Abstract
To evaluate potency and safety of 14-day bismuth–furazolidone quadruple regimens and to compare efficacies of five proton pump inhibitors (PPIs) for the initial eradication of Helicobacter pylori (H. pylori), 175 eligible patients were enrolled and randomly assigned to 14-day quadruple regimens consisting of bismuth (400 mg), amoxicillin (1 g), furazolidone (100 mg), and a PPI, twice a day. PPIs used were Group A (pantoprazole capsules, 40 mg), Group B (pantoprazole tablets, 40 mg), Group C (lansoprazole, 30 mg), Group D (esomeprazole, 20 mg), and Group E (rabeprazole, 10 mg). H. pylori status was reassessed by 13C urea breath test on day 56 as the primary outcome. Gastrointestinal symptoms, parenteral side effects, compliance, and stool type were recorded simultaneously. The total eradication rates were 86.9% (152/175 [95% CI 80.9–91.5%]) and 95.6% (152/159 [91.1–98.2%]) by intention-to-treat (ITT) and per-protocol (PP) analysis. The efficacies of Group A, B, C, D, and E by ITT analysis were 91.4% (32/35 [76.9–98.2%]), 85.7% (30/35 [69.7–95.2%]), 88.6% (31/35 [73.3–96.8%]), 85.7% (30/35 [69.7–95.2%]), and 82.9% (29/35 [66.4–93.4%]) (p > 0.05). In the PP analysis, the efficacies were 97.0% (32/33), 93.8% (30/32), 93.9% (31/33), 100% (30/30), and 93.5% (29/31) (p > 0.05). Gastrointestinal symptoms and stool type were improved significantly (p < 0.05). Total side effects rate and poor compliance rate were 15.7% (25/159) and 5.0% (8/159). Fourteen-day bismuth–furazolidone quadruple regimens are of high potency and safety for the initial eradication of H. pylori. Efficacies of different PPIs and different dosages (9–32 mg omeprazole equivalents) showed no significant difference. The appropriate PPI can thus be chosen by clinicians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Helicobacter pylori (H. pylori), a Gram-negative bacterium that colonizes adjacent to the gastric mucosa, is reported to be associated with a range of gastrointestinal diseases, such as gastritis, peptic ulcer, gastric cancer, and mucosa-associated lymphoid tissue lymphoma [1]. The prevalence of H. pylori infection differs from region to region. Nearly half of the world’s population is infected with H. pylori [2]. Although the prevalence of H. pylori infection has decreased on a global scale, it remains a serious concern in China with a prevalence of 66% among rural populations and 47% among urban populations [3]. The Kyoto global consensus report on H. pylori gastritis has defined it as an infectious disease and suggested that all populations infected with H. pylori should receive eradication treatment [4]. The recent fifth Chinese national consensus report on the management of H. pylori infection, in accordance with the Maastricht V/Florence consensus report, recommended a test-and-treat strategy for uninvestigated dyspepsia patients for the consideration of regional prevalence and cost–benefit ratio [5, 6]. Therefore, the selection of a cost-effective and safe regimen for the initial eradication of H. pylori is urgently needed in China.
Standard triple regimens failed to reach 80% efficacy due to antibiotic resistance, poor compliance, high gastric acidity, high bacterial load, and cytochrome P450 2C19(CYP2C19) polymorphism [7]. Resistance rates toward clarithromycin, metronidazole, levofloxacin, amoxicillin, and furazolidone in the southeast coastal region of China were 21.5, 95.4, 20.6, 0.1, and 0.1%, respectively [8]. A recent multi-region prospective 7-year study in China reported no resistance toward furazolidone among H. pylori strains isolated from 1117 patients [9]. As a broad-spectrum nitrofuran antimicrobial, furazolidone is not available in many western countries due to concern of potential carcinogenicity. However, the carcinogenic effects of furazolidone have only been observed in animals. The WHO listed furazolidone as a Class III carcinogen, and there is no direct evidence to support its carcinogenic effect in humans [10]. Besides, bismuth can eradicate H. pylori and synergize with other antimicrobials as a topical antimicrobial [11]. Based on the supply and availability of bismuth and furazolidone, bismuth–furazolidone quadruple regimen consisting of a proton pump inhibitor (PPI), amoxicillin, furazolidone, and bismuth is the most popular regimen in the southeast coastal region of China among the seven regimens recommended by the fifth Chinese national consensus report.
