Abstract
The globulin–platelet model (GP) is a new noninvasive liver fibrosis model developed in chronic hepatitis B (CHB) patients. This study aimed to evaluate the diagnostic performance of GP model for liver fibrosis and cirrhosis in CHB patients with high HBV DNA and mildly elevated alanine transaminase (ALT) levels. We enrolled 316 CHB patients with HBV DNA ≥ 4 log 10 copies/mL and 40 IU/L < ALT ≤ 80 IU/L. The GP, aspartate transaminase-to-platelet ratio index (APRI) and fibrosis index based on four factors (FIB-4) were calculated. Using liver histology as a gold standard, the diagnostic performances of noninvasive fibrosis models were compared by the area under receiver operating characteristic curves (AUROCs). Of 316 patients, 146 (46.2%), 64 (20.3%) and 40 (12.7%) were classified as having significant fibrosis, severe fibrosis and cirrhosis, respectively. To predict significant fibrosis, the AUROC of GP was lower than APRI (0.64 vs 0.76, p < 0.001) and equivalent to FIB-4 (0.64 vs 0.66, p = 0.366). To predict severe fibrosis, the AUROC of GP was equivalent to APRI (0.82 vs 0.79, p = 0.409) and FIB-4 (0.82 vs 0.77, p = 0.224). To predict cirrhosis, the AUROC of GP was higher than APRI (0.91 vs 0.84, p = 0.033) and FIB-4 (0.91 vs 0.80, p = 0.004). GP is a more accurate noninvasive fibrosis model than APRI and FIB-4 to diagnose cirrhosis in CHB patients with high HBV DNA and mildly elevated ALT levels. The clinical application of GP model may reduce the need for liver biopsy in CHB patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic hepatitis B virus (HBV) infection is a global public health problem, and approximately 240 million people are estimated to be infected with HBV in the world [1]. In chronic hepatitis B (CHB) patients without antiviral therapy, the cumulative incidences of cirrhosis and hepatocellular carcinoma (HCC) at 5 years were 8–20 and 2–5%, respectively [2]. Cirrhosis and HCC occurred more frequently in CHB patients with significant liver histological changes including significant inflammation and significant fibrosis [3]. In general, patients with more than a twofold increase in alanine transaminase (ALT) levels were regarded as having significant liver inflammation [4]. However, significant liver fibrosis was difficult to evaluate by a single serum marker.
The liver biopsy is the gold standard for the assessment of liver fibrosis, but it is an invasive procedure [5]. In addition, liver biopsy is very difficult to perform in routine practice in resource-constrained settings because of its expensive cost. Thus, noninvasive tests to evaluate liver fibrosis are particularly needed. The FibroScan device is an excellent tool for the diagnosis of liver fibrosis; however, it is expensive and often only accessible in several hospitals in developing country [6]. Besides FibroScan, fibrosis model based on routine laboratory tests was another noninvasive diagnostic method for liver fibrosis. In recent years, numerous noninvasive liver fibrosis models have been developed, among which the aspartate transaminase (AST)-to-platelet ratio index (APRI) and fibrosis index based on four factors (FIB-4) have been recommended for the detection of liver fibrosis and cirrhosis by the WHO HBV guidelines [7].
The globulin–platelet (GP) model is a new noninvasive liver fibrosis model. To date, only two studies have evaluated the GP model in the diagnosis of liver fibrosis and cirrhosis in CHB patients. Liu et al. [8] found that the GP model had a correlation with liver fibrosis (r = 0.47, p < 0.001), and the area under receiver operating characteristic (ROC) curve (AUROC) of GP model for minimal fibrosis and cirrhosis was 0.76 and 0.78, respectively. Coskun et al. [9] found that the AUROC of GP model for significant fibrosis was 0.74. However, both studies [8, 9] have some limitations. Initially, both studies have relatively small sample sizes (114 and 228 patients, respectively). Secondly, according to the WHO HBV guidelines, the noninvasive fibrosis models should be used alongside clinical criteria and other laboratory criteria (ALT and HBV DNA levels), which has been considered as more accurate to predict liver fibrosis and cirrhosis than noninvasive fibrosis models alone [7]. However, both previous studies evaluated the diagnostic performance of GP model without considering ALT and HBV DNA levels. Thirdly, the different histological scoring systems have been used: Liver fibrosis was classified into five stages according to METAVIR scoring system in the study by Liu et al. [8] and seven stages according to Scheuer scoring system in the study by Coskun et al. [9].
