Abstract
Autoimmune uveitis (AU), an inflammatory non-infectious process of the vascular layer of the eye, can lead to visual impairment and, in the absence of a timely diagnosis and suitable therapy, can even result in total blindness. The majority of AU cases are idiopathic, whereas fewer than 20 % are associated with systemic diseases. The clinical severity of AU depends on whether the anterior, intermediate, or posterior part of the uvea is involved and may range from almost asymptomatic to rapidly sight-threatening forms. Race, genetic background, and environmental factors can also influence the clinical picture. The pathogenetic mechanism of AU is still poorly defined, given its remarkable heterogeneity and the many discrepancies between experimental and human uveitis. Even so, the onset of AU is thought to be related to an aberrant T cell-mediated immune response, triggered by inflammation and directed against retinal or cross-reactive antigens. B cells may also play a role in uveal antigen presentation and in the subsequent activation of T cells. The management of AU remains a challenge for clinicians, especially because of the paucity of randomized clinical trials that have systematically evaluated the effectiveness of different drugs. In addition to topical treatment, several different therapeutic options are available, although a standardized regimen is thus far lacking. Current guidelines recommend corticosteroids as the first-line therapy for patients with active AU. Immunosuppressive drugs may be subsequently required to treat steroid-resistant AU and for steroid-sparing purposes. The recent introduction of biological agents, such as those targeting tumor necrosis factor-α, is expected to remarkably increase the percentages of responders and to prevent irreversible sight impairment. This paper reviews the clinical features of AU and its crucial pathogenetic targets in relation to the current therapeutic perspectives. Also, the largest clinical trials conducted in the last 12 years for the treatment of AU are summarized and critically discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uveitis is an inflammatory disorder involving the pigmented vascular coat of the eyeball. This term, however, is now used more extensively to describe many forms of intraocular inflammation affecting not only the uveal tract but also the retina and its vessels, the optic nerve, and the vitreous. Uveitis is a major cause of severe visual impairment and accounts for 10–15 % of all cases of total blindness in the USA [1]. Its prevalence is estimated to be 115 cases per 100,000 people, with an incidence in the USA of 52.4/100,000 person-years [1]. Uveitis is more frequently diagnosed in younger adults: In our own experience, 48 out of 104 (46.1 %) difficult-to-treat patients with AU, followed up at a single tertiary reference center, were of <40 years at presentation [2]. There is a slightly higher prevalence in females (M/F ratio 0.68 to 0.95) [3–6], with few exceptions (M/F ratio 1.06 to 1.08) [7, 8].
The disease is classified as: (a) infectious, when an obvious infectious agent is recognized (e.g., Herpes viruses, Toxoplasma gondii, Candida albicans, Borrelia burgdorferi, Mycobacterium tuberculosis, Chlamydia trachomatis); (b) non-infectious, when it is believed to be autoimmune or immune-mediated in origin. Although the distinction between autoimmune and immune-mediated is vague, the former is linked to an autoimmune reaction to self-proteins (autoantigens), while the latter is primarily due to an aberrant activation of the innate inflammatory response to environmental triggers (microbial) or to autologous immune changes (tissue damage) [9]. Thus, the term autoimmune uveitis (AU) indicates an isolated type of autoimmune uveal inflammation that can occur without other autoimmune manifestations (idiopathic autoimmune uveitis: I-AU) or in the context of a known systemic autoimmune disease (systemic disease-associated autoimmune uveitis: SDA-AU) [10]. Sometimes, mainly in elderly patients, uveitis is the first presentation of an occult malignancy (“masquerade syndrome”), e.g., a central nervous system lymphoma [11, 12].
In a large cross-sectional study comprising 2619 patients with uveitis, 62.8 % of them had I-AU, 18.5 % had SDA-AU, and the remaining 18.7 % infectious uveitis [6]. These results are consistent with our cohort of 104 difficult-to-treat patients AU patients [2], with a higher prevalence of I-AU (72.1 %) compared with SDA-AU (27.9 %). Owing to this heterogeneity, the diagnosis and the treatment of AU are frequently challenging.
The aim of this paper is to provide an overview of the clinical features, pathogenetic clues, and therapeutic approach to AU.
