Abstract
This study compared virologic response to entecavir monotherapy and de novo lamivudine plus adefovir (LAM + ADV) combination therapy in patients with chronic hepatitis B (CHB) with high viral load (HVL). Hepatitis B e antigen (HBeAg)-positive patients [hepatitis B virus (HBV) DNA levels >1 × 107 copies/ml] were assigned to LAM + ADV or entecavir treatment. The primary efficacy endpoint measure of the multicenter prospective cohort study was proportion of patients with CHB with virologic response, defined as HBV DNA <300 copies/ml at week 48. During treatment, 39.1 % (18/46) of patients in the LAM + ADV group and 48.1 % (25/52) of those in the entecavir group achieved virologic response in week 48 (P = 0.37). A baseline alanine aminotransferase (ALT) level ≥5 × ULN (upper limit of normal) or baseline serum HBV DNA level <8 log10 IU/ml could predict virologic response at week 48 (P = 0.025). The mean reduction in HBV DNA was comparable (P = 0.45); no significant difference was found in the proportion of ALT normalization (P = 0.46) or HBeAg seroconversion (P = 0.88). Two cases of genotypic resistance were found (rtM204 V + rtL180 M and rtA181T/V) in the LAM + ADV group, with a resistance rate of 4.3 %; there was no genotypic resistance in the entecavir group (P = 0.13). De novo LAM + ADV combination therapy is as effective as entecavir monotherapy in HBeAg-positive patients with CHB with HVL. Moreover, genotypic resistance was only found in the LAM + ADV group at week 48. Baseline ALT levels ≥5 ULN or baseline serum HBV DNA levels <8 log10 IU/ml were favorable predictors of virologic response in CHB with HVL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic hepatitis B virus (HBV) infection affects an estimated 400 million people worldwide and remains a serious clinical problem because of its potential adverse outcomes, including cirrhosis, hepatic decompensation, and hepatocellular carcinoma. The goals of therapy in chronic hepatitis B (CHB) are to suppress HBV replication in a sustained manner to achieve hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive patients and eventually prevent disease progression. Long-term therapy with oral nucleos(t)ide analogs (NUCs) is an effective strategy for CHB patients.
Available evidence suggests that CHB patients with high viral load (HVL) at baseline are less likely to achieve virologic response and more likely to be associated with high resistance than those with lower viral load [1, 2]. In a study involving 1006 CHB patients with a median follow-up period of 7.7 years, 8.5 % of patients developed hepatocellular carcinoma, and HVL was proved as a risk factor [3]. In HBeAg-positive patients, serum HBV DNA levels at baseline <9 log10 copies/ml or alanine aminotransferase (ALT) levels ≥2 ULN (upper limit of normal) were confirmed as strong predictors of favorable virologic and serological outcomes at week 104, including undetectable serum HBV DNA by PCR assay and HBeAg seroconversion [4]. Until now, antiviral therapy of HBV infection with HVL remains a clinical challenge. It is unclear what kind of treatment adaptation is optimal for CHB with HVL.
Treatment options for CHB with HVL recommended by Chinese CHB clinical practice guidelines suggest de novo combination therapy and potent agents with a high genetic barrier to resistance [5]. Lamivudine (LAM), the first oral agent approved for treatment of CHB, has a well-established safety and efficacy profile [6]. However, the clinical benefit is difficult to sustain over a long-term treatment, owing to the selection of HBV mutants to resistance, which occur at rates of 14–32 % annually [7]. Management of LAM-resistant CHB requires rescue therapy with appropriate complementary drugs without cross-resistance, such as adefovir dipivoxil (ADV). Entecavir (ETV), with potent HBV inhibition and a high barrier to resistance, is initially recommended as a first-line NUC in most guidelines. However, it is still unclear which treatment adaptation is better for naïve CHB patients with HVL treated with de novo combination therapy or potent agent monotherapy. Therefore, the aims of this multicenter prospective cohort study were to investigate the one-year efficacy of de novo combination therapy of LAM with ADV (LAM + ADV) and ETV monotherapy in any potential differences for treatment-naïve CHB patients with HVL.