The GP model must be evaluated in a large cohort of CHB patients before it can be considered for common use. Combining other laboratory markers (ALT and HBV DNA levels) with the GP model might increase the diagnostic performance for liver fibrosis and cirrhosis. Therefore, we evaluated the diagnostic performance of the GP model for significant fibrosis, severe fibrosis and cirrhosis in CHB patients with high HBV DNA and mildly elevated ALT levels.
Materials and methods
Patients
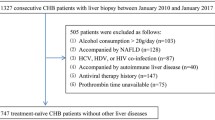
We retrospectively collected the clinical data of 1327 consecutive CHB patients who underwent liver biopsies at Shanghai Public Health Clinical Center, Shanghai, China, between January 2010 and January 2017. CHB was defined as the persistent presence of HBsAg for more than 6 months [10]. The upper limit of normal (ULN) of ALT is 40 IU/L in Asian-Pacific region, and the mildly elevated ALT is defined as ULN < ALT ≤ 2 ULN. Inclusion criteria were HBV DNA ≥ 104 copies/mL and 40 IU/L < ALT ≤ 80 IU/L. The exclusion criteria were co-infection with hepatitis C virus, hepatitis D virus or HIV; a history of alcohol consumption (>20 g/day); concomitant other chronic liver diseases, such as nonalcoholic fatty liver disease, autoimmune liver disease; a history of antiviral therapy. Finally, 316 treatment-naïve CHB patients with high HBV DNA and mildly elevated ALT levels were included.
Liver histological examination
Ultrasonography-guided percutaneous liver biopsy was performed under local anesthesia. Liver samples of minimum length 15 mm were formalin-fixed and paraffin-embedded. A minimum of six portal tracts is considered sufficient for liver fibrosis scoring. Liver fibrosis was classified into five stages according to METAVIR scoring system: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; and F4, cirrhosis [11]. Significant fibrosis, severe fibrosis and cirrhosis were defined as METAVIR fibrosis stage ≥F2, ≥F3 and =F4, respectively.
Routine laboratory tests
Fasting blood samples were obtained the day before liver biopsies. The serological markers of HBV were detected with enzyme-linked immune-sorbent assay kits (ARCHITECT i2000 SR; Abbott, Wiesbaden, Germany). The liver function tests including ALT, AST and globulin were measured by full automated biochemistry analyzer (7600 Series; Hitachi, Tokyo, Japan). Platelet count was detected with automated hematology analyzer (XT-2000i, Sysmex, Kobe, Japan). HBV DNA was quantified by real-time PCR (ABI 7500; Applied Biosystems, Foster City, USA) with a detection limit of 500 copies/mL.
Noninvasive fibrosis models calculation
The formulas for noninvasive fibrosis models are as follows:
-
1.
\( {\text{GP}} = {\text{Globulin}}\,\left( {{\text{g}}/{\text{dL}}} \right) \times 100/{\text{platelet}}\,{\text{count}}\,\left( { \times 10^{9} /{\text{L}}} \right); \)
-
2.
\( {\text{APRI}} = \left( {{\text{AST}}\,\left( {{\text{IU}}/{\text{L}}} \right)/{\text{ULN}}\,{\text{of}}\,{\text{AST}}} \right)/{\text{platelet}}\,{\text{count}}\,\left( {10^{9} /{\text{L}}} \right) \times 100;\,{\text{ULN}}\,{\text{of}}\,{\text{AST}} = 40\,{\text{IU}}/{\text{L}}. \)
-
3.
