Abstract
Markers of oxidative stress and antioxidant status in relation to inflammatory mediators in septic patients (SPs) during the course of sepsis and after recovery were analysed. Patients were 30 critically ill adults in severe sepsis/septic shock, 19 of which completed 3 samplings (S1: within 24 h after onset of sepsis, S7: 7 days after S1, R7: 7 days after clinical recovery). Comparing SPs with healthy controls (HCs), enhanced C-reactive protein, procalcitonin, bilirubin and CuZn-superoxide dismutase activity were found at S1 only. Oxidized low-density lipoprotein, conjugated dienes and nitrotyrosine were increased at S1, culminated at S7 and reverted nearly to HC levels at R7. Reduced catalase activity and serum amyloid were observed at S1 and endured until R7. Increase in IL-6, IL-10 and tumour necrosis factor alpha (TNF-α) with accompanying decrease in apolipoprotein A1, high-density lipoprotein (HDL) cholesterol, selenium, zinc, albumin, paraoxonase 1 and glutathione peroxidase 1 activity appeared at S1 and persisted until R7. TNF-α, IL-10 and markers of oxidative stress were in negative correlation with HDL cholesterol and albumin at R7. After clinical recovery, increased cytokines and decreased antioxidants were accompanied by lower albumin and HDL cholesterol levels. During this important and beneficial period of tissue repair, patients with prolonged persistence of this status are probably more vulnerable to secondary infections and should be dealt with as constituting a high-risk population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is defined as systemic inflammatory response syndrome (SIRS) in the presence of infection progressing with varying degrees of severity [1]. The inflammatory processes, in principle beneficial, can be deregulated in patients with severe sepsis and septic shock that correspond to extensive exhaustion of individual functional reserves and development of organ dysfunction. These patients require intensive care in order to improve survival [2, 3]. The following phase often taking place in a post-intensive care unit (ICU) setting is accordingly important, too, for patients’ successful recovery.
With their capacity to interact with the innate and adaptive immune responses, lymphocytes play a central role in modulating the sepsis response. This process occurs as interplay of changes in the expression and activity of numerous endogenous mediators of pro- and anti-inflammatory processes [4] in order to eliminate the insult and renew homoeostasis. SIRS, typically present in early sepsis and lasting 3–5 days, entails a predominately pro-inflammatory period [5, 6]. This usually is followed by the development of so-called compensatory anti-inflammatory response syndrome (CARS), a complex but incompletely defined pattern of immunologic responses to promote tissue renewal and attenuate pro-inflammatory reaction of the host which, when unbalanced under severe infection, can result in anergy and immunoparalysis with increased susceptibility to developing a new infection [7]. Although these definitions and the timing of both events are useful in clinical description, it has become apparent that CARS is not simply the cessation of SIRS; CARS can exist in parallel with SIRS, and both processes often take place together as mixed antagonist response syndrome (MARS) [8]. In inflammatory conditions as well as in ageing, the redox balance shifts to more oxidized state reflecting activation of oxidative as well as reductive processes, leading to a new balance which normalizes during recovery. In sepsis, the activation of leucocytes and release of mediators are inevitably accompanied by increased production of reactive oxygen and nitrogen species (RONS). RONS are well recognized for their dual role as beneficial and/or deleterious species. Beneficial effects occur at low-to-moderate RONS concentrations and involve a physiological role in cellular responses, as, for example, in defending against infectious agents and in the functioning of a number of cellular signalling pathways [9, 10].
Individuals who survive severe sepsis beyond intensive care and are clinically recovered continue to undergo intense tissue healing. In this period, cytokines/growth factors are operative in tissue rebuilding and reinforcement, steering substrates from peripheral tissues or food to healing tissues and also countering oxidative stress [11]. This essentially beneficial but very energy- and substrate-consuming process should ensure a successful recovery. On the other hand, metabolically exhausted patients in recovery phase remain susceptible to secondary complications with negative impacts for their long-term prognoses. Recent clinical studies have shown that increased levels of interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and IL-10 persisting after clinical recovery from sepsis—rather than their initial peaks—are more characteristic of those patients who ultimately have further complications or who die [12]. This tends to be the case especially in elderly patients [13]. It would be useful, therefore, to determine appropriate markers for identifying those patients who are at risk.
There have been many studies directed to predicting the severity of sepsis and patients’ long-term outcomes using the levels of individual cytokines and other inflammatory modulators. Mostly, these have been analysed in the early phase of the disease [14]. In our opinion, this approach does not provide a tool for assessing risk to these patients after recovery or appropriate individual follow-up. The design of this study, therefore, emerges from the findings of the aforementioned clinical study monitoring the basic pro-inflammatory (IL-6, TNF-α) and anti-inflammatory (IL-10) cytokines in a population of patients with severe sepsis and after recovery together with their clinical outcomes [12, 15].