As a component of bismuth-containing quadruple regimens, PPIs play an important role in the eradication of H. pylori. Different drugs, doses, dosing intervals, dosing in relation of meals, formulations, frequencies and therapy durations are factors that influence the outcome of eradication therapy [11, 12]. Numerous studies have reported a comparison among efficacies of two or three PPIs in standard triple regimens [13,14,15,16,17]; however, prospective clinical trial studying the efficacies of four or more PPIs in bismuth–furazolidone quadruple regimens is still lacking. In this study, we prospectively compared the efficacies of five PPIs in bismuth–furazolidone quadruple regimens (PPI + amoxicillin + furazolidone + bismuth). Simultaneously, changes in gastrointestinal symptoms and stool type after the eradication of H. pylori were recorded. Parenteral side effects and compliance with different regimens were compared.
We aimed to evaluate the potency and safety of 14-day bismuth–furazolidone quadruple regimens for the initial eradication of H. pylori and to provide credible evidence to justify the clinical use of different PPIs.
Methods
Patients
This prospective, open-label, randomized trial (registration number: ChiCTR-IPR-16010286, http://www.chictr.org.cn/showproj.aspx?proj=17499) was performed at Sir Run Run Shaw Hospital, Zhejiang Province, China from December 2016 to August 2017. Research data were stored and shared in ResMan Research Manager. Patients aged between 18 and 70 years were enrolled if they were diagnosed positive for H. pylori gastritis in the past month by gastroscopy and histological examination. Patients with any of the following criteria were excluded from this study: previous H. pylori eradication treatment failure; confirmed or suspected upper gastrointestinal malignant tumor as identified by gastroscopy and tissue biopsy; peptic ulcer or other upper gastrointestinal lesions; the use of antacids or gastric mucosal protective agents in the past 2 weeks; the use of antibiotics or probiotics in the past month; known allergy to drugs in this study; a history of gastric surgery or intestinal disease; decompensated cardiac, liver, renal, or pulmonary illness; thyroid disease or diabetes mellitus; pregnant and lactating women; and non-cooperative patients.
Procedure
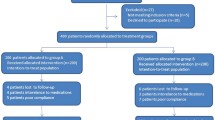
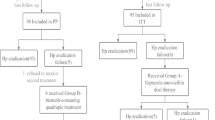
In total, 175 eligible patients were enrolled and randomly assigned to receive one of the five 14-day bismuth–furazolidone quadruple regimens. The randomization sequence was generated by a computer algorithm. The quadruple regimens included PPI + amoxicillin (1000 mg, b.i.d) + furazolidone (100 mg, b.i.d) + colloidal bismuth pectin (0.4 g, b.i.d). The PPIs used in the five regimens were Group A (SOMAC, pantoprazole sodium enteric-coated capsules; Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd.; 40 mg, b.i.d), Group B (Pantoloc, pantoprazole enteric-coated tablets; Takeda GmbH, Oranienburg; 40 mg, b.i.d), Group C (Takepron, lansoprazole enteric-coated capsules; Takeda Pharmaceutical Co., Ltd.; 30 mg, b.i.d), Group D (Nexium, esomeprazole magnesium enteric-coated tablets; AstraZeneca Pharmaceutical Co., Ltd.; 20 mg, b.i.d), and Group E (Pariet, rabeprazole sodium enteric-coated tablets; Eisai Co., Ltd.; 10 mg, b.i.d). H. pylori status was reassessed by 13C urea breath test 6 weeks after the completion of treatment (day 56) as the primary outcome of this study; H. pylori-negative status indicated the success of eradication treatment. Gastrointestinal symptoms in patients were assessed by investigators on day 0 and day 56 using a simplified Gastrointestinal Symptom Rating Scale (GSRS). The simplified GSRS includes 15 items, and each item contains scores from 0 to 3(0 for none, 1 for mild, 2 for moderate, and 3 for severe). The Bristol Stool Scale (BSS) was used to record chief stool type in the previous month on day 0 and day 56. Type 1 to type 7 transited gradually from hard stool to liquid stool, type 4 being the optimal stool type. Parenteral adverse effects and compliance were recorded simultaneously (Fig. 1). Patients who took less than 80% of the total dose were poorly compliant.