Nosographic setting and epidemiological data
The most widely used classification of uveitis was devised by the International Uveitis Study Group (IUSG) since 1987 [13], based on the anatomic location of the inflammatory process. It includes (a) anterior uveitis (iritis, iridocyclitis, and anterior cyclitis), (b) intermediate uveitis (pars planitis, posterior cyclitis, and hyalitis), (c) posterior uveitis (focal, multifocal, or diffuse choroiditis, chorioretinitis, retinitis, and diffuse neuroretinitis), and (d) panuveitis (involving the anterior chamber, the vitreous, the retina, and the choroid). Several years later, the Standardization of the Uveitis Nomenclature (SUN) Working Group [14] emphasized the validity of the anatomic classification and added new standards for grading anatomic location and inflammatory activity. These may be useful in clinical trials for testing the effectiveness of novel treatments. Nevertheless, ambiguities remain, such as in pars planitis, neuroretinitis, and anterior/intermediate uveitis [15].
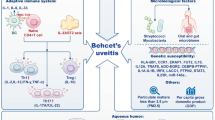
Because the patterns of AU differ in various geographic regions according to race, genetic background, and environmental factors, it is hard to establish a universal classification system [16]. In the already-mentioned cross-sectional study [6] of 2619 patients with uveitis, the most common anatomic form was anterior uveitis (59.9 %), followed by posterior (18.3 %), intermediate (14.8 %), and panuveitis (7 %). Anterior and intermediate AU account for the majority of idiopathic forms in both Eastern and Western countries and may be associated with Behçet’s disease [17], spondyloarthropathies [18, 19], and juvenile idiopathic arthritis [20]. Posterior AU is mainly idiopathic in Western countries [21] and is associated with Behçet’s disease in Turkey [22] and Iran [23]. In our own experience with 104 difficult-to-treat patients, the different systemic diseases that were associated to AU are reported in Fig. 1.
Clinical features
Obviously, the clinical picture of AU varies according to which part of the uvea is involved. Symptoms can range from eye redness and pain to light sensitivity, blurred vision (distinctive of anterior uveitis) or declining visual acuity (retinitis or neuroretinitis). Some patients (often those with childhood posterior uveitis) may be asymptomatic. Symptoms usually occur suddenly and worsen rapidly, although sometimes they develop gradually and become chronic or recurrent [24]. They may affect one eye or both eyes. Based on the observation of 104 difficult-to-treat patients with AU, who had been referred to our tertiary reference center and had been followed up for a median of 4.8 years, the most frequent complications, at disease onset or later, were cataract (24 %), retinal neovascularization (16.3 %), chorioretinal scars (10.6 %), cystoid macular edema (8.6 %), glaucoma/hypertension (7.7 %), epiretinal membranes (4.8 %), and retinal detachment (3.8 %) [2]. AU can involve only the eye, or it can be part of a systemic disease whose identification is helpful for the choice of treatment.
Several conditions can be related to AU: Some are known systemic diseases, while others are rare syndromes restricted to the eye. One first example is birdshot chorioretinopathy, a rare posterior bilateral uveitis characterized by distinctive, multiple, hypo-pigmented choroidal and retinal areas of inflammation. At least 96 %, and possibly all patients, share the major histocompatibility antigen HLA-A29 [25]: Patients who are HLA-A29 carriers exhibit a relative risk ratio of 49.9 for birdshot chorioretinopathy [26]. Another example is sympathetic ophthalmia, also called sympathetic uveitis, a rare bilateral granulomatous panuveitis that occurs following a penetrating injury to one eye. Its pathophysiology is still unclear, but it has been hypothesized that the disrupted integrity of the injured eye leads to an autoimmune hypersensitivity reaction against the exposed ocular antigens in the injured eye as well as in the sympathizing eye [27].
Many systemic rheumatologic disorders can be associated with uveitis: It is known, for example, that the most frequent extra-articular manifestation in spondyloarthritis is eye involvement, which occurs in percentages ranging from 25 to 30 % in patients with psoriatic arthritis to approximately 33 % in those with ankylosing spondylitis [28, 29]. The occurrence of AU usually precedes systemic involvement [30], and its prevalence increases with the duration of disease in HLA-B27-positive patients [31, 32]. Typical eye involvement is sudden-onset, unilateral, anterior uveitis (iridocyclitis), characterized by painful red eye with photophobia and blurred vision together with a strong tendency for recurrent attacks, also in the contralateral eye.