In our study, we selected group of patients in the diagnostic category early severe sepsis/septic shock with similar mortality rate [12, 15]. While this study had used hospital discharge as the time for last sampling, we considered that as inappropriate due to the bias caused by such organizational aspects of the healthcare system as the accessibility of follow-up care. The timing of recovery in our study, 7 days after cessation of all clinical signs of inflammation, reflects the time differences among individual subjects in the progress of their illnesses. This timing enabled us to capture data from patients in similar stages of recovery, regardless of the duration of their sepsis and occurrence of subsequent inflammatory complications.
The aim of the study presented here was to describe inflammatory processes of severe sepsis and/or septic shock during SIRS, during CARS and 7 days after clinical recovery in a carefully selected group of ICU patients. The analysis of inflammatory mediators together with oxidative stress markers and antioxidant status would help to confirm clinical stages of sepsis while emphasizing the persistence of risk after recovery (usually after discharge from the ICU or hospital) that should be addressed via standard follow-up measures to determine patient status and the choice of appropriate interventions for ameliorating the prognosis.
Patients and methods
This prospective study was carried out in the medical adult ICU of the General Teaching Hospital, Charles University in Prague. The study protocol was approved by the institutional review board and the ethics committee of the General Teaching Hospital in Prague. Written informed consent was obtained from all participants included in the study.
Patients
The population under study consisted of two groups: 30 septic patients (SPs) and 30 age- and sex-matched healthy controls (HCs). Sepsis was defined according to the Society of Critical Care Medicine/American College of Chest Physicians (SCCM/ACCP) definitions [1]. SPs needed to fulfil the following additional inclusion criteria: APACHE II score >10 and serum C-reactive protein (CRP) concentration >20 mg/l. Exclusion criteria for SPs were the following: antioxidant therapy, chronic dialysis, history of diabetes, generalized tumours, immunosuppressive therapy, non-steroidal anti-inflammatory drugs therapy and chemotherapy. Sepsis was treated according to guidelines [16]. HCs were defined as individuals without a known major disease.
Data collection
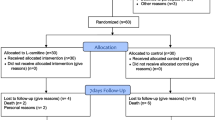
Samples from SPs were collected three times in various phases of inflammatory process (Fig. 1): during the first 24 h after ICU admission (S1), 7 days after S1 (S7) and 7 days after recovery (R7). R7 was defined as the seventh day after cessation of septic clinical signs and CRP < 20 mg/l. Samples from the HC group were obtained once. From the group of 30 SPs, 8 patients died due to sepsis and 3 SPs were lost from follow-up because they never fully recovered from sepsis. Thus, all three samplings were available from 19 patients. These SPs were compared with the group of 19 sex- and age-matched HCs. The main source of sepsis was lung, occurring in 13 cases. In all study participants, the medical history and intake of any medicaments were documented at study entry. The sequential organ failure assessment (SOFA) score was calculated from laboratory and clinical parameters in SPs for the first 7 days after ICU admission [17]. Blood was taken after overnight fasting from an arterial line (SPs) or by puncturing a peripheral vein (HCs).
The concentrations of CRP, procalcitonin (PCT), IL-6, IL-10, TNF-α, serum amyloid A (SAA), oxidized low-density lipoprotein (ox-LDL), albumin, bilirubin, uric acid, Cu, Zn, Fe, Se, vitamins A and E, and lipid parameters, as well as paraoxonase 1 (PON1), activity were measured in serum. Serum was prepared (after coagulation in Vacutainer tubes) by centrifugation at 3500 rpm at 4 °C for 10 min. Conjugated dienes (CD) were measured in precipitated low-density lipoprotein (LDL). Activities of antioxidant enzymes were measured in haemolysed erythrocytes. The samples were stored at −80 °C until assay. All samples were marked with unique identification numbers, and data were merged only after assays had been completed.
Laboratory measurements
The routine biochemical tests were conducted at the Central Biochemical Laboratory of the General University Hospital in Prague. CRP concentration was measured by immunoturbidimetric method using a K-ASSAY CRP kit (Kamiya Biomedical Company, Seattle, WA, USA) on a Roche/Hitachi Modular SWA analyser (Tokyo, Japan). PCT concentration was measured by immunoluminometric assay using a BRAHMS PCT LIA kit (catalogue number 54.1; Brahms Diagnostica, Berlin, Germany). Cytokines (IL-6, IL-10 and TNF-α) were analysed using Fluorokine MAP kits (R&D Systems, Minneapolis, MN, USA) and a Luminex®100 analyser (Luminex, Austin, TX, USA). SAA concentration was analysed using a solid-phase sandwich ELISA kit (Invitrogen, Carlsbad, CA, USA). The arylesterase activity of PON1 was measured according to the method as previously described by Eckerson et al. while using phenylacetate as a substrate [18]. The rate of phenol generation was monitored spectrophotometrically at 270 nm. The arylesterase activity of PON1 was calculated using the molar extinction coefficient of the produced phenol (1310 M−1 cm−1) and expressed as U/ml of serum. The ox-LDL measurement was performed using an oxidized LDL ELISA kit (Mercodia, Uppsala, Sweden). Activities of antioxidant enzymes were determined by spectrophotometric kinetic methods as previously described [19], and concentration of CD/LDL was measured according to Ahotupa et al. [20]. The concentration of nitrotyrosine was measured using a solid-phase sandwich ELISA kit (Biovendor, Brno, Czech Republic). The concentration of nitrites and nitrates in serum was determined by the Griess reaction according to a method previously published [21].