Study design. R, randomly assigned; SOMAC, pantoprazole sodium enteric-coated capsules; Pantoloc, pantoprazole enteric-coated tablets; Takepron, lansoprazole enteric-coated capsules; Nexium, esomeprazole magnesium enteric-coated tablets; Pariet, rabeprazole sodium enteric-coated tablets; UBT, 13C urea breath test; GSRS, gastrointestinal symptom rating scale; BSS, bristol stool scale
Statistical analysis
The primary outcome was assessed by intention-to-treat (ITT) and per-protocol (PP) analysis. All patients who violated the protocol, such as those lost to follow-up or withdrew, were excluded from the PP analysis. In the ITT analysis, patients who dropped out were considered as treatment failures. Categorical data were analyzed by χ2 test or Fisher’s exact test, and 95% confidence intervals were calculated for ITT and PP analyses. Baseline continuous data were presented as mean ± standard deviation (SD) and were analyzed by one-way ANOVA. GSRS scores were presented as the mean ± standard error (SE). Absolute values of BSS scores subtracting 4 were taken and reported as the mean ± standard error (SE). Differences in the two scores above-mentioned between day 0 and day 56 were both compared first by all subjects and then by subgroups with Wilcoxon signed rank test. All statistical tests were two-tailed; p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patient data
In total, 175 eligible patients (73 male/102 female) were enrolled and randomly assigned to five bismuth–furazolidone quadruple regimens. Each regimen included 35 patients at baseline. Finally, 159 patients (90.9%) completed the study and 16 patients (9.1%) dropped out (Fig. 2). No significant difference was observed among the five groups with regard to baseline characteristics (Table 1).
Eradication rates of different regimens
The total eradication rate of the 14-day bismuth–furazolidone quadruple regimens was 86.9% (152/175 [95% CI 80.9–91.5%]) by ITT analysis and 95.6% (152/159 [91.1–98.2%]) by PP analysis. The eradication rates of the five regimens by ITT analysis were as follows: SOMAC, 91.4% (32/35 [76.9–98.2%]); Pantoloc, 85.7% (30/35 [69.7–95.2%]); Takepron, 88.6% (31/35 [73.3–96.8%]); Nexium, 85.7% (30/35 [69.7–95.2%]); Pariet, 82.9% (29/35 [66.4–93.4%]). In the PP analysis, the eradication rates were as follows: SOMAC, 97.0% (32/33 [84.2–99.9%]); Pantoloc, 93.8% (30/32 [79.2–99.2%]); Takepron, 93.9% (31/33 [79.8–99.3%]); Nexium, 100% (30/30 [88.4–100%]); Pariet, 93.5% (29/31 [78.6–99.2%]). No significant difference was observed in both the ITT analysis and PP analysis (Table 2).
Dose–response effect of different PPIs
All of the PPIs used in our study were converted to omeprazole equivalents based on research on PPI equivalents [18, 19]. 40 mg pantoprazole, 10 mg rabeprazole, 30 mg lansoprazole and 20 mg Nexium were equivalent to 9, 18, 27 and 32 mg omeprazole equivalent, respectively. Finally, efficacies of 9, 18 mg and approximately 30 mg omeprazole equivalents were compared and no significant difference was observed (Table 3).
Change of gastrointestinal symptoms and stool type after eradication treatment for H. pylori
The total GSRS score decreased significantly from day 0 to day 56(5.48 ± 0.32 vs. 2.76 ± 0.24, p < 0.05), and 12 of 15 gastrointestinal symptoms were observed to have prominent remission (p < 0.05). Only the scores of increased flatus (p = 0.050), hard stools (p = 0.105) and urgent need for defecation (p = 0.280) decreased without statistical significance (Table 4). Detailed symptom changes in each group were listed in Supplementary material 1. Lansoprazole and rabeprazole were observed to relieve more symptoms than pantoprazole and esomeprazole. Furthermore, the stool type was improved distinctly after eradication treatment when compared to baseline by all subjects (p < 0.01) (Table 5). When compared in each group, Group B (Pantoloc, pantoprazole enteric-coated tablets) reported most prominent stool improvement.