Subjects and methods
Subjects
The multicenter prospective cohort study was conducted from July 2011 to September 2013. Patients with CHB aged 16–61 years were enrolled if they were hepatitis B surface antigen (HBsAg)-positive for at least 6 months and HBeAg-positive with HVL at baseline (defined as serum HBV DNA levels >1 × 107 copies/ml), accompanied by ALT levels greater than the ULN, documented on two separate occasions at least 2 weeks apart, with the latest ALT ≥2 × ULN. Patients were excluded if they had received previous treatment with interferon or NUCs, if they confirmed any evidence of pregnancy, if they had metabolic liver disease or markers of co-infections with hepatitis C virus, hepatitis D virus or HIV, or if they had autoimmune hepatitis, heavy alcohol abuse, liver cirrhosis, or hepatocellular carcinoma. The Institutional Review Board of Nanfang Hospital, Southern Medical University, had approved the study (ID: ZHF2011206). Each enrolled patient provided informed consent.
Of the 120 patients who met the enrollment criteria and were included at the four hospitals, 98 patients were treated with the study drugs and completed the 48-week follow-up (Fig. 1). The most common reasons for withdrawal were patient’s request (LAM + ADV, 9; ETV, 8), self-withdrawal (LAM + ADV, 2; ETV, 1), and pregnancy (LAM + ADV, 1; ETV, 1). Because patients might have taken economic burden into consideration of NUC choice, the treatment was suggested by clinical physicians and decided by patients. Forty-six (46.9 %) of the 98 patients received LAM plus ADV combination therapy, and the remaining 52 (53.1 %) patients received ETV monotherapy. The demographic and baseline characteristics of the two groups are shown in Table 1. The proportion of men to women was not significantly different between the two study groups (P = 0.56). Groups were similar in terms of ALT levels (217.9 ± 124.9 vs. 216.1 ± 163.9 U/l, P = 0.95) and serum HBV DNA levels (7.88 ± 0.65 vs. 7.91 ± 0.75 log10 IU/ml, P = 0.84).
Laboratory tests
Serum ALT levels were measured by automated techniques. Levels of HBV serological markers were determined using a commercially available radioimmunoassay (ARCHITECT i2000SR, Abbott Laboratories, 100 Abbott Park Road, Illinois, USA). Serum HBV DNA levels were measured using real-time PCR quantification, the Cobas Ampliprep/Cobas TaqMan, version 2.0 (CAP/CTM, Roche Molecular Systems, Inc., Pleasanton, CA, USA). The manufacturer reports an HBV DNA linear range of 20–1.7 × 108 IU/ml (1 IU/ml = 5.82 copies/ml).
Follow-up
The enrolled patients were followed up four teaching hospitals: Nanfang Hospital of Southern Medical University in Guangzhou (45 patients), Southwest Hospital of the Third Military Medical University in Chongqing (20 patients), the Second Xiangya Hospital of Central South University in Changsha (19 patients), and the Affiliated Sir Run Run Shaw Hospital of Zhejiang University in Hangzhou (14 patients).
Serum ALT levels, levels of HBV serological markers, and serum HBV DNA levels were evaluated every 12 weeks from baseline to week 48. Levels of HBV serological markers and serum HBV DNA levels were measured at the central laboratory (Nanfang Hospital), and serum ALT levels were assessed at local laboratories according to standard procedures.
Efficacy and safety endpoints
The primary efficacy endpoint measure of the study was the proportion of CHB patients with virologic response, defined as HBV DNA <300 copies/ml (52 IU/ml, 1.72 log10 IU/ml) at week 48. Secondary efficacy endpoint measures included mean serum HBV DNA reduction and the proportions of patients with ALT normalization, HBeAg loss or seroconversion, virologic breakthrough, primary non-response, and genotypic resistance. Safety analysis included all patients who enrolled and received study medication and had at least one safety assessment since the baseline. Safety assessment included assessment of adverse events and laboratory abnormalities. The change in blood creatinine concentration in patients receiving ADV treatment was also calculated.
Primary non-response was defined as a decrease in serum HBV DNA <2 log10 IU/ml after at least 24 weeks of therapy [8]. Virologic breakthrough was defined as a confirmed increase in HBV DNA ≥1 log10 compared with the nadir after achieving an initial response during antiviral therapy [9]. Genotypic resistance was defined as detection of mutations in the HBV genome that are known to confer resistance during antiviral treatment [10].
Statistical analysis
Continuous variables were expressed as mean and standard deviation, and categorical variables were expressed as percentages. The HBV DNA levels were expressed in logarithmic units (log10 IU/ml). The Chi-square test and t test were applied when appropriate, to determine whether the results were statistically different. The statistical significance of all tests was set as P < 0.05 by two-tailed tests. Data analyses and quality control procedures were performed using SPSS for Windows, version 13.0 (SPSS Inc. 233 South Wacker Drive, 11th Floor, Chicago, USA).
Results
Virologic response
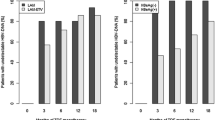
Virologic response at week 24 was generally consistent with the response at week 48 (Table 2). In the 24-week analysis, 26.1 (12/46) and 26.9 % (14/52) (P = 0.93) of patients in the LAM + ADV and ETV groups, respectively, achieved complete viral response, defined as HBV DNA <300 copies/ml. This viral control trend was maintained and continued to week 48, with 39.1 (18/46) and 48.1 % (25/52) in the LAM + ADV and ETV groups, respectively, achieving complete viral suppression (P = 0.37).
The viral quantification curves were almost identical for each treatment (Fig. 2). No statistically significant difference in HBV DNA decrease was observed between groups at week 24 (5.36 ± 1.48 vs. 5.27 ± 1.16 log10 IU/ml, P = 0.72) and week 48 (5.69 ± 1.31 vs. 5.87 ± 1.06 log10 IU/ml, P = 0.45), respectively.
Mean HBV DNA curves of two study groups. In the LAM + ADV group, the mean HBV DNA level decreased from 7.88 ± 0.65 at baseline to 2.52 ± 1.33 log10 IU/ml at week 24 and 2.19 ± 1.41 log10 IU/ml at week 48. Similarly, in the ETV group, the mean HBV DNA level decreased from 7.91 ± 0.75 log10 IU/ml to 2.65 ± 1.30 log10 IU/ml and 2.04 ± 1.04 log10 IU/ml in the two groups, respectively. ADV adefovir dipivoxil, ETV entecavir, HBV hepatitis B virus, LAM lamivudine
Biochemical and serologic responses
There were no significant differences between the two treatment groups in biochemical endpoints at week 24 and week 48. Normalization of ALT levels was achieved in 76.1 (35/46) and 71.2 % (37/52) of patients in the LAM + ADV and ETV groups, respectively, at week 24 (P = 0.58). At week 48, 91.3 % (42/46) of patients who received LAM + ADV and 86.5 % (45/52) of patients who received ETV achieved ALT level normalization (P = 0.46).
We had observed progressively increasing rates of HBeAg seroconversion in the LAM + ADV group and similar results in the ETV group (Fig. 3). At week 24, 6.5 % (3/46) of patients in the LAM + ADV group and 7.7 % (4/52) in the ETV group experienced HBeAg seroconversion (P = 0.82). At week 48, 13.0 % (6/46) in the LAM + ADV group and 11.5 % (6/52) in the ETV group achieved HBeAg seroconversion (P = 0.82).
Cumulative HBeAg seroconversion over time. Among 46 patients in the LAM + ADV group, an accumulated 1, 3, 3, and 6 patients, respectively, experienced HBeAg seroconversion at each follow-up visit; for the ETV group, accumulated 1, 4, 4, and 6 patients experienced HBeAg seroconversion. ADV adefovir dipivoxil, ETV entecavir, HBeAG hepatitis B e antigen, LAM lamivudine
Primary non-response, virologic breakthrough, and resistance
Three patients in the LAM + ADV group and one patient in the ETV group experienced primary non-response at week 24 (6.5 vs. 1.9 %, P = 0.25), as defined by the guidelines of the American Association for the Study of Liver Disease. However, we continued to observe serum HBV DNA levels with further treatment and found that all patients in both treatment groups who experienced primary non-response at week 24 achieved HBV DNA decrease >2 log10 IU/ml at week 48.
Among the patients enrolled (n = 98), there were eight episodes of confirmed virologic breakthrough (five in the LAM + ADV group and three in the ETV group) at week 48. Virologic resistance was assessed by evaluating genotypic changes using HBV polymerase/reverse transcriptase assay in patients who experienced virologic breakthrough. Three patients in the LAM + ADV group and two patients in the ETV group admitted that they administered the drugs irregularly. Only two cases of resistance were found (rtM204 V + rtL180 M and rtA181T/V, respectively) in the LAM + ADV group with a corresponding resistance rate of 4.3 %, compared with absence of genotypic resistance in the ETV group (P = 0.13).
Analysis of baseline ALT levels or baseline HBV DNA levels as predictors of 48-week treatment response
We also explored the relationships between baseline characteristics and the outcomes of virologic response at week 48. A total of 43 patients experienced virologic response at week 48 with baseline ALT levels of 259.6 ± 163.1 U/l and baseline serum HBV DNA levels of 7.67 ± 0.78 log10 IU/ml, significantly different from the other patients with baseline ALT levels of 183.6 ± 122.80 U/l (P = 0.01) and baseline serum HBV DNA levels of 8.08 ± 0.58 log10 IU/ml (P = 0.004). Furthermore, a multivariate logistic regression analysis was designed to aid in the prediction of virologic response at week 48 included baseline characteristics and type of treatment (Table 3). The baseline ALT levels ≥5 ULN or baseline serum HBV DNA levels <8 log10 IU/ml were strong predictors for the virologic response at week 48 (P = 0.046 and P = 0.029, respectively). Odds ratio for baseline ALT levels ≥5 ULN versus <5 ULN was 2.35 (95 % confidence interval (CI) 1.02–5.46), and that for baseline serum HBV DNA levels <8 versus ≥8 log10 IU/ml was 2.56 (95 % CI 1.10–5.94).
Drug safety
Both LAM + ADV and ETV were well tolerated through the 48 weeks. No serious adverse events were identified during 48-week treatment in the two groups. No patients experienced an on-treatment hepatic flare or liver failure during treatment periods. No renal relative adverse events occurred that were attributed to the study drug by the clinical investigators. According to the changes in blood creatinine concentration in patients in the LAM + ADV group, we confirmed an absence of renal impairment, with blood creatinine concentrations greater than 1.2 mg/dl.
Discussion
Studies have shown that a high HBV DNA level is an independent predictor of LAM and telbivudine resistance and increased the risk of hepatocellular carcinoma [3, 4, 7, 11]. These results contributed to the determination of CHB patients with HVL as a clinical challenge to improve the efficacy of antiviral therapy and achieve desirable therapeutic endpoints. The optimal treatment strategy for CHB patients with HVL needed to be supported by further study. This study is the first multicenter, prospective cohort study to evaluate the efficacy and safety of combination therapy of LAM with ADV and ETV monotherapy.
Yuen et al. have recently reported a study of treatment-naïve CHB patients who received ETV for 3 years. In their study, 90.2 % of patients with baseline HBV DNA <5.9 log10 copies/ml had achieved virologic response at week 52, in contrast to only 66.2 % of patients with HVL (≥8 log10 copies/ml) [12]. Other studies have confirmed that a serum HBV DNA level <9 log10 copies/ml at baseline is a strong predictor for virologic outcome at week 104 [4]. Data from the present 48-week study suggest, too, that CHB patients with HVL are less likely to achieve virologic response than normal CHB patients. In a cohort of 70 patients with HVL (HBV DNA ≥107 copies/ml), Jianchun [13] reported that the virologic response of combination therapy of LAM with ADV is better than ETV monotherapy. However, he admitted that the limitation of sample size resulted in biased data. Moreover, the study tested the serum HBV DNA levels by using general PCR assay, the lower limit of which is 1000 copies/ml, which was hardly sufficient to ensure the reality of the virologic response [14]. In a meta-analysis published in Virology Journal, Liu et al. [15] included four randomized controlled trials and cohorts study to determine the efficacy of LAM plus ADV compared with ETV monotherapy in the treatment of chronic hepatitis B patients. The meta-analysis indicated that combination therapy (LAM + ADV) was more effective than switching to entecavir monotherapy and had longer-lasting effects in the treatment of CHB. However, in the included studies, Yu included 32 HBeAg-negative patients (30.8 %) at baseline [16]. Also, a total of 26 HBeAg-negative patients (36.6 %) and 22 patients (30.9 %) with cirrhosis were included in Wang’s study with the serum HBV DNA levels being only between 5 and 6 log10 copies/ml [17]. In another study by Wei, the baseline serum HBV DNA levels were not reported and treatment groups were not compared to show the balanced at baseline with respect to demographics and other characteristics [18]. More importantly, this meta-analysis tested the serum HBV DNA levels using general PCR assay, while guidelines from the Asian Pacific Association for the Study of the Liver suggest Cobas TaqMan real-time PCR assay (the lower limit of which is 20 IU/ml) [10]. Our study included HBeAg-positive patients with HVL and tested the serum HBV DNA levels before and after treatment using Cobas TaqMan real-time PCR. The results indicated that there was no statistical difference in virologic response between combination therapy with LAM + ADV and ETV monotherapy. These results suggest that both LAM + ADV combination therapy and ETV monotherapy can be effective in treating patients with CHB with HVL. However, the major limitations of the study include the relatively limited number of CHB patients and the relatively short observation period which may influence the evaluation of long-term efficacy. It is necessary to be concerned to prolong and enlarge the study in the next period.
Based on data from the study, combination therapy did not induce a higher serological response. At week 24 and week 48, HBeAg seroconversion rates were similar between the LAM + ADV group and the ETV group (6.5 vs. 7.7 %, 13.0 vs. 11.5 %). A study confirmed that serum HBV DNA levels ≤9 log10 copies/ml is a positive predictor of HBeAg seroconversion with Peg-interferon-α2a treatment [19]. Among patients with CHB who received NUC treatment, studies showed that 22.2 % of patients with CHB treated with ETV experience HBeAg seroconversion at week 48, compared with 13 % of patients treated using ADV and 13–29 % of patients using LAM [12, 20, 21]. In the present studies, only 11.5 % of patients treated with ETV achieved HBeAg seroconversion compared with 13.0 % of patients treated with LAM + ADV. Patients with CHB with HVL should be regarded as a negative factor to HBeAg seroconversion in NUC treatment as are patients with CHB for interferon treatment.
The experience of virologic primary non-response or virologic breakthrough is an important incident in anti-HBV treatment. Clinical practice guidelines for the treatment of CHB have recognized that resistance may result in primary non-response or virologic breakthrough on therapy [9]. In addition, NUC-related HBV resistance is associated with prior treatment with NUC or treatment-naïve patients with HVL [1]. A report involving four multicenter, controlled, phase 3 trials indicated that high baseline HBV DNA level, high body mass index, and male sex were significant and independent predictors for developing tyrosine-methionine-aspartate-aspartate (YMDD) variants in LAM treatment (P < 0.03) [7]. The four patients (three in the LAM + ADV group and one in the ETV group) in our study who experienced primary non-virologic response all had a partial virologic response with mean HBV DNA reduction of 3.36 ± 1.05 log10 IU/ml (range 2.36–4.78 log10 IU/ml) during the next 24-week treatment. A genotypic resistance test was performed in eight patients (five in the LAM + ADV group and three in the ETV group) who experienced virologic breakthrough. Finally, one case with rtM204V + rtL180M mutation and one case with rtA181T/V mutation were confirmed from the LAM + ADV group. The three patients from the LAM + ADV group admitted that they had administered the drugs irregularly, and frequently missed doses. Our results suggest that ensuring patients’ adherence to drug treatment regimens might contribute to virologic breakthrough more frequently [21, 22]. Meanwhile, for virologic breakthrough, it is necessary to monitor closely and identify patients with CHB with HVL, especially those with biochemical breakthrough characterized by an increased serum ALT level. Although the difference in primary non-response between LAM + ADV combination therapy and ETV monotherapy was not significant, it is worth considering that a 3.5-fold difference in proportion of patients who experienced primary non-response in ETV group (1.9 %) and LAM + ADV group (6.5 %). We acknowledge that the number of patients for the current study is limited and a large number of the CHB patients with HVL are still need to be investigated.
Renal laboratory abnormalities reported with 30 mg daily ADV were not found with the 10-mg dosage during the 1-year study period, as the results from this study showed. However, another study reported that the cumulative incidences of renal impairment at 1, 3, and 5 years were 1.4, 7.5, and 10.5 %, respectively, defined as estimated glomerular filtration rate <50 ml/(min 1.73 m2) [22]. It is suggested that, however, renal function should still be noted among patients with CHB receiving LAM + ADV therapy.
The 2-year-long GLOBE trial identified that baseline ALT level ≥2 ULN is a strong predictor of better efficacy outcomes at week 104 in telbivudine treatment [4]. In this study, we found that a high ALT levels at baseline (≥5 ULN) or n relative low HBV DNA levels (<8 log10 IU/ml) were significant predictors of a favorable virologic response at week 48. This suggests that, compared with patients with general CHB, those patients with CHB with HVL need a higher ALT level at baseline to achieve a better virologic outcome in antiviral treatment.
A previous cost-effectiveness study by Veenstra et al. [23] has reported a benefit for ETV versus LAM + ADV for HBeAg-seropositive CHB patients, supporting the use of ETV as first-line therapy. Although the price of the LAM + ADV and ETV is different in the USA and China, the conclusion is still valuable to those Chinese CHB patients with HVL. In Guangzhou of China, the cost of the ETV (921 yen/month) is greater than that of the LAM + ADV (889 yen/month), and a cost-effectiveness analysis is necessary in order to provide knowledge that may facilitate an informed choice between the two treatments.
One limitation of this study was the lack of randomization of patients. Because the drug in the study was not free, patients might have taken economic burden into consideration when choosing NUCs. However, the baseline demographics were similar and comparable in the two groups. Another limitation was the relatively large number of patients who dropped out of the study. A total of 98 patients from 120 patients were included in the efficacy analysis, with a dropout rate of 18.3 %, still less than the upper limit of 20 %, and it is unlikely to contribute to the biased results in this study.
In conclusion, this study showed that combination therapy of LAM with ADV and ETV monotherapy can be used both to suppress HBV replication and to improve clinical outcomes in 48 weeks of treatment. However, for patients with CHB with HVL who received LAM + ADV combination therapy, it is still necessary to be aware of the possibility of HBV mutation to drug-resistant forms. We must pay close attention to the incidence of primary non-response and virologic breakthrough. Baseline ALT levels ≥5 ULN or HBV DNA levels <8 log10 IU/ml were favorable predictors of virologic response at week 48 in CHB with HVL. As for the CHB patients with HVL in a long-term treatment, the optimal treatment strategy still needs to be evaluated.
Abbreviations
- ADV:
-
Adefovir dipivoxil
- ALT:
-
Alanine aminotransferase
- CHB:
-
Chronic hepatitis B
- ETV:
-
Entecavir
- HBeAb:
-
Hepatitis B e antibody
- HBeAg:
-
Hepatitis B e antigen
- HBsAb:
-
Hepatitis B surface antibody
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HVL:
-
High baseline viral load
- LAM:
-
Lamivudine
- NUC:
-
Nucleos(t)ide analog
- PCR:
-
Polymerase chain reaction
- ULN:
-
Upper limit of normal
- YMDD:
-
Tyrosine-methionine-aspartate-aspartate
References
Zoulim F, Locarnini S. Management of treatment failure in chronic hepatitis B. J Hepatol. 2012;56(Suppl 1):S112–22.
Zoutendijk R, Reijnders JG, Brown A, Zoulim F, Mutimer D, Deterding K, Petersen J, Hofmann WP, Buti M, Santantonio T, van Bommel F, Pradat P, Oo Y, Luetgehetmann M, Berg T, Hansen BE, Wedemeyer H, Janssen HL. Entecavir treatment for chronic hepatitis B: adaptation is not needed for the majority of naive patients with a partial virological response. Hepatology. 2011;54:443–51.
Chan HL, Tse CH, Mo F, Koh J, Wong VW, Wong GL, Lam CS, Yeo W, Sung JJ, Mok TS. High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol. 2008;26:177–82.
Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, Manns M, Bzowej N, Niu J, Han SH, Hwang SG, Cakaloglu Y, Tong MJ, Papatheodoridis G, Chen Y, Brown NA, Albanis E, Galil K, Naoumov NV. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486–95.
Chinese Society of Hepatology, CMA. The guideline of prevention and treatment for chronic hepatitis B (2010 version). Chin J Hepatol. 2011;19:13–24.
Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, Stephenson SL, Gray DF. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–8.
Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687–96.
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2.
European Association for the Study of the Liver. Clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85.
Liaw Y, Kao J, Piratvisuth T, Chan HLY, Chien R. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–61.
Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N, Naoumov NV, Di Bisceglie AM, Zeuzem S, Moon YM, Goodman Z, Chao G, Constance BF, Brown NA. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–88.
Yuen MF, Seto WK, Fung J, Wong DK, Yuen JC, Lai CL. Three years of continuous entecavir therapy in treatment-naive chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106:1264–71.
Jian-chun Z. De novo combination therapy with lamivudine and adefovir dipivoxil versus entecavir monotherapy for na(i)ve chronic hepatitis B patients with high viral loads. Zhong Hua Lin Chuang Gan Ran Bing Xue Za Zhi. 2012;5:142–4.
Cai SH, Lv FF, Zhang YH, Jiang YG, Peng J. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis. 2014;14:85.
Liu F, Wang X, Wei F, Hu H, Zhang D, Hu P, Ren H. Efficacy and resistance in de novo combination lamivudine and adefovir dipivoxil therapy versus entecavir monotherapy for the treatment-naive patients with chronic hepatitis B: a meta-analysis. Virol J. 2014;11:59.
Yu JH, Shi JP, Wu J, Li XO, Guo JC, Xun YH, Zhao C, Jin J, Xu AF, Lou GQ. Efficacy and safety of lamivudine plus adefovir combination therapy and entecavir monotherapy for chronic hepatitis B patients. Zhonghua Gan Zang Bing Za Zhi. 2011;19:88–92.
Wang LC, Chen EQ, Cao J, Liu L, Zheng L, Li DJ, Xu L, Lei XZ, Liu C, Tang H. De novo combination of lamivudine and adefovir versus entecavir monotherapy for the treatment of naive HBeAg-negative chronic hepatitis B patients. Hepatol Int. 2011;5:671–6.
Wei W, Huang Z. Effect of entecavir and lamivudine combined with adefovir in treatment of e antigen-positive chronic hepatitis B. Chin J Hosp Infect. 2012;22(19):4335–45.
Cooksley G, Manns M, Lau G, Liaw YF, Marcellin P, Chow WC, Thongsawat S, Gane E, Fried MW, Zahm F. Effect of genotype and other baseline factors on response to peginterferon alpha-2a (40 kDa)(PEGASYS (R)) in HBeAg-positive chronic hepatitis B: results from a large, randomised study. J Hepatol. 2005;42(Suppl 2):30–1.
Marcellin P, Chang TT, Lim SG, Sievert W, Tong M, Arterburn S, Borroto-Esoda K, Frederick D, Rousseau F. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48:750–8.
Yao GB, Zhu M, Cui ZY, Wang BE, Yao JL, Zeng MD. A 7-year study of lamivudine therapy for hepatitis B virus e antigen-positive chronic hepatitis B patients in China. J Dig Dis. 2009;10:131–7.
Tanaka M, Suzuki F, Seko Y, Hara T, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y, Saitoh S, Arase Y, Ikeda K, Kobayashi M, Kumada H. Renal dysfunction and hypophosphatemia during long-term lamivudine plus adefovir dipivoxil therapy in patients with chronic hepatitis B. J Gastroenterol. 2014;49(3):470–80.
Veenstra DL, Sullivan SD, Clarke L, Iloeje UH, Tafesse E, Di Bisceglie A, Kowdley KV, Gish RG. Cost effectiveness of entecavir versus lamivudine with adefovir salvage in HBeAg-positive chronic hepatitis B. Pharmacoeconomics. 2007;25:963–77.
Acknowledgments
This study was supported by Grants from Bristol-Myers Squibb and the Chinese Foundation for Hepatitis Prevention and Control (GHF 2011206).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The Institutional Review Board of Nanfang Hospital, Southern Medical University, had approved the study (ID: ZHF2011206). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for inclusion in the study.
Conflict of interest
Shaohang Cai, Tao Yu, Yegui Jiang, Yonghong Zhang, Fangfang Lv, and Jie Peng declare that they have no financial or personal relationships with other people or organizations that could inappropriately influence this work.
Rights and permissions
About this article
Cite this article
Cai, S., Yu, T., Jiang, Y. et al. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med 16, 429–436 (2016). https://doi.org/10.1007/s10238-015-0373-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0373-2