Statistical analysis
Data are expressed as mean ± standard deviation (SD) for parametric and as median and interquartile range (25th–75th percentiles) for nonparametric variables. Normality of data distribution was tested using the Shapiro–Wilk test. Differences between SPs and HCs were evaluated by t test, while for nonparametric analysis the Mann–Whitney U test was used. Friedman ANOVA was used for dependent analysis. Multivariate discriminant analysis (MDA) based on analysis of the relationships among multiple variables simultaneously was used. MDA was carried out in a stepwise manner using the minimum Wilks λ. At each step in the process, that variable having the most discriminating power was identified and its coefficient determined. All statistical analyses were performed using version 8.0 of Statistica software from StatSoft (2007, Czech Republic). The cut-off for statistical significance was set at P < 0.05.
Results
Basic characteristics
Table 1 summarizes the demographic and clinical characteristics of 19 SPs at all three samplings and 19 sex- and age-matched HCs. Hospital mortality was 27 %.
Acute-phase response markers
Serum PCT and CRP concentrations were increased at S1, but no significant difference was observed at S7 compared to HCs. Increased concentrations of interleukins (IL-6, IL-10, TNF-α) persisted from S1 until R7. The IL-10/TNF-α ratio gradually decreased, mainly due to a decline in IL-10 concentration (S1 = 0.350 [0.173–0.511]; S7 = 0.235 [0.125–0.321]; R7 = 0.128 [0.078–0.279]; HC = 0.091 [0.001–0.098]; P < 0.001 S1 vs. HC; P < 0.01 S7 vs. HC). SOFA gradually decreased from S1 until S7 (Fig. 2).
Changes in inflammatory markers and SOFA in the course of sepsis. S1 septic patients enrolled within 24 h after onset of sepsis, S7 septic patients 7 days after S1, R7 septic patients 7 days after recovery, HCs healthy controls. PCT procalcitonin, CRP C-reactive protein, TNF-α tumour necrosis factor alpha, IL-6 interleukin-6, IL-10 interleukin-10. Data presented as median and interquartile range (25th–75th percentile). *S1 or S7 or R7 versus HC; + S1 or S7 versus R7; ◦S1 versus S7. ***, +++, °°° P < 0.001, **, ++, °°P < 0.01, *, +, °P < 0.05; SOFA: *1 versus all other days, ◦ 2, 3 or 5 versus 7, x6 versus 2
Serum markers of oxidative stress
The levels of ox-LDL, CD and nitrotyrosine increased at S1, culminated at S7 and returned to HC values at R7. Enhanced serum concentration of nitrites/nitrates was observed only at S7 (Fig. 3).
Changes in activities of oxidative stress parameters in the course of sepsis. S1 septic patients enrolled within 24 h after onset of sepsis, S7 septic patients 7 days after S1, R7 septic patients 7 days after recovery, HCs healthy controls. Ox-LDL oxidized low-density lipoproteins, CD conjugated dienes in precipitated LDL, LDL-C low-density lipoprotein cholesterol, NT 3-nitrotyrosine. Data presented as mean ± SD. *S1 or S7 or R7 versus HC; + S1 or S7 versus R7 ***, +++ P < 0.001, **, ++ P < 0.01, *, + P < 0.05
Antioxidant capacity
CuZn-superoxide dismutase (CuZnSOD) activity was increased at S1 and returned to HC values already at S7. A decline in catalase (CAT) activity was found at S1 and S7, but this returned to HC levels at R7 while the decrease in glutathione peroxidase 1 (GPX1) activity persisted in all three samplings. No significant difference in glutathione reductase (GR) activity between HCs and individual SP samplings was found (Fig. 4).
Changes in activities of antioxidant enzymes in the course of sepsis. S1 septic patients enrolled within 24 h after onset of sepsis, S7 septic patients 7 days after S1, R7 septic patients 7 days after recovery, HCs healthy controls. CuZnSOD CuZn-superoxide dismutase, CAT catalase, GPX1 glutathione peroxidase 1, GR glutathione reductase. Data presented as mean ± SD. *S1 or S7 or R7 versus HC; + S1 or S7 versus R7; S1 versus S7. ***, +++, °°°P < 0.001, **, ++, °°P < 0.01, *, +, °P < 0.05
Table 2 presents non-enzymatic antioxidants and cofactors of antioxidant enzymes. Decrease in concentrations of vitamin A, vitamin E and bilirubin was found at S1 only, while decrease in Zn was observed at both S1 and S7. Significant decline of uric acid and a rise in Cu were observed only at S7 compared to HCs. Nevertheless, all these returned nearly to HC values at R7. On the other hand, substantial decrease in transferrin, Fe, Se and ferritin concentrations, as well decrease in albumin concentration, was observed already at S1, still persisted 7 days after recovery (R7), and never reached HC levels. The CRP/albumin ratio gradually decreased from S1 until R7, while there remained differences versus HCs (S1 = 4.47 [2.2–12.7]; S7 = 1.0 [0.43–4.04]; R7 = 0.51 [0.21–0.76]; HC = 0.045 [0.042–0.187]; P < 0.05).