Parenteral adverse effects and compliance
The rate of total parenteral adverse effects was 15.7% (25/159), and a significant difference was observed among the five groups (p = 0.018). In terms of individual parenteral adverse effects, the rates of fever were significantly different among the different groups (p < 0.05) (Table 6). Further subgroup analysis revealed that the rate of parenteral adverse effects was higher with SOMAC (33.3%) than Takepron (6.1%, p = 0.005), Nexium (10.0%, p = 0.026) and Pariet (9.7%, p = 0.022). The parenteral adverse effects in this study included pruritus, neuritis, headache, dizziness, and fever. Pruritus occurred most frequently in this study. These adverse effects were self-limited. The total rate of poor compliance was 5.0% (8/159) and different regimens showed similar outcome (Table 6).
Discussion
Because standard triple therapies have failed to achieve acceptable efficacies due to the high rate of antibiotic resistance, a 14-day bismuth-containing quadruple regimen was recommended as first-line therapy for the initial eradication of H. pylori. According to a real-world Asia–Pacific H. pylori survey, it is the most commonly used regimen in China [20]. A recent large-sample study, which investigated the susceptibility of 16 antibiotics in China, reported that resistance rates toward amoxicillin and furazolidone were 1.58 and 1.49%, respectively; however, resistance rates toward clarithromycin and metronidazole were 22.73 and 92.53%, respectively [21]. Furazolidone was more frequently used in China and Iran. Although 200 mg b.i.d was the recommended dosage of furazolidone in furazolidone–bismuth quadruple therapy in Iran, 14-day regimens including 100 mg q.i.d furazolidone and 250 mg q.i.d tetracycline achieved the optimal outcome with a success rate of 94.5% by ITT and PP analyses in Iran [12]. Lessons from China showed that best 14-day furazolidone–bismuth quadruple regimen consisting of 100 mg t.i.d furazolidone and 1 g t.i.d amoxicillin could achieve a cure rate of 95.2% by ITT analysis and 99.0% by PP analysis [22]. A satisfactory outcome was observed with a total eradication rate of 86.9% by ITT analysis and 95.6% by PP analysis for initial eradication treatment in our study, revealing that 100 mg b.i.d furazolidone and 1 g b.i.d amoxicillin were adequate to eradicating H. pylori with appropriate regimens. Furthermore, there are still very few studies of colloidal bismuth pectin and the comparison between different bismuth preparations is also far from adequate. Only a study from China compared the efficacies of colloidal bismuth pectin and colloidal bismuth subcitrate by year 1999 [23]. Accordingly, our study confirmed that twice-daily 14-day bismuth–furazolidone quadruple regimens incorporating 1 g amoxicillin, 100 mg furazolidone, 400 mg colloidal bismuth pectin and a PPI were of high potency. However, Dore et al. [24] found that 10-day bismuth-containing quadruple regimens were not inferior to 14-day bismuth-containing quadruple regimens. A clinical trial conducted in China [25] reported that 10-day bismuth–furazolidone quadruple therapy was effective for H. pylori infection. Therefore, further studies are needed to identify the most cost-effective duration of bismuth–furazolidone quadruple treatment.
PPI is another vital component of bismuth-containing quadruple regimens apart from antibiotics and bismuth. PPIs that target the gastric H+/K+-ATPase were first introduced in 1989 [7]. They inhibit gastric acid secretion and increase gastric pH. They contribute to the eradication of H. pylori by lowering the minimum inhibitory concentration [26] and increasing the stability and transport of antibiotics from the plasma to the gastric mucosa, thereby increasing the concentration of antibiotics and increasing the sensitivity of H. pylori to antibiotics [27,28,29]. These effects mainly depend on the dosage of PPIs and CYP2C19 polymorphism, which is closely associated with their metabolism [7, 30].
In our study, the efficacies of four regimens were acceptable by ITT analysis according to the evaluation system proposed by Graham DY [31]. The eradication rate of the Pariet-bismuth quadruple regimen was 82.9% by ITT analysis and 93.5% by PP analysis. However, the efficacies of five PPI-based bismuth-containing quadruple regimens in this study were not significantly different by ITT or PP analysis. In addition, the efficacy of rabeprazole has been reported to be similar to those of other PPIs [15, 32]. In order to further explore the relationship between PPI dosage and eradicating rate, we converted dosages of PPIs to omeprazole equivalents. The outcome showed that dosage didn’t significantly influence the eradicating rate in the range of 9–32 mg omeprazole equivalent. Therefore, the efficacies of the five PPIs in bismuth-containing quadruple regimens studied may be considered equivalent. As the primary outcome is reliable, there is no need to double the dosages of PPIs considering the economic burden of patients.