\( {\text{FIB}} - 4 = \left( {{\text{age}}\,\left( {\text{years}} \right) \times {\text{AST}}\,\left( {{\text{IU}}/{\text{L}}} \right)} \right)/\left( {{\text{platelet}}\,{\text{count}}\,\left( {10^{9} /{\text{L}}} \right) \times \left( {{\text{ALT}}\,\left( {{\text{IU}}/{\text{L}}} \right)} \right)^{1/2} } \right). \)
Statistical analysis
Normality tests of data were performed by Kolmogorov–Smirnov test. The data were presented as follows: normal distribution data as mean ± standard deviation, non-normal distribution continuous data as median [interquartile range (IQR)] and categorical variables as number (percentage). The correlations of noninvasive fibrosis models with METAVIR fibrosis stages were analyzed using Spearman’s test. The diagnostic performances of noninvasive fibrosis models were evaluated by the ROC curves [12]. The ROC curve analysis and Z test were, respectively, used to compute and compare AUROCs. Two sets of cutoffs were calculated: (1) maximizing Youden’s index (sensitivity + specificity 1) or (2) obtaining a sensitivity of at least 90%. Diagnostic accuracy was evaluated by sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR) and negative likelihood ratio (NLR). All significance tests were two-tailed, and p < 0.05 was considered statistically significant. All statistical analyses were carried out using the SPSS statistical software version 15.0 (SPSS Inc. Chicago, IL, USA) and MedCalc statistical software version 16.1 (MedCalc Software bvba, Ostend, Belgium).
Results
Baseline characteristics of the study population
The baseline characteristics of the study population are shown in Table 1. The majority of the enrolled patients were male (65.8%) and HBeAg positive (67.1%). The median age, HBV DNA, ALT, AST, globulin and platelet count were 34 years (IQR 28–39), 6.5 log 10 copies/mL (IQR 5.8–7.7), 54 IU/L (IQR 46–61), 35 IU/L (IQR 28–42), 3.0 g/dL (IQR 2.7–3.2) and 172 × 109/L (IQR 144–202), respectively. The median GP, APRI and FIB-4 scores were 1.7 (IQR 1.5–2.0), 0.53 (IQR 0.40–0.82) and 0.86 (IQR 0.64–1.17), respectively.
The METAVIR inflammation stage was distributed as follows: A0 = 14 (4.4%); A1 = 122 (38.6%); A2 = 142 (44.9%); and A3 = 38 (12.1%). The METAVIR fibrosis stage was distributed as follows: F0 = 14 (4.4%); F1 = 156 (49.4%); F2 = 82 (25.9%); F3 = 24 (7.6%); and F4 = 40 (12.7%). Of 316 enrolled patients, 146 (46.2%), 64 (20.3%) and 40 (12.7%) were classified as having significant fibrosis, severe fibrosis and cirrhosis, respectively.
Correlations between noninvasive fibrosis models and METAVIR fibrosis stages
The correlations between noninvasive fibrosis models and METAVIR fibrosis stages were analyzed to evaluate whether noninvasive fibrosis models are suitable for predicting liver fibrosis (Table 2). The globulin levels had a positive correlation with METAVIR fibrosis stages (correlation coefficient r = 0.37, p < 0.001), and platelet count was negatively correlated (r = −0.35, p < 0.001). The METAVIR fibrosis stages were positively correlated with GP (r = 0.44, p < 0.001), APRI (r = 0.50, p < 0.001) and FIB-4 (r = 0.42, p < 0.001), resulting in higher median GP, APRI and FIB-4 scores with increasing METAVIR fibrosis stages (Fig. 1).
Diagnostic performances of noninvasive fibrosis models for liver fibrosis and cirrhosis
The ROC curves of noninvasive fibrosis models for significant fibrosis, severe fibrosis and cirrhosis are shown in Fig. 2. To predict significant fibrosis, the AUROC of GP was lower than APRI (0.64 vs 0.76, p < 0.001) and equivalent to FIB-4 (0.64 vs 0.66, p = 0.366) (Table 3). To predict severe fibrosis, the AUROC of GP was equivalent to APRI (0.82 vs 0.79, p = 0.409) and FIB-4 (0.82 vs 0.77, p = 0.224) (Table 3). To predict cirrhosis, the AUROC of GP was higher than APRI (0.91 vs 0.84, p = 0.033) and FIB-4 (0.91 vs 0.80, p = 0.004) (Table 3).