Plasma lipids and associated proteins
Figure 5 demonstrates a marked drop in high-density lipoprotein cholesterol (HDL-C) concentration which appeared at the onset (S1) and persisted until recovery (R7) in positive correlation with apolipoprotein A1 (Apo-A1) (R = 0.899, 0.925, 0.736) and albumin (R = 0.517, 0.622, 0.490) at S1, S7 and R7, respectively, and in positive correlation with PON1 only at S1 (R = 0.613). SAA concentration was significantly increased at S1 and S7 and reached nearly HC levels at R7; this was closely tracked by decrease in PON1 activity. Negative correlation between SAA and HDL-C was observed at R7 (R = −0.500). For the markers of lipid peroxidation, ox-LDL and CD, negative correlation with HDL-C was found at S1 (R = −0.576, −0.632) and at S7 (R = −0.682, −0.622), respectively. Negative correlation of HDL-C with CD was observed at R7 (R = −0.490).
Changes in PON1 activity and associated parameters in the course of sepsis. S1 septic patients enrolled within 24 h after onset of sepsis, S7 septic patients 7 days after S1, R7 septic patients 7 days after recovery, HC healthy controls. PON1 paraoxonase 1—arylesterase activity, SAA serum amyloid A, Apo-A1 apolipoprotein A1, HDL-C high-density lipoprotein cholesterol. Data presented as mean ± SD. *S1 or S7 or R7 versus HC, + S1 or S7 versus R7, ◦S1 versus S7. ***, +++, °°°P < 0.001, **, ++, °°P < 0.01, *, +, °P < 0.05
Significant decrease in concentrations (mmol/l) of TC (S1 = 3.3 [2.5–3.5]; S7 = 3.7 [2.8–4.3]; R7 = 4.4 [4.0–5.2]; HC = 5.7 [4.8–6.7]) and LDL cholesterol (S1 = 1.8 [1.2–2.2]; S7 = 2.2 [1.1–2.4]; R7 = 2.9 [2.2–3.2]; HC = 3.7 [3.0–4.3]) appeared already at S1 and persisted until R7.
Significant transient increase in triglyceride concentration was observed at S7 ([mmol/l], S1 = 1.3 [0.8–1.9]; S7 = 1.8 [1.1–2.4] (P < 0.05); R7 = 1.5 [1.1–2.1]; HC = 1.5 [1.0–1.7]).
Relationships among individual parameters
The positive correlation of albumin with HDL-C and a negative correlation with TNF-α and IL-10; negative correlation of HDL-C with TNF-α; positive correlations of PON1 with CD and GPX-1 with ox-LDL at R7 are presented in Fig. 6.
Correlations at R7 (septic patients 7 days after recovery); TNF-α tumour necrosis factor alpha, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, PON1 paraoxonase 1—arylesterase activity, GPX1 glutathione peroxidase 1, Ox-LDL oxidized low-density lipoproteins, CD conjugated dienes
Using MDA, we created two discriminate models that contained dependent variables (albumin and HDL) and the independent variables, for both models were antioxidant enzymes (CAT, CuZnSOD, GPX1, GR, PON1), markers of oxidative stress (CD, ox-LDL) and markers of inflammation (TNF-α, Il-6, IL-10). The variables with the most discriminating power in the case of albumin were TNF-α (P < 0.001), IL-10 (P = 0.028) and ox-LDL (P = 0.013). In the case of HDL, these were TNF-α (P = 0.030) and ox-LDL (P = 0.019).
Discussion
Despite the difference in timing [15], we confirmed the persistence of increased levels of cytokines after the cessation of sepsis at R7. The authors of this clinical study had concluded that despite his or her clinical recovery, a patient leaving hospital with heightened levels of cytokines is exposed to increased risk of death during the ensuing year. All patients that have been subject to true sepsis go through a recovery phase during which many plasma constituents still differ from those of not-stressed patients. In fact, the cytokine/growth factor response and the change in membrane permeability take months to normalize. Especially in the first 6-week period, the body cannot adequately generate a renewed pro-inflammatory response after a repeated challenge (referred to as the “second hit phenomenon”) [22].