As gastrointestinal symptoms and microbiota dysbiosis are commonly reported side effects of the eradication treatment of H. pylori, we specifically compared gastrointestinal symptoms and stool type before and after eradication treatment. Total GSRS scores and the majority of the gastrointestinal symptoms significantly improved on day 56 (p < 0.05), indicating that the eradication treatment is effective for alleviating symptoms of H. pylori gastritis. Interestingly, most symptoms were strikingly relieved with Pariet-bismuth quadruple regimen although the eradication rate of this regimen was lowest in this study. Meanwhile, least symptoms were reported to be relieved with Nexium-bismuth quadruple regimen while this regimen achieved 100% eradication rate by PP analysis. A study in Turkey [33] compared symptomatic relief after standard triple therapy with that after sequential therapy using GSRS, and gastrointestinal symptoms were significantly resolved after 12 months. Our study is limited by its short observation period. We observed a significant improvement in the stool type (p = 0.003), especially diarrhea. Our analysis was supported by changes in the GSRS scores, which showed that frequencies of increased passage of stools, decreased passage of stools, loose stools, and the feeling of incomplete evacuation were decreased after eradication therapy. This outcome alleviated our concerns regarding gut microbiota dysbiosis after 14-day treatment with antibiotics. However, further studies are needed to evaluate specific changes in gut microbiota.
Side effects and compliance are key points that influence eradication success. Side effects of furazolidone and bismuth are always an issue in the process of H. pylori eradication therapy. Totally 25 patients (15.7%) reported parenteral side effects in this study, and they differed significantly among the five regimens. The side effects rate was between that reported in similar studies from Iran (approximately 38%) and China (9.4%) [34, 35]. Further analysis revealed that patients assigned to SOMAC-bismuth quadruple regimen experienced parenteral side effects at a higher frequency than those assigned to Takepron-, Nexium- and Pariet-bismuth quadruple regimens. However, patient compliance in this study was satisfactory despite the adverse effects. Only 5% of the patients were poorly compliant, and this rate did not differ significantly among the different regimens. The good compliance might have been partly due to good health education as the effect of furazolidone can be influenced by some drugs and foods. We informed the participants of possible adverse effects and the importance of good compliance at baseline. Additionally, it is noteworthy that the SOMAC-bismuth quadruple regimen was effective despite parenteral adverse effects. The application of this regimen can significantly reduce the cost of medical treatment and increase the treatment rate. Therefore, further studies on the efficacy and safety of the SOMAC-bismuth quadruple regimen in China are worthwhile.
Our study is limited in some aspects. The sample size of each of the five compared regimens was limited. Additionally, this study was not double-blind. The observation period was short, and further studies are needed to confirm the optimal duration of eradication treatment.
Conclusions
Fourteen-day bismuth–furazolidone quadruple regimens are of high potency and safety in the southeast coastal region of China. Efficacies of different PPIs and different dosages ranging from 9 to 32 mg omeprazole equivalents for the initial eradication of H. pylori showed no significant difference. The appropriate PPI can thus be chosen by the clinician. Further large-scale study is needed to explore the long-term effect of bismuth–furazolidone quadruple regimens and optimize the duration of treatment.
Abbreviations
- H. pylori :
-
Helicobacter pylori
- PPI:
-
Proton pump inhibitor
- GSRS:
-
Gastrointestinal symptom rating scale
- BSS:
-
Bristol stool scale
- ITT:
-
Intention-to-treat
- PP:
-
Per-protocol
- BMI:
-
Body mass index
- b.i.d:
-
Bis in diē/twice a day
- t.i.d:
-
Ter in diē/three times a day
- q.i.d:
-
Quater in diē/four times a day
References
Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–39. https://doi.org/10.1038/ajg.2016.563.
Cave DR. Transmission and epidemiology of Helicobacter pylori. Am J Med. 1996;100(5A):12S–8S Discussion 7S-8S.
Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:8. https://doi.org/10.1186/s13099-016-0091-7.
Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67. https://doi.org/10.1136/gutjnl-2015-309252.
Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30. https://doi.org/10.1136/gutjnl-2016-312288.
Chinese Society of G, Chinese Study Group on Helicobacter p, Peptic U, Liu G, Xie J, Lu ZR, et al. Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Zhonghua Nei Ke Za Zhi. 2017;56(7):532–45. https://doi.org/10.3760/cma.j.issn.0578-1426.2017.07.014.
Kuo CH, Lu CY, Shih HY, Liu CJ, Wu MC, Hu HM, et al. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol. 2014;20(43):16029–36. https://doi.org/10.3748/wjg.v20.i43.16029.
Su P, Li Y, Li H, Zhang J, Lin L, Wang Q, et al. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013;18(4):274–9. https://doi.org/10.1111/hel.12046.
Liu DS, Wang YH, Zeng ZR, Zhang ZY, Lu H, Xu JM, et al. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: a multi-region prospective 7-year study. Clin Microbiol Infect. 2017. https://doi.org/10.1016/j.cmi.2017.11.010.
Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol. 2012;18(1):1–2. https://doi.org/10.4103/1319-3767.91724.
Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol. 2013;25(10):1134–40. https://doi.org/10.1097/MEG.0b013e3283633b57.
Mohammadi M, Attaran B, Malekzadeh R, Graham DY. Furazolidone, an underutilized drug for H. pylori eradication: lessons from Iran. Dig Dis Sci. 2017;62(8):1890–6. https://doi.org/10.1007/s10620-017-4628-5.
Catalano F, Terminella C, Branciforte G, Bentivegna C, Brogna A, Scalia A. Eradication therapy with rabeprazole versus omeprazole in the treatment of active duodenal ulcer. Digestion. 2002;66(3):154–9. https://doi.org/10.1159/000066756.
Choi HS, Park DI, Hwang SJ, Park JS, Kim HJ, Cho YK, et al. Double-dose, new-generation proton pump inhibitors do not improve Helicobacter pylori eradication rate. Helicobacter. 2007;12(6):638–42. https://doi.org/10.1111/j.1523-5378.2007.00556.x.
Lee VW, Chau TS, Chan AK, Lee KK, Waye MM, Ling TK, et al. Pharmacogenetics of esomeprazole or rabeprazole-based triple therapy in Helicobacter pylori eradication in Hong Kong non-ulcer dyspepsia Chinese subjects. J Clin Pharm Ther. 2010;35(3):343–50. https://doi.org/10.1111/j.1365-2710.2009.01088.x.
Pan X, Li Y, Qiu Y, Tang Q, Qian B, Yao L, et al. Efficacy and tolerability of first-line triple therapy with levofloxacin and amoxicillin plus esomeprazole or rabeprazole for the eradication of Helicobacter pylori infection and the effect of CYP2C19 genotype: a 1-week, randomized, open-label study in Chinese adults. Clin Ther. 2010;32(12):2003–11. https://doi.org/10.1016/j.clinthera.2010.11.005.
Keum B, Lee SW, Kim SY, Kim JM, Choung RS, Yim HJ, et al. Comparison of Helicobacter pylori eradication rate according to different PPI-based triple therapy–omeprazole, rabeprazole, esomeprazole and lansoprazole. Korean J Gastroenterol = Taehan Sohwagi Hakhoe chi. 2005;46(6):433–9.
Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2017. https://doi.org/10.1016/j.cgh.2017.09.033.
Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65(1):19–31. https://doi.org/10.1007/s00228-008-0576-5.
Chuah YY, Wu DC, Chuah SK, Yang JC, Lee TH, Yeh HZ, et al. Real-world practice and expectation of Asia-Pacific physicians and patients in Helicobacter pylori eradication (REAP-HP survey). Helicobacter. 2017. https://doi.org/10.1111/hel.12380.
Shao Y, Lu R, Yang Y, Xu Q, Wang B, Ye G. Antibiotic resistance of Helicobacter pylori to 16 antibiotics in clinical patients. J Clin Lab Anal. 2017. https://doi.org/10.1002/jcla.22339.
Liang X, Xu X, Zheng Q, Zhang W, Sun Q, Liu W, et al. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol. 2013;11(7):802–7. https://doi.org/10.1016/j.cgh.2013.01.008.
Nie Y, Li Y, Wu H, Sha W, Du H, Dai S, et al. Colloidal bismuth pectin: an alternative to bismuth subcitrate for the treatment of Helicobacter pylori–positive duodenal ulcer. Helicobacter. 1999;4(2):128–34.
Dore MP, Farina V, Cuccu M, Mameli L, Massarelli G, Graham DY. Twice-a-day bismuth-containing quadruple therapy for Helicobacter pylori eradication: a randomized trial of 10 and 14 days. Helicobacter. 2011;16(4):295–300. https://doi.org/10.1111/j.1523-5378.2011.00857.x.
Lu Z, Xie Y, Lu N, Zhou H, Liu Z, Zhu X, et al. Efficacy of triple versus quadruple furazolidone-based eradication regimens for Helicobacter pylori infection. Zhonghua Yi Xue Za Zhi. 2014;94(8):572–5.
Labenz J. Current role of acid suppressants in Helicobacter pylori eradication therapy. Best Pract Res Clin Gastroenterol. 2001;15(3):413–31. https://doi.org/10.1053/bega.2001.0188.
Kita T, Tanigawara Y, Aoyama N, Hohda T, Saijoh Y, Komada F, et al. CYP2C19 genotype related effect of omeprazole on intragastric pH and antimicrobial stability. Pharm Res. 2001;18(5):615–21.
Gisbert JP. Potent gastric acid inhibition in Helicobacter pylori eradication. Drugs. 2005;65(Suppl 1):83–96.
Calvet X, Gomollon F. What is potent acid inhibition, and how can it be achieved? Drugs. 2005;65(Suppl 1):13–23.
Sahara S, Sugimoto M, Uotani T, Ichikawa H, Yamade M, Iwaizumi M, et al. Twice-daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19 rapid metabolisers compared with twice-daily omeprazole, rabeprazole or lansoprazole. Aliment Pharmacol Ther. 2013;38(9):1129–37. https://doi.org/10.1111/apt.12492.
Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12(2):177–86. https://doi.org/10.1016/j.cgh.2013.05.028 Discussion e12-3.
Liu MK, Wu IC, Lu CY, Kuo CH, Yu FJ, Liu CJ, et al. Randomized trial comparing rabeprazole- versus lansoprazole-based Helicobacter pylori eradication regimens. Kaohsiung J Med Sci. 2013;29(7):379–84. https://doi.org/10.1016/j.kjms.2012.11.006.
Sarikaya M, Dogan Z, Ergul B, Filik L. Functional dyspepsia symptom resolution after Helicobacter pylori eradication with two different regimens. Prz Gastroenterol. 2014;9(1):49–52. https://doi.org/10.5114/pg.2014.40851.
Mokhtare M, Hosseini V, Tirgar Fakheri H, Maleki I, Taghvaei T, Valizadeh SM, et al. Comparison of quadruple and triple Furazolidone containing regimens on eradication of Helicobacter pylori. Med J Islam Repub Iran. 2015;29:195.
Xie Y, Zhu Y, Zhou H, Lu ZF, Yang Z, Shu X, et al. Furazolidone-based triple and quadruple eradication therapy for Helicobacter pylori infection. World J Gastroenterol. 2014;20(32):11415–21. https://doi.org/10.3748/wjg.v20.i32.11415.
Acknowledgements
We thank our patients and colleagues at Sir Run Run Shaw Hospital, Zhejiang University. This study was funded by Zhejiang Province Key Science and Technology Innovation Team (2013TD13), Science Foundation of Zhejiang Traditional Medicine Bureau (2012ZB098, 2017ZB063) and Natural Science Foundation of Zhejiang Province (LY18H160011).
Author information
Authors and Affiliations
Contributions
SC and AL contributed to the study design. LC and JH organized the study, enrolled participants and performed the writing of the manuscript. LW and QG performed the data entry and statistical analysis. XC, YL, YD and HH performed the preliminary screening of participants. HC and YC conducted the 13C urea breath test. All authors have participated in the discussion of the manuscript and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics approval
This study was approved by the Ethics Committee of Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University (20161206-21).
Informed consent
Written informed consent was obtained from all participants before enrollment.
Additional information
Luyi Chen and Jiamin He have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, L., He, J., Wang, L. et al. Efficacies of different proton pump inhibitor-based 14-day bismuth–furazolidone quadruple regimens for the initial eradication of Helicobacter pylori in the southeast coastal region of China: an open-label, randomized clinical trial. Clin Exp Med 18, 569–576 (2018). https://doi.org/10.1007/s10238-018-0510-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-018-0510-9