Diagnostic thresholds of noninvasive fibrosis models for liver fibrosis and cirrhosis
Diagnostic thresholds of noninvasive fibrosis models for liver fibrosis and cirrhosis are presented in Table 4. By maximizing Youden’s index, the optimal cutoffs of GP were 2.0 for the diagnosis of significant fibrosis (the corresponding sensitivity, specificity, PPV and NPV were 38, 96, 90 and 65%, respectively) and 2.6 for cirrhosis (the corresponding sensitivity, specificity, PPV and NPV were 80, 93, 62 and 97%, respectively). Using a sensitivity of at least 90%, the optimal cutoffs of GP were 1.2 for the diagnosis of significant fibrosis (the corresponding sensitivity, specificity, PPV and NPV were 90, 7, 46 and 86%, respectively) and 1.9 for cirrhosis (the corresponding sensitivity, specificity, PPV and NPV were 90, 83, 43 and 98%, respectively).
Discussion
Routine assessment of CHB patients is needed to guide management and indicate the need for treatment. The routine assessment generally includes detection of HBeAg status; measuring ALT levels to help determine liver inflammation; quantification of HBV DNA levels; and stage of liver fibrosis [7]. Patients with significant liver fibrosis need antiviral therapy as a priority in order to prevent further disease progression. Liver biopsy is considered the gold standard method to assess the degree of fibrosis, but it is not widely used in resource-limited settings because of its high cost, invasiveness, patient discomfort, risk of complications, sampling error, as well as the need for expert histological interpretation [7]. In recent years, the noninvasive methods for assessing the stage of liver fibrosis are supplanting liver biopsy. Blood fibrosis models including APRI and FIB-4, as well as commercial models such as FibroTest, can be estimated, or FibroScan performed to assess liver fibrosis and cirrhosis [6, 13, 14].
This study aimed to evaluate the diagnostic performance of GP model for liver fibrosis and cirrhosis in CHB patients with high HBV DNA and mildly elevated ALT levels. According to the WHO HBV guidelines, APRI, FIB-4 and FibroTest have been recommended as the preferred noninvasive test to assess for the presence of liver fibrosis in resource-limited settings [7]. FibroTest is patented and must be performed in laboratories that meet certain quality standards [15]. On the contrary, APRI and FIB-4 are associated with lower costs and can be performed in an outpatient setting. Therefore, we used APRI and FIB-4 as comparisons when evaluating the diagnostic performance of GP model. We found that the GP model did not show advantages than APRI and FIB-4 in identifying significant fibrosis (AUROC: 0.64 for GP, 0.76 for APRI and 0.66 for FIB-4). However, the AUROC of GP model was higher than APRI (0.91 vs 0.84, p = 0.033) and FIB-4 (0.91 vs 0.80, p = 0.004) to predict cirrhosis. The GP model might be the most precise model for diagnosing cirrhosis in CHB patients with high HBV DNA and mildly elevated ALT levels compared with APRI and FIB-4.
The GP model is a new noninvasive liver fibrosis model, which was developed in a cohort of 114 CHB patients [8]. Liu et al. [8] first reported that the AUROC of GP model was higher than APRI (0.76 vs 0.64, p < 0.05) and equivalent to FIB-4 (0.76 vs 0.72, p > 0.05) for diagnosing minimal fibrosis and equivalent to APRI (0.78 vs 0.70, p > 0.05) and FIB-4 (0.78 vs 0.77, p > 0.05) for diagnosing cirrhosis. Using a cutoff value of GP < 1.68, the sensitivity, specificity, PPV and NPV were 72.4, 69.6, 71.2 and 70.8%, respectively, for the diagnosis of minimal fibrosis. Using a cutoff value of GP > 2.53, the sensitivity, specificity, PPV and NPV were 72.7, 84.5, 33.4 and 96.7%, respectively, for the diagnosis of cirrhosis [8]. Coskun et al. [9] then found that there was no significant difference between the AUROC of GP model and that of FIB-4 (0.74 vs 0.75, p > 0.05) for diagnosing significant fibrosis. Using a cutoff value of GP > 1.5, the sensitivity, specificity, PPV and NPV were 75.2, 62.8, 62 and 75%, respectively, for the diagnosis of significant fibrosis [9].
However, two previous studies about GP model have relatively small sample sizes, which might have resulted in statistical bias. Secondly, both studies evaluated the diagnostic performance of GP model for liver fibrosis and cirrhosis without considering ALT and HBV DNA levels. In the letters to the editor, Afyon et al. [16] emphasized better results for predicting the severity of liver fibrosis may be achieved by using serum HBV DNA levels combined with GP model in CHB patients, but large-scale studies are required. In this study, we first evaluated the diagnostic performance of GP model for significant fibrosis and cirrhosis combining with HBV DNA and ALT levels in a relatively large cohort of 316 CHB patients. We found that GP is a more accurate noninvasive fibrosis model to stage cirrhosis in CHB patients with high HBV DNA and mildly elevated ALT levels compared with APRI and FIB-4. As the presence of significant fibrosis was considered a priority criterion for initiation of antiviral therapy, the primary outcome assessment in this study was for the diagnosis of significant fibrosis and cirrhosis, rather than minimal fibrosis.
In previous studies, globulin and platelet counts demonstrated predictive value for liver fibrosis in CHB patients. Schmilovitz-Weiss et al. [17] reported that serum globulin level (OR 5.97, p < 0.001) and platelet count (OR 0.98, p = 0.001) were independent predictors of significant fibrosis in CHB patients. In the study by Coskun et al. [9], liver fibrosis positively correlated with globulin level (r = 0.34, p < 0.001) and inversely correlated with platelet counts (r = −0.34, p < 0.001). In our study, the globulin levels had a positive correlation with METAVIR fibrosis stages (r = 0.37, p < 0.001), and platelet count was negatively correlated (r = −0.35, p < 0.001). Our results supported that serum globulin increased, while platelets decreased with liver fibrosis progression, and they can serve as noninvasive markers of liver fibrosis.
The GP model consists of serum globulin and platelet count, which are more readily available in resource-limited settings, are associated with lower costs and can be performed in an outpatient setting. The use of GP model could help with the optimal selection of CHB patients for antiviral therapy and reduce the need for liver biopsy. In this study, for the diagnosis of cirrhosis, the GP model had significantly better sensitivity (80 vs 73%) and specificity (93 vs 83%) than APRI. However, the GP model has a high specificity (96%) but low sensitivity (38%) for the diagnosis of significant fibrosis at its optimal cutoff value and, therefore, many CHB patients with significant fibrosis may be missed.
The WHO HBV guidelines recommended use two cutoff points for diagnosing specific fibrosis stages: A cutoff with high specificity (i.e., fewer false-positive results) is used to diagnose persons with fibrosis and a cutoff with high sensitivity (i.e., fewer false-negative results) to rule out the presence of a particular stage of fibrosis 7. As well as diagnosing significant fibrosis and cirrhosis, it is important to exclude the presence of significant fibrosis or cirrhosis and establish patients who were not candidate for anti-HBV therapy. In order to establish who is safely excluded from anti-HBV therapy, we obtained a set of cutoffs for GP, APRI and FIB-4 using a sensitivity of at least 90%. Using sensitivity of at least 90%, the cutoffs of GP were 1.2 for the diagnosis of significant fibrosis (the NPV was 86%) and 1.9 for cirrhosis (the NPV was 93%).
There were some limitations in this study. First, the retrospective design of this study might have caused selective bias [18]. Thus, the diagnostic performance of GP model needs to be confirmed in prospective studies. Second, we cannot compare the performance of FibroTest to GP model because the calculation of FibroTest is patented. Third, we cannot compare the performance of FibroScan to GP model because the FibroScan measurement is unavailable for most patients in our cohort. In fact, the FibroScan device is expensive (€34000 for the portable machine and €5000 for the annual maintenance) and only accessible in specialized centers in developing countries including China.
In conclusion, this study showed that GP model shows obvious advantages for the diagnosis of cirrhosis compared with APRI and FIB-4 in CHB patients with high HBV DNA and mildly elevated ALT levels, thus warranting its widespread use for this specific population. However, the GP model showed a limited value in identifying significant fibrosis compared with APRI. Therefore, we recommended that APRI could be used to predict significant fibrosis, and GP model could be used to predict cirrhosis in CHB patients with high HBV DNA and mildly elevated ALT levels. In clinical practice, diagnosing cirrhosis or significant fibrosis needs a combined assessment of clinical features and serum fibrosis models. Liver biopsy would still be needed when there are discordances between clinical symptoms and the extent of fibrosis assessed by noninvasive models.
References
Inoue T, Tanaka Y. Hepatitis B virus and its sexually transmitted infection—an update. Microb Cell. 2016;3(9):420–37.
Lin J, Wu JF, Zhang Q, Zhang HW, Cao GW. Virus-related liver cirrhosis: molecular basis and therapeutic options. World J Gastroenterol. 2014;20(21):6457–69.
Li ZQ, Hu CL, Yu P, et al. The development of hepatocarcinoma after long-term antivirus treatment of Chinese patients with chronic hepatitis B virus infection: Incidence, long-term outcomes and predictive factors. Clin Res Hepatol Gastroenterol. 2017;41(3):311–8.
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–83.
Shah ND, Sasatomi E, Baron TH. Endoscopic ultrasound-guided parenchymal liver biopsy: single center experience of a new dedicated core needle. Clin Gastroenterol Hepatol. 2017;15(5):784–6.
Yu JH, Lee JI. Current role of transient elastography in the management of chronic hepatitis B patients. Ultrasonography. 2017;36(2):86–94.
Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: World Health Organization; 2015.
Liu XD, Wu JL, Liang J, Zhang T, Sheng QS. Globulin–platelet model predicts minimal fibrosis and cirrhosis in chronic hepatitis B virus infected patients. World J Gastroenterol. 2012;18(22):2784–92.
Coskun BD, Altinkaya E, Sevinc E, et al. The diagnostic value of a globulin/platelet model for evaluating liver fibrosis in chronic hepatitis B patients. Rev Esp Enferm Dig. 2015;107(12):740–4.
Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–93.
Albeck MJ, Borgesen SE. ROC-curve analysis. A statistical method for the evaluation of diagnostic tests. Ugeskr Laeger. 1990;152(23):1650–3.
Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142(6):1293–302.
Park SH, Kim CH, Kim DJ, et al. Usefulness of multiple biomarkers for the prediction of significant fibrosis in chronic hepatitis B. J Clin Gastroenterol. 2011;45(4):361–5.
Park MS, Kim SU, Kim BK, et al. Prognostic value of the combined use of transient elastography and fibrotest in patients with chronic hepatitis B. Liver Int. 2015;35(2):455–62.
Afyon M. Could viral load combined with indirect serum markers be an option for predicting the degree of liver fibrosis in treatment-naive chronic hepatitis B patients? Rev Esp Enferm Dig. 2017;109(2):166–7.
Schmilovitz-Weiss H, Tovar A, Halpern M, et al. Predictive value of serum globulin levels for the extent of hepatic fibrosis in patients with chronic hepatitis B infection. J Viral Hepat. 2006;13(10):671–7.
Choi BC. Sensitivity and specificity of a single diagnostic test in the presence of work-up bias. J Clin Epidemiol. 1992;45(6):581–6.
Acknowledgements
This study was supported by Grant No. SHDC12015129 from the ShenKang Development Center of Shanghai, Grant No. 17411969700 from the Science and Technology Commission of Shanghai and Grant No. 13401902100 from the Science and Technology Commission of Shanghai.
Author information
Authors and Affiliations
Contributions
Qiang Li contributed to study concept and design and drafting of the manuscript. Liang Chen, Chuan Lu, Weixia Li, Yuxian Huang and Qiang Li helped in analysis and interpretation of data. Liang Chen critically revised the manuscript for important intellectual content.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval and Informed consent
All patients signed the informed consent before liver biopsy, and all clinical procedures were in accordance with the Helsinki Declaration of 1975, as revised in 1983. The study protocol was permitted by the Ethics Committee of Shanghai Public Health Clinical Center.
Additional information
Qiang Li is acting as the submission’s guarantor and takes responsibility for the integrity of the work as a whole, from inception to published article.
Rights and permissions
About this article
Cite this article
Li, Q., Lu, C., Li, W. et al. Globulin–platelet model predicts significant fibrosis and cirrhosis in CHB patients with high HBV DNA and mildly elevated alanine transaminase levels. Clin Exp Med 18, 71–78 (2018). https://doi.org/10.1007/s10238-017-0472-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-017-0472-3