TNF-α and IL-6 are known to mediate mainly the pro-inflammatory phase, while IL-10 is considered to be an anti-inflammatory mediator in principle [8]. In our group of patients, the significant decrease in the IL-10/TNF-α ratio was caused mainly by gradual decrease in the level of the anti-inflammatory IL-10, whereas the pro-inflammatory TNF-α declined between S1 and S7 but remained practically unchanged thereafter. We cannot exclude the possibility of having missed the peak of the IL-10 concentration between S1 and S7, but, according to the observation of Gogos et al. [23], this is unlikely to be the case in survivors. We also confirmed the typical rapid decline of IL-6 in sepsis survivors reported earlier [24]. Therefore, we found no evidence to support the classical two-phase model of sepsis in this study. We showed that increased cytokine levels persist 7 days after the cessation of septic clinical signs and patients’ cytokine concentrations are still far from the values of age- and sex-matched HCs.
We have shown strong correlation of TNF-α and IL-10 with albumin and ox-LDL over time of the study. This might be explained by pleiotrophic role of TNF-α (the catabolic in peripheral tissues and anabolic in healing tissues, immune system and liver) [25]. The persistently high levels of IL-10 suggest that IL-10 could play more than an anti-inflammatory role long after the primary event. Cytokines are also involved in the stopping of the primary inflammatory activity by regulating the expression and stability of cyclooxygenase-2 [26] that produces inflammation resolving eicosanoids and docosanoids (resolvins, etc.) [27].
In the cases of the main acute-phase response markers (CRP, PCT), the enhanced concentrations were observed only at S1. This corresponds with other studies showing particularly PCT to be a typical marker of early sepsis [28]. CRP and PCT are short-lived acute-phase proteins only correlating with the sepsis severity in the beginning of the insult, suggesting that our surviving patients were adequately treated. In this respect in non-survivors, the four patients that were still alive at S7 and died later, median PCT was still increased compared to survivors in our study (4.0 vs. 0.4 mg/l, P < 0.01). This may indicate that PCT has only a role in clearance of the primary insult. When increased after longer follow-up might indicate that the inflammatory focus (infection, trauma) is still active.
We had supposed that increased levels of cytokines during the monitored times would be reflected by concomitant rearrangement of redox status and that inspired us to analyse the markers of oxidative damage together with the levels of enzymatic and non-enzymatic antioxidants. The lipid peroxidation markers ox-LDL and CD were elevated at S1, persisted at heightened levels until S7, and then both returned nearly to the values in their HC ranges at R7. Similarly, a study by Behnes et al. [29] had reported increased concentration of ox-LDL in patients with severe sepsis during the first week of illness. Moreover, endotoxin administration was shown to cause a sharp rise in plasma CD levels in the porcine model of burn and sepsis [30]. Another study had shown increased thiobarbituric acid reactive substances and protein carbonyls to be markers of lipid peroxidation and protein oxidation, respectively. While in that study thiobarbituric acid reactive substances had normalized during the first 7 days of sepsis, increased protein carbonyls still had persisted 3 months after the onset of sepsis, probably due to the slow protein turnover [31]. In our study, the increased level of nitrotyrosine appeared already at S1 and persisted until S7; the nitrites/nitrates were increased only at S7. Both these parameters normalized after recovery at R7. The observed rise in these nitrogen compounds accords with previous studies on septic shock patients indicating elevated nitric oxide and reactive nitrogen species formation during the generalized inflammatory response [31]. The observed shift between initiation of the increase in nitrotyrosine and nitrites/nitrates is in line with results of Strand et al. [32], who had shown that the peak concentration of nitrotyrosine need not coincide with the peak of nitrites/nitrates during septic shock.
We confirmed reduced antioxidant defence capacity in septic critically ill patients, as reported by Crimi et al. [9]. Moreover, we found that the reduction of some antioxidant components lasted until R7. In our study, increased CuZnSOD and decreased CAT and GPX1 activities in erythrocytes were observed at S1. While CuZnSOD normalized already at S7, the decrease in GPX1 and the declining trend in CAT activities persisted still at R7. Likewise, Warner et al. [33] had found increased activity of CuZnSOD in erythrocytes at the onset of sepsis. In paediatric sepsis, too, there has been observed an apparent trend towards increased CuZnSOD activity in erythrocytes [34]. CuZnSOD is one of the most important antioxidant enzymes responsible for decomposition of the superoxide radical while producing H2O2 that is further transformed to H2O by the CAT and GPX1 action [35]. It is important to note that the increase in CuZnSOD activity observed in the early stage of sepsis cannot in principle result from the rise in protein amount because mature erythrocytes possess no transcriptional apparatus. Rather, it results from the activity’s stimulation [36]. We propose that the increase in CuZnSOD activity in combination with simultaneous decrease in CAT and GPX1 activities may intensify the H2O2 accumulation with subsequent spontaneous formation of highly reactive hydroxyl radicals causing escalation of oxidative damage. Therefore, the increased CuZnSOD activity at S1 may act predominantly as a pro-oxidant [37]. The short-lived increase in CuZnSOD activity may predominantly play a role in the beginning of sepsis for supporting clearance of the debris and bacterial products.
Results published on erythrocyte CAT in sepsis are rather controversial for our study. Warner et al. [33] and Leff et al. [38] had described increased CAT activity in plasma and red blood cells during sepsis. The decrease in CAT activity observed in our group of SPs could possibly be explained by high severity of the illness.
We found decreased GPX1 activity during sepsis and after recovery. The main reasons could be a low level of glutathione (GSH) [39] as well as the observed decline in Se concentration during sepsis. Reduced GSH acts as a reducing substrate of GPX1. Selenium, bound in the active site of the enzyme in the form of one SeCys residue, is a cofactor essential for GPX1’s activity [40]. Accordingly, suppressed GPX1 activity was accompanied by decrease in Se concentration lasting until R7. Supplementation with Se has been shown to improve antioxidant capacity, as demonstrated by increased GPX1 activity [41]. With a view to the decrease in GPX1 activity at R7, we also must consider the possibility of relatively prolonged regeneration of the enzyme due to the slow turnover of mature erythrocytes. The enzyme has been shown to protect red blood cells against haemoglobin oxidation and haemolysis [42], and for that reason the diminished antioxidant capacity of erythrocytes could impact upon the patient outcome in case of any secondary insult.
In accordance with other studies [10, 43, 44], we report decreased serum concentrations of vitamins E and A at S1. These vitamins are lipid phase antioxidants, crucial for preventing lipid peroxidation [45]. We also observed a persistence of decreased albumin level at R7, which underscores the low antioxidant capacity [35]. The long-persisting inflammatory response including increased capillary permeability leads to an increase in the third space, together with the distribution volume of albumin, which is diluted to low concentration. This is well acknowledged to be a predictor of bad outcome, complications and mortality [46]. Albumin is an indicator of capillary leakage, probably playing a role in getting the cells, the proteins and other substances at the place where healing occurs. All these substances are therefore playing useful roles and indicate that the process of proliferation and regeneration is still active. This low albumin level is also in part responsible for the decreases in vitamins, trace elements and electrolytes like Ca/Zn because albumin is a binding protein for many of these substances [47, 48]. Nevertheless, we have to take into account that a low total Ca/Zn does not always indicate a deficiency, but that only the low ionized fraction does. This active part which may be normal was not measured in this study.
An important finding of our study, therefore, is the revelation that in the septic state increased amounts of peroxidation products are made. The antioxidative response dealing with these products appears to worsen antioxidant status (at S1 and S7). At R7, the peroxidation products are nearly normalized and accompanied by a persisting low, but for the moment probably sufficient, level of antioxidants. The antioxidant capacity for further insult could nevertheless be diminished.
As for lipids, our observation of hypocholesterolaemia is in line with studies presenting the decrease in cholesterol level in septic, surgical and other critically ill patients [49]. Decreased HDL-C accords with findings from other studies on SPs [50]. Given HDL’s various beneficial functions, such as its pivotal role in reverse cholesterol transport and its anti-inflammatory, antioxidant and antithrombotic properties [51], its decreased level may impair immune response to sepsis and during recovery. Several clinical and experimental studies suggest that high circulating levels of various cytokines are associated with low cholesterol level during acute illness [52]. Accordingly, the drop in HDL-C is in strong negative correlation with the persisting increase in TNF-α and IL-10 found in this study. The correlation of HDL-C with albumin and CRP at S1 points to HDL’s being an acute-phase reactant [53]. Changes in HDL synthesis are mediated by cytokines produced in response to a variety of stimuli in multiple cell types that include macrophages, monocytes, T lymphocytes, endothelial and parenchymal cells [54]. Nevertheless, recent evidence suggests that it is the change in the functional properties of HDL particles rather than merely their concentration, measured as HDL-C, which properly reflects the life-saving importance of HDL for acute or chronically ill patients [55]. We have shown that the decline of HDL-C was followed by decrease in Apo-A1 and PON1 as well as increase in SAA. Moreover, the changes in these HDL-associated proteins continued to persist after clinical recovery from sepsis. HDL-associated PON1 is considered to be another antioxidant enzyme playing an important role in defence against oxidative stress [56, 57]. We confirmed our pilot study’s finding that had shown a decline in PON1 activity during sepsis [58]. Simultaneously, other authors had reported decrease in PON1 activity in patients at the onset of sepsis [59, 60]. They had demonstrated that antioxidant effect of HDL on LDL oxidative modification is mediated by HDL-bound PON1. The ability of PON1 to protect LDL against oxidation consists in the interaction of oxidized lipids with free sulfhydryl groups of the enzyme accompanied by its inactivation [61]. There is some evidence suggesting that apolipoproteins are also responsible for the antioxidant properties of HDL [62]. In this study, the decrease in HDL-C, PON1 and apo-A1 was closely followed by a marked increase in SAA concentration persisting until R7. It is known that during inflammation SAA replaces Apo-A1 and displaces PON1 from the association with HDL, which event is accompanied by decrease in PON1’s activity [63]. These changes transform originally anti-inflammatory HDL into pro-inflammatory HDL. McGillicuddy et al. [64] reported that acute-phase HDL enriched in SAA has an impaired capacity to remove cholesterol from macrophages during acute endotoxemia, thus indicating impaired reverse cholesterol transport during inflammation. Our patients also show signs of hypertriglyceridemia that correspond to some studies that have observed increased serum TAG levels in patients with infection [65, 66]. The hypertriglyceridemic effect induced by sepsis/inflammation is mostly alarming landmark of extreme severity of illness and bad prognosis.
Conclusions
The persistence of enhanced TNF-α and IL-10 concentrations in combination with decreased antioxidant level is in correlation with the decrease in albumin and HDL-C concentrations during the recovery phase. This finding corresponds with the well-known and often neglected poorer outcome of these high-risk severe sepsis/septic shock survivors, which might be explained by a prolonged combined presence of persisting low-grade inflammation and hypometabolic state. Moreover, the present results suggest that determination of the HDL-C and albumin concentrations is relatively affordable and provides satisfactory monitoring of these high-risk patients during the recovery period. Nevertheless, to confirm this assumption, a larger, long-term outcome study should be undertaken. In addition, such simple interventions as balanced nutritional supplementation and physiotherapy as well as the prevention and vigorous treatment of subsequent complications in various post-ICU settings (internal medicine, general practice, long-term care) might comprise a promising approach to improving the outcomes of patients discharged after severe sepsis. The follow-up care effort and resources should be directed to facilitating sufficient functional recovery.
References
Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–6.
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10.
van Ruler O, Schultz MJ, Reitsma JB, Gouma DJ, Boermeester MA. Has mortality from sepsis improved and what to expect from new treatment modalities: review of current insights. Surg Infect (Larchmt). 2009;10:339–48.
Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15.
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50.
Gullo A, Bianco N, Berlot G. Management of severe sepsis and septic shock: challenges and recommendations. Crit Care Clin. 2006;22:489–501.
Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am. 2008;55:647–68.
Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29:617–25.
Crimi E, Sica V, Williams-Ignarro S, et al. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006;40:398–406.
Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med. 1995;23:646–51.
Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49.
Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–63.
Linner A, Sunden-Cullberg J, Johansson L, Hjelmqvist H, Norrby-Teglund A, Treutiger CJ. Short- and long-term mortality in severe sepsis/septic shock in a setting with low antibiotic resistance: a prospective observational study in a Swedish university hospital. Front Public Health. 2013;1:51.
Lichtenstern C, Brenner T, Bardenheuer HJ, Weigand MA. Predictors of survival in sepsis: what is the best inflammatory marker to measure? Curr Opin Infect Dis. 2012;25:328–36.
Yende S, D’Angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–7.
Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8.
Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983;35:1126–38.
Kodydkova J, Vavrova L, Zeman M, et al. Antioxidative enzymes and increased oxidative stress in depressive women. Clin Biochem. 2009;42:1368–74.
Ahotupa M, Ruutu M, Mantyla E. Simple methods of quantifying oxidation products and antioxidant potential of low density lipoproteins. Clin Biochem. 1996;29:139–44.
Guevara I, Iwanejko J, Dembinska-Kiec A, et al. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin Chim Acta. 1998;274:177–88.
Lasanianos NG, Kanakaris NK, Dimitriou R, Pape HC, Giannoudis PV. Second hit phenomenon: existing evidence of clinical implications. Injury. 2011;42:617–29.
Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–80.
Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27:1262–4.
Sethu S, Melendez AJ. New developments on the TNFalpha-mediated signalling pathways. Biosci Rep. 2011;31:63–76.
Huang ZF, Massey JB, Via DP. Differential regulation of cyclooxygenase-2 (COX-2) mRNA stability by interleukin-1 beta (IL-1 beta) and tumor necrosis factor-alpha (TNF-alpha) in human in vitro differentiated macrophages. Biochem Pharmacol. 2000;59:187–94.
Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101.
Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother. 2011;66(Suppl 2):33–40.
Behnes M, Brueckmann M, Liebe V, et al. Levels of oxidized low-density lipoproteins are increased in patients with severe sepsis. J Crit Care. 2008;23:537–41.
Baron P, Traber LD, Traber DL, et al. Gut failure and translocation following burn and sepsis. J Surg Res. 1994;57:197–204.
Andresen M, Regueira T, Bruhn A, et al. Lipoperoxidation and protein oxidative damage exhibit different kinetics during septic shock. Mediators Inflamm. 2008;2008:168652.
Strand OA, Leone A, Giercksky KE, Kirkeboen KA. Nitric oxide indices in human septic shock. Crit Care Med. 2000;28:2779–85.
Warner A, Bencosme A, Healy D, Verme C. Prognostic role of antioxidant enzymes in sepsis: preliminary assessment. Clin Chem. 1995;41:867–71.
Cherian S, Jameson S, Rajarajeswari C, et al. Oxidative stress in sepsis in children. Indian J Med Res. 2007;125:143–8.
Halliwell B, Gutteridge JMC. Free radicals in biology and medicine, 4th edn. New York: Oxford University Press Inc.; 2007. p. 123–124, 347–348.
Taylor DE, Piantadosi CA. Oxidative metabolism in sepsis and sepsis syndrome. J Crit Care. 1995;10:122–35.
Bulger EM, Maier RV. Antioxidants in critical illness. Arch Surg. 2001;136:1201–7.
Leff JA, Parsons PE, Day CE, et al. Increased serum catalase activity in septic patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1992;146:985–9.
Andersen HR, Nielsen JB, Nielsen F, Grandjean P. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43:562–8.
Ding L, Liu Z, Zhu Z, Luo G, Zhao D, Ni J. Biochemical characterization of selenium-containing catalytic antibody as a cytosolic glutathione peroxidase mimic. Biochem J. 1998;332(Pt 1):251–5.
Forceville X, Vitoux D, Gauzit R, Combes A, Lahilaire P, Chappuis P. Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit Care Med. 1998;26:1536–44.
Mills GC. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957;229:189–97.
Ogilvie AC, Groeneveld AB, Straub JP, Thijs LG. Plasma lipid peroxides and antioxidants in human septic shock. Intensive Care Med. 1991;17:40–4.
Weber SU, Lehmann LE, Schewe JC, et al. Low serum alpha-tocopherol and selenium are associated with accelerated apoptosis in severe sepsis. BioFactors. 2008;33:107–19.
Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–86.
Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42.
Kragh-Hansen U, Minchiotti L, Galliano M, Peters T Jr. Human serum albumin isoforms: genetic and molecular aspects and functional consequences. Biochim Biophys Acta. 2013;1830:5405–17.
Evans TW. Review article: albumin as a drug–biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002;16(Suppl 5):6–11.
Chiarla C, Giovannini I, Giuliante F, et al. Severe hypocholesterolemia in surgical patients, sepsis, and critical illness. J Crit Care. 2010;25:361.
van Leeuwen HJ, Heezius EC, Dallinga GM, van Strijp JA, Verhoef J, van Kessel KP. Lipoprotein metabolism in patients with severe sepsis. Crit Care Med. 2003;31:1359–66.
Tabet F, Rye KA. High-density lipoproteins, inflammation and oxidative stress. Clin Sci (Lond). 2009;116:87–98.
Gordon BR, Parker TS, Levine DM, et al. Relationship of hypolipidemia to cytokine concentrations and outcomes in critically ill surgical patients. Crit Care Med. 2001;29:1563–8.
Luthold S, Berneis K, Bady P, Muller B. Effects of infectious disease on plasma lipids and their diagnostic significance in critical illness. Eur J Clin Invest. 2007;37:573–9.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54.
Marsche G, Saemann MD, Heinemann A, Holzer M. Inflammation alters HDL composition and function: implications for HDL-raising therapies. Pharmacol Ther. 2013;137:341–51.
Mackness B, Hine D, McElduff P, Mackness M. High C-reactive protein and low paraoxonase1 in diabetes as risk factors for coronary heart disease. Atherosclerosis. 2006;186:396–401.
Precourt LP, Amre D, Denis MC, et al. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis. 2011;214:20–36.
Novak F, Vavrova L, Kodydkova J, et al. Decreased paraoxonase activity in critically ill patients with sepsis. Clin Exp Med. 2010;10:21–5.
Kedage V, Muttigi MS, Shetty MS, et al. Serum paraoxonase 1 activity status in patients with liver disorders. Saudi J Gastroenterol. 2010;16:79–83.
Draganov D, Teiber J, Watson C, et al. PON1 and oxidative stress in human sepsis and an animal model of sepsis. Adv Exp Med Biol. 2010;660:89–97.
Aviram M, Rosenblat M, Billecke S, et al. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999;26:892–904.
Garner B, Waldeck AR, Witting PK, Rye KA, Stocker R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem. 1998;273:6088–95.
James RW, Deakin SP. The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity. Free Radic Biol Med. 2004;37:1986–94.
McGillicuddy FC, de la Llera MM, Hinkle CC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–45.
Sammalkorpi K, Valtonen V, Kerttula Y, Nikkila E, Taskinen MR. Changes in serum lipoprotein pattern induced by acute infections. Metabolism. 1988;37:859–65.
Gallin JI, Kaye D, O’Leary WM. Serum lipids in infection. N Engl J Med. 1969;281:1081–6.
Acknowledgments
This study was supported by a research grant from the Ministry of Health of the Czech Republic (Project No. IGA NT/13236-4/2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Vavrova, L., Rychlikova, J., Mrackova, M. et al. Increased inflammatory markers with altered antioxidant status persist after clinical recovery from severe sepsis: a correlation with low HDL cholesterol and albumin. Clin Exp Med 16, 557–569 (2016). https://doi.org/10.1007/s10238-015-0390-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0390-1