Eye involvement in Behçet’s disease occurs in 30–70 % of patients, with a more severe progression in male and young patients (disease onset <40 years). Generally, eye involvement appears 2–3 years after recognition of the disease; only in a quarter of cases, it is the first presenting feature [33, 34]. The typical form is a chronic, relapsing, bilateral uveitis affecting both anterior and posterior segments of the uvea (panuveitis). Retinal vasculitis and vitreitis are the most frequent findings in posterior uveitis, while anterior uveitis with hypopyon is peculiar to ocular Behçet’s disease, although it is diagnosed in only one-third of patients [33]. Ocular involvement in Behçet’s disease is characterized by explosive attacks of severe inflammation, and about 25 % of patients with retinal involvement develop serious complications (hemorrhage, thrombosis, macular disease), leading to blindness. Loss of visual acuity in association with neurological disease is the major cause of morbidity and disability in Behçet’s disease.
Pediatric uveitis affects around 10 % of children with juvenile idiopathic arthritis, with a strong prevalence among girls (80 %) [35]. Among the different phenotypes of juvenile idiopathic arthritis, the oligoarticular type is most frequently associated with anterior uveitis, positivity of antinuclear antibodies, and the presence of DRB1*0801 (DRw8) allele [36]. The majority of patients usually present a bilateral iridocyclitis within 4 years from the onset of the disease, with a typically indolent and chronic course that is defined as “white uveitis” [35, 37]. Both asymptomatic and untreated forms can progress to total blindness [38]. The most frequent complications of uveitis associated with juvenile idiopathic arthritis are band keratopathy, posterior synechiae, and ocular hypertension [37].
AU is a frequent complication of systemic sarcoidosis, occurring in 20–50 % of these patients [39]. Because an isolated ocular sarcoidosis rarely develops in older patients, it is often misdiagnosed as primary intraocular lymphoma [40]. Sarcoidosis can involve any part of the eye, including the orbit and the lacrimal gland. It usually presents with bilateral fat keratic precipitates, iris nodules, and anterior and posterior synechiae. Long-term complications are common, and cystoid macular edema is the worst consequence.
Vogt–Koyanagi–Harada syndrome is a systemic autoimmune disease characterized by a bilateral, chronic, diffuse, granulomatous panuveitis that is frequently associated with other systemic disorders, including those with neurological, auditory, and tegumentary manifestations [41, 42]. It is also one of the most common forms of uveitis among Chinese and Japanese patients [7]. More rarely, AU is associated with other autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, Wegener’s granulomatosis, Sjögren’s syndrome, polyarteritis nodosa, primary anti-phospholipid syndrome [43], autoimmune hepatitis [44], multiple sclerosis [45], inflammatory bowel diseases [46], and celiac disease [47].
Finally, several drugs with indications in diseases pertaining to different areas of medicine (such as cidofovir, anti-mycobacterial antibiotics, bisphosphonates, sulfonamides, tumor necrosis factor-α inhibitors, oral fluoroquinolones, and anti-vascular endothelial growth factor monoclonal antibodies) may uncommonly be associated with uveitis [48, 49]. Often overlooked or misdiagnosed in the past, drug-induced uveitis is now increasingly recognized, thus becoming an important step of the uveitis diagnostic algorithm. Although the mechanisms underlying drug-induced intraocular inflammation are poorly understood, direct or immune-mediated toxic effects may be involved [50]. In 1981, Naranjo et al. [51] established several criteria to determine the potential causal relationship between medication and adverse reaction. These criteria are still used to distinguish four categories of causal likelihood (i.e., definite, probable, possible, and doubtful) according to a numerical score (0–13). Patients with drug-induced uveitis commonly present with moderate-to-severe non-granulomatous anterior uveitis, scleritis and episcleritis that develop 1–3 weeks after the administration of a systemic, intraocular, or topical drug. Discontinuation of the suspected drug(s) and/or addition of topic corticosteroids and cycloplegic therapy is usually followed by resolution of the ocular inflammation [48].
Pathogenetic clues
The delicate nature of the ocular tissues has greatly limited the use of invasive techniques aimed at obtaining tissue specimens, and this has obviously hampered the studies of the pathogenetic mechanisms underlying the heterogeneous spectrum of AU. In addition, the experimental models of AU, in spite of their undisputable usefulness, do not reproduce exactly the complex relationships between the immune system and the eye [9]. Nevertheless, it has been hypothesized that a number of inflammatory processes may induce an aberrant T cell-mediated immune response that breaks down the blood–retinal barrier and acts against retinal antigens or cross-reactive antigens [52, 53]. The retinal antigens involved are most probably components of melanocytes or tyrosinase or tyrosinase-related proteins [54, 55], such as retinal arrestin (S-antigen), inter-photoreceptor retinoid-binding protein, recoverin, melanin proteins and their products, and rhodopsin [56].
Retinal antigen-specific CD4+ T cells account for the pivotal role of inflammation in the development and maintenance of the disease [54]. Clinical studies in humans and in animal models have shown specific roles for several T cell effector phenotypes and their cytokine pathways: T helper 1 (producing interleukin-2 and interferon-γ) and T helper 2 cells (producing interleukins 4, 5, and 13) exert both pathogenetic and protective roles, whereas T helper 9 (interleukins 9 and 10) and T helper 17 cells (interleukins 17A, 21, and 22) play exclusively pathogenetic roles [53]. Recently, T helper 17 cells have been implicated in the uveal pathology based on the observation that, in mice, the deletion of signal transducers and activators of transcription 3 (STAT 3) and retinoic acid receptor-related orphan nuclear receptors (RORγt and RORα) in CD4+ T cells abrogates T helper 17 cell differentiation and prevents the onset of posterior AU [57]. Moreover, CD4+ T cells isolated from experimental AU exhibit high levels of death receptor 3, which promotes the increase in interleukin-17 [58] and serum interleukin-17A levels in active uveitis [59]. Particularly, T helper 17 cells seem to be involved in the end phase of experimental AU, in which blockade of interferon-γ and interleukin-4 in wild-type mice resulted in the exacerbation of AU at a later phase marked by increased interleukin-17 production, whereas AU severity was significantly reduced in interleukin-17(−/−) mice [60]. Although either the Th1 or the Th17 effector response can independently drive uveitis, it is likely that in human uveitis, the conditions related to the early events play a pivotal role in determining the dominant effector T cell response [61]. Additional studies on the pathogenesis of AU have been focused on the involvement of innate immune response, especially during the first phase of anterior AU. This evidence and the absence of specific autoantigens suggest that AU can be considered an auto-inflammatory condition [52].
Toll-like receptors 2 and 4 (TLR2 and TLR4) have been shown to be constitutively expressed by antigen-presenting cells of normal uvea, retina, sclera, and conjunctiva [62] and to serve as the link between innate and cell-mediated immune response [63]. In secondary lymphoid tissues, bacterial components such as the peptidoglycan of gram-positive bacteria and lipopolysaccharides of gram-negative bacteria bind to TLR2 and TLR4, respectively, leading to the activation of the nuclear factor kappa light chain enhancer of activated B cells (NF-kB) pathway and to the production of pro-inflammatory cytokines IL-1, IL-2, IL-6, and TNF-α [64, 65]. These responses induce naïve T cells to differentiate into two main subsets of autoreactive effector CD4+ T cells, namely T helper 1 and T helper 17 [53, 65]. Importantly, in TLR4 gene-deficient mice, experimental anterior AU cannot be induced, as shown by clinical manifestations, histological changes, and expression of the downstream signal transduction molecules MyD88 and NF-kB, all of which are absent in these animals [65]. The possible implications of TLR2 and TLR4 polymorphisms in the Caucasian populations have not yet been clarified [63].
Following activation and polyclonal expansion, T helper 1 and T helper 17 cells escape peripheral tolerance mechanisms and move to the eyes, where they probably reduce and inhibit CD4+CD25+ regulatory T cells (Tregs) [66, 67]. This is likely to occur especially in patients with Behçet’s disease [68] and Vogt–Koyanagi–Harada syndrome [69]. An increase in the frequency and immunoregulatory activity of Tregs has been demonstrated during the development of experimental AU, in which Tregs are able to induce the regression of inter-photoreceptor retinoid-binding protein-induced AU in mice [70]. In addition, in a series of AU patients the Treg lymphocyte population increased and the functional state of these cells was restored following therapy-induced improvement [71].
Experimental models indicate that the complement pathway and complement regulatory proteins (Cregs), including Crry (complement-related gene/protein y, 512 antigen), are involved in AU. In fact, antibodies against factor B prevent the development of anterior AU in mice, inhibiting specific CD4+ T cell proliferation in vitro and reducing tumor necrosis factor-α and interferon-γ production [72]. Furthermore, complement inhibition by recombinant Crry linked to the Fc fragment of rat immunoglobulin G (Crry-Ig) reduces the levels of C3, the membrane attack complex, chemokines (IFN-γ), and adhesion molecules (ICAM-1, LECAM-1, and IP-10) in the eye and rapidly improves experimental anterior AU [73]. Similarly, C3−/− mice are less susceptible of developing AU [74]. Complement (via C5a) can also activate macrophages; studies in mice have indeed demonstrated the hyper-expression of CD200 receptors on retinal resident macrophages, which promote a permanent inhibitory state [75]. The AU that develops in CD200−/− mice is characterized by extensive macrophage infiltrates with IFN-γ-mediated nitric oxide and IL-6 production [76]. Specific polymorphisms of complement components are also associated with the development of AU, such as complement factor H gene in posterior and intermediate AU [77] and C2 component and complement factor B in anterior AU [78].
Finally, adhesion molecules promote T cell migration into the eyes through the blood–retinal barrier, as shown by the administration of monoclonal antibodies against ICAM-1, LFA-1 [79], VLA-4, and VCAM-1 [80] and of anti-α4-integrin inhibitor peptide [81], all of which prevent the development of experimental AU. T cells move into the eye also following breakdown of the blood–retinal barrier, in response to the actions of vascular endothelial growth factor, tumor necrosis factor-α, and interleukin-1β on the tight junctions between endothelial cells [82].
The role of B cells in AU is poorly understood [9]. B cell depletion induced by the administration of an anti-CD20 monoclonal antibody (rituximab) has been shown to be effective in patients with chronic anterior uveitis resistant to corticosteroids and conventional immunosuppressive therapy [83], in Behçet’s disease-associated uveitis [84], and in juvenile idiopathic arthritis [85]. These observations suggest that B cells may be involved in uveal antigen presentation and the subsequent activation of T cells.
Treatment of autoimmune uveitis
The abovementioned pathogenetic pathways provide the rationale for the therapeutic use of immunosuppressants, cytokines, and biological drugs at various levels in AU (Fig. 2). The most commonly used drugs act on one or more key points in the proposed pathogenetic pathway: (1) Corticosteroids and cyclophosphamide block NF-kB signaling activated by TLR2 and TLR4 (peripheral lymph nodes) in addition to acting on activated CD4+ cells; (2) anakinra (IL-1 receptor antagonist) and tocilizumab (anti-interleukin-6) neutralize specific cytokines of the innate immune response (peripheral lymph nodes); (3) cyclosporin A, tacrolimus, and voclosporin inhibit CD4+ T cells and the nuclear factor of activated T cells; azathioprine, methotrexate, and mycophenolate mofetil suppress T cells and activate B cells (peripheral lymph nodes); (4) interferon-α has anti-proliferative activity on T cells; (5) fingolimod, by modulating the sphingosine-1-phosphate receptor, is able to halt T cell discharge by lymph nodes; (6) natalizumab (anti-α4-integrin) prevents T cell migration through the endothelium in experimental AU; (7) anti-tumor necrosis factor-α (infliximab, adalimumab, certolizumab, and etanercept) and anti-interleukin-2 (daclizumab) inhibit, respectively, tumor necrosis factor-α and interleukin-2 produced by activated T helper 1 cells (peripheral lymph nodes); (8) secukinumab specifically targets interleukin-17 produced by T helper 17 cells; and (9) ustekinumab targets the p40 subunit common to both IL-12 and IL-23 that are involved in Th1 and Th17 responses, respectively; (10) anti-vascular endothelial growth factor antibodies (e.g., bevacizumab, ranizumab) neutralize vascular endothelial growth factor derived from the nuclear factor of activated T cell pathway as a consequence of the production of hypoxia-induced factor 1.
Hypothetical pathogenetic model of autoimmune uveitis, loosely inspired to that proposed by Dr. Caspi [9]. The potential therapeutic targets and the available drugs that can potentially be used in relation to each critical checkpoint are indicated. Retinal antigen-specific T cells that have not been eliminated in the thymus escape the control of nTregs and differentiate into pathogenic effector T cells that migrate into the eye. Biological drugs are included in the rounded blocks, anti-inflammatory, and immunosuppressive drugs in the rectangular block. IFN-α2, interferon-α2; Th1, T helper cells 1; Th17, T helper cells 17; APC, antigen-presenting cells; Treg, T regulatory cells; nTreg, naturally occurring T regulatory cells, VEGF, vascular endothelial growth factor; PMN, polymorphonuclear leukocytes; anti-S1p R, anti-sphingosine-1-phosphate receptor; anti-IL-1R, anti-interleukin-1 receptor; anti-IL-2 Rα, anti-interleukin-2 receptor α; anti-IL-6, anti-interleukin-6
A standard therapeutic regimen for the treatment of AU has yet to be assessed in large, randomized, controlled clinical trials [86]. Current guidelines for the use of immunosuppressive drugs [87] recommend corticosteroids as the first-line therapy for patients who present with active uveitis and the subsequent addition of immunosuppressive agents in the management of resistant AU, also with steroid-sparing purpose.
Table 1 summarizes the largest clinical trials published in the last 10 years [88–102] on systemic immunosuppressive treatment of AU, excluding those with <20 patients. The selected trials include I-AU and SDA-AU, in adults and children. The response to treatment was assessed in terms of percentages of patients achieving disease control/inactivity, significant improvement in clinical parameters such as visual acuity, and the most frequent adverse events that occurred during treatment. The following conclusions can be drawn from these studies:
-
(a)
Most of the available studies (11/15) were retrospective non-comparative analyses [88–96, 100, 102], and only four were prospective clinical trials [97–99, 101].
-
(b)
Eight studies enrolled more than 100 patients [88, 90, 92–95, 99, 101], the two largest consisting of 682 [101] and 384 patients [92].
-
(c)
Out of 15 studies, four focused on SDA-AU (juvenile idiopathic arthritis, Behçet’s disease [91, 96, 98, 101]); three dealt with idiopathic AU, defined as “non-infectious” or “autoimmune” [93–95], and the remainder considered both I-AU and SDA-AU (Behçet’s disease, juvenile idiopathic arthritis, Vogt–Koyanagi–Harada syndrome, spondyloarthropathy, inflammatory bowel disease, and sarcoidosis) [88–90, 92, 97, 99, 100, 102].
-
(d)
With the exception of five studies [88, 89, 91, 100, 101], the average study length was only 6 or 12 months. At 6 months, cyclophosphamide and mycophenolate mofetil seemed to be the most effective immunosuppressive drugs, achieving disease control in 49.2 % [94] and 53.1 % [95] of patients, respectively. At 12 months, the use of azathioprine, cyclosporin A, cyclophosphamide, mycophenolate mofetil, methotrexate, and infliximab resulted in disease control in 49 % or more of the patients enrolled in the various trials [90–98]. In particular, among patients with SDA-AU, 76.5 % achieved inactivity with azathioprine alone within 1 year [96]; in those with I-AU, 76.4 % reached disease control with cyclophosphamide [94] and 73.1 % with mycophenolate mofetil [95].
-
(e)
At 12 months, anti-tumor necrosis factor-α antibodies were found to be effective drugs in the control of disease activity (from 69.0 to 93.7 % with infliximab [97, 98, 102] and from 61.8 to 94.1 % with adalimumab [97, 99, 100]).
-
(f)
All immunosuppressants and monoclonal antibodies allowed a reduction in steroid administration, but at 12 months, the major steroid-sparing effect was obtained with anti-tumor necrosis factor-α antibodies [97], followed by cyclophosphamide [94], methotrexate [92], and mycophenolate mofetil [95].
-
(g)
The incidence of overall side effects ranged from 11.8 % with mycophenolate mofetil to 46 % with infliximab. Azathioprine was mainly associated with gastrointestinal effects [90, 96]; cyclosporine A with renal toxicity and hypertension [91, 93]; cyclophosphamide with fatigue, nausea, headache, leukopenia, and cystitis/hematuria [89, 94]; and methotrexate and mