–10 days) testing of multiple therapies on the same tumor and can thus be described as an efficient precision medicine platform. However, it is to be noted that understanding of its stromal environment and interaction with the avian immune system needs further exploration [31].
HER2 expression in colon cancer was studied using a PDX mouse model created by grafting of tumor in nonobese diabetic (NOD)/Prkdcscid/IL-2Rγnull (NPG) mice. In this preclinical development, HER2-specific chimeric antigen receptor T cells were shown to result in regression and even elimination of the CRC xenograft with an apparent survival advantage observed in the mice when compared to the mice transplanted with green fluorescent protein T cells. This established that the chimeric antigen receptor T cell treatment may be a promising therapy for solid tumor clearance and that the PDX model is an effective tool to study the effects of chimeric antigen receptor T cells [35].
A recent analysis demonstrated that one of the most apparent CRC molecular subtypes is the epithelial subtype, which is shown to be overall ineffective in establishing PDX models. The study also concluded that tumor cell proliferation was linked to the successful establishment of PDX models and could distinguish between the patients with poor clinical outcomes within consensus molecular subtype 2 (CMS2) group, which is strongly underrepresented. Notably, there was a good relation between proliferation and recurrence rate specifically in the CMS2 group, and there is an assumption that it may not be a homogeneous subtype [36]. Genetic alterations of three primary CRCs and xenograft tissues in PDX showed that there was a preservation of key mutations detected in the primary tumor site, with 14 out of 17 somatic mutations identified [37].
PDX: challenges and alternative approaches
PDX models, although valuable tools, have several limitations including being very resource intensive. Models used in immune-deficient mice occasionally create an environment unsuitable to adequately test immunotherapies. Notably, drug performance in the xenografts derived from cancer cell lines is not always directly related to the predictive clinical efficacy. Moreover, human microvasculature and stroma are eventually replaced by the murine counterparts, which may cause difficulties when observing treatments that target microvasculature or stroma. This reveals a requirement for creating a humanized PDX model, whereby native murine immune system is ablated and replaced by human hematopoietic stem cells or tumor-infiltrating lymphocytes into immunodeficient mice. This newer model has the potential to overcome the issues seen in immunodeficiency in spite of the species-specific differences in the immunoresponse and can also be used to comprehensively study the interaction of immune system with the microbiome. This becomes especially relevant in the case of CRC where the human gut microbiome activity is known to accelerate cancer growth [29].
Another emerging methodology to enable personalized therapies is the autologous cell transplantation (ACT), which involves sequential introduction of tumor cells and tumor-infiltrating T cells from the same patient to the mouse model [38]. Jespersen and colleagues reported that PDX models from patients who clinically responded to ACT showed positive reduction in tumor, while in patients who did not show any response, the models were ineffective, showing high correlation between the model and clinical response and opening up possibilities for combination therapy [39].
A co-clinical trial referred to as “Avatar” model has been considered to create personalized medicine regimen [40, 41]. It consists of creating a PDX model from a patient and administering it with the same treatment as offered to the patient. Kopetz et al. studied the Avatar model on patients with metastatic BRAF-mutated CRC and were administered vemurafenib, an oral BRAF inhibitor. By observation of the PDX model, they identified that the apparent cause of acquired resistance was co-occurring KRAS and NRAS mutations at low allele frequency in a subset of the patient tumors (median 0.21% allele frequency), stressing the viability of the Avatar model to create a better and personalized therapeutic regimen [42]. Since drug response can be widely heterogeneous, to overcome the limited number of PDX models possible and achieve high throughput, one possible methodology is to use “one animal per model per treatment” preclinical trial setting as reported by Gao et al. [43], in which 1075 models were evaluated relating to a large number of common solid cancers.
Although very insightful, the PDX-patient co-clinical trial is extremely resource intensive in terms of time, labor and capital. A possible solution lies in creating a model from 3D-cultured multicellular aggregates, grown from the stem cells or isolated organ progenitors of the patient and referred to as patient-derived organoids (PDOs) [44]. PDOs cultured from stem cells retain the characteristics of their parent stroma and have their organizational functionality [45]. PDOs from 71 patients with CRC or gastroesophageal cancers had the molecular signature of the tumor organoids related to drug screening results as there was 88% accuracy of predicting positive drug response and 100% accuracy for predicting whether they would not respond to anticancer drugs [46]. Another study also confirmed the phenotypic and genotypic matching of the PDOs in CRC and gastroesophageal cancer patients (recruited in phase ½ clinical trials), and the result was matched with the drug screening results [47].
In the future, to study the clinical efficacy of novel drugs and different therapies, ideal models should possess the cost and scalability of cancer cell lines while providing the predictive capability of PDXs. 2D and 3D organoids appear to be a possible solution going forward in this direction.
Conclusion
The advanced stages of CRC tumors are very aggressive and represent a notable clinical challenge, in which the low 5-year survival rate for metastatic CRC indicates the essential need for discovering new therapeutic trials and preclinical models. The PDX models of CRC introduce clinically valuable properties and are essential for more individualized strategies of therapy. In addition, the genetic profile of each cancer of each individual should be treated using a personalized strategy. Moreover, the therapeutic strategies have been transitioned from the cytotoxic drugs to more precise and accurate modalities which include the targeted therapies. PDX models of CRC have introduced a promising approach in the development of oncologic drugs. These models present certain limitations, including the use of animals, limited engraftment efficiency and high costs. In this context, patient-derived organoids might be way forward to overcome these limitations.
References
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91. https://doi.org/10.1136/gutjnl-2015-310912.
DeVita VT Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–53. https://doi.org/10.1158/0008-5472.can-07-6611.
Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–7. https://doi.org/10.1055/s-0029-1242458.
Zhang Y, Yuan J, Zhang HY, et al. Natural resistance to apoptosis correlates with resistance to chemotherapy in colorectal cancer cells. Clin Exp Med. 2012;12(2):97–103. https://doi.org/10.1007/s10238-011-0146-5.
Abaan OD, Polley EC, Davis SR, et al. The exomes of the NCI-60 panel: a genomic resource for cancer biology and systems pharmacology. Cancer Res. 2013;73(14):4372–82. https://doi.org/10.1158/0008-5472.can-12-3342.
Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. https://doi.org/10.1158/2159-8290.cd-14-0001.
Lasabova Z, Kalman M, Holubekova V, et al. Mutation analysis of POLE gene in patients with early-onset colorectal cancer revealed a rare silent variant within the endonuclease domain with potential effect on splicing. Clin Exp Med. 2019;19(3):393–400. https://doi.org/10.1007/s10238-019-00558-7.
Oh BY, Hong HK, Lee WY, Cho YB. Animal models of colorectal cancer with liver metastasis. Cancer Lett. 2017;387:114–20. https://doi.org/10.1016/j.canlet.2016.01.048.
Hood L, Flores M. A personal view on systems medicine and the emergence of proactive P4 medicine: predictive, preventive, personalized and participatory. N Biotechnol. 2012;29(6):613–24. https://doi.org/10.1016/j.nbt.2012.03.004.
Krzyszczyk P, Acevedo A, Davidoff EJ, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 2018;6(3–4):79–100. https://doi.org/10.1142/s2339547818300020.
Burney IA, Lakhtakia R. Precision medicine: where have we reached and where are we headed? Sultan Qaboos Univ Med J. 2017;17(3):e255–8. https://doi.org/10.18295/squmj.2017.17.03.001.
Byrne AT, Alferez DG, Amant F, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17(4):254–68. https://doi.org/10.1038/nrc.2016.140.
Galimi F, Torti D, Sassi F, et al. Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer: response to met inhibition in patient xenografts and pathologic correlations. Clin Cancer Res. 2011;17(10):3146–56. https://doi.org/10.1158/1078-0432.ccr-10-3377.
Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1(6):508–23. https://doi.org/10.1158/2159-8290.cd-11-0109.
Julien S, Merino-Trigo A, Lacroix L, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18(19):5314–28. https://doi.org/10.1158/1078-0432.ccr-12-0372.
Burgenske DM, Monsma DJ, Dylewski D, et al. Establishment of genetically diverse patient-derived xenografts of colorectal cancer. Am J Cancer Res. 2014;4(6):824–37.
Fujii M, Shimokawa M, Date S, et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18(6):827–38. https://doi.org/10.1016/j.stem.2016.04.003.
Yao YM, Donoho GP, Iversen PW, et al. Mouse PDX trial suggests synergy of concurrent inhibition of RAF and EGFR in colorectal cancer with BRAF or KRAS mutations. Clin Cancer Res. 2017;23(18):5547–60. https://doi.org/10.1158/1078-0432.ccr-16-3250.
Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–86. https://doi.org/10.1016/j.semcancer.2005.05.004.
Puig I, Chicote I, Tenbaum SP, et al. A personalized preclinical model to evaluate the metastatic potential of patient-derived colon cancer initiating cells. Clin Cancer Res. 2013;19(24):6787–801. https://doi.org/10.1158/1078-0432.ccr-12-1740.
Williams SA, Anderson WC, Santaguida MT, Dylla SJ. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab Invest. 2013;93(9):970–82. https://doi.org/10.1038/labinvest.2013.92.
Fu XY, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88(20):9345–9. https://doi.org/10.1073/pnas.88.20.9345.
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. https://doi.org/10.1016/0092-8674(90)90186-i.
Nakarai C, Osawa K, Akiyama M, et al. Expression of AKR1C3 and CNN3 as markers for detection of lymph node metastases in colorectal cancer. Clin Exp Med. 2015;15(3):333–41. https://doi.org/10.1007/s10238-014-0298-1.
Vanova B, Kalman M, Jasek K, et al. Droplet digital PCR revealed high concordance between primary tumors and lymph node metastases in multiplex screening of KRAS mutations in colorectal cancer. Clin Exp Med. 2019;19(2):219–24. https://doi.org/10.1007/s10238-019-00545-y.
Fichtner I, Slisow W, Gill J, et al. Anticancer drug response and expression of molecular markers in early-passage xenotransplanted colon carcinomas. Eur J Cancer. 2004;40(2):298–307. https://doi.org/10.1016/j.ejca.2003.10.011.
Sareeboot T, Punyarit P, Petmitr S. DNA amplification on chromosome correlated with poor prognosis in colorectal cancer. Clin Exp Med. 2011;11(2):97–103. https://doi.org/10.1007/s10238-010-0107-4.
Qiu Y, Cai G, Zhou B, et al. A distinct metabolic signature of human colorectal cancer with prognostic potential. Clin Cancer Res. 2014;20(8):2136–46. https://doi.org/10.1158/1078-0432.ccr-13-1939.
Nakatsu G, Li X, Zhou H, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727. https://doi.org/10.1038/ncomms9727.
Isella C, Brundu F, Bellomo SE, et al. Selective analysis of cancer-cell intrinsic transcriptional traits defines novel clinically relevant subtypes of colorectal cancer. Nat Commun. 2017;8:15107. https://doi.org/10.1038/ncomms15107.
DeBord LC, Pathak RR, Villaneuva M, et al. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am J Cancer Res. 2018;8(8):1642.
Nunes M, Vrignaud P, Vacher S, et al. Evaluating patient-derived colorectal cancer xenografts as preclinical models by comparison with patient clinical data. Cancer Res. 2015;75(8):1560–6. https://doi.org/10.1158/0008-5472.can-14-1590.
Jung J, Seol HS, Chang S. The generation and application of patient-derived xenograft model for cancer research. Cancer Res Treat Off J Korean Cancer Assoc. 2018;50(1):1.
Maekawa H, Miyoshi H, Yamaura T, et al. A chemosensitivity study of colorectal cancer using xenografts of patient-derived tumor-initiating cells. Mol Cancer Ther. 2018;17(10):2187–96.
Teng R, Zhao J, Zhao Y, et al. Chimeric antigen receptor–modified T cells repressed solid tumors and their relapse in an established patient-derived colon carcinoma xenograft model. J Immunother. 2019;42(2):33.
Prasetyanti PR, van Hooff SR, van Herwaarden T, et al. Capturing colorectal cancer inter-tumor heterogeneity in patient-derived xenograft (PDX) models. Int J Cancer. 2019;144(2):366–71.
Yu SM, Jung S-H, Chung Y-J. Comparison of the genetic alterations between primary colorectal cancers and their corresponding patient-derived xenograft tissues. Genomics Inform. 2018;16(2):30.
De La Rochere P, Guil-Luna S, Decaudin D, Azar G, Sidhu SS, Piaggio E. Humanized mice for the study of immuno-oncology. Trends Immunol. 2018;39(9):748–63. https://doi.org/10.1016/j.it.2018.07.001.
Jespersen H, Lindberg MF, Donia M, et al. Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nat Commun. 2017;8(1):707. https://doi.org/10.1038/s41467-017-00786-z.
Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(34):4023–31. https://doi.org/10.1200/JCO.2015.63.2471.
Malaney P, Nicosia SV, Davé V. One mouse, one patient paradigm: new avatars of personalized cancer therapy. Cancer Lett. 2014;344(1):1–12. https://doi.org/10.1016/j.canlet.2013.10.010.
Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33(34):4032–8. https://doi.org/10.1200/jco.2015.63.2497.
Gao H, Korn JM, Ferretti S, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318–25. https://doi.org/10.1038/nm.3954.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. https://doi.org/10.1126/science.1247125.
van de Wetering M, Francies HE, Francis JM, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–45. https://doi.org/10.1016/j.cell.2015.03.053.
Yang H, Sun L, Liu M, Mao Y. Patient-derived organoids: a promising model for personalized cancer treatment. Oxford: Oxford University Press; 2018.
Vlachogiannis G, Hedayat S, Vatsiou A, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920–6.
Acknowledgements
This work was supported by Zhejiang Provincial Science and Technology Projects (Nos. 2017C37159 to YL)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, J., Lin, Y. Patient-derived xenograft models for personalized medicine in colorectal cancer. Clin Exp Med 20, 167–172 (2020). https://doi.org/10.1007/s10238-020-00609-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-020-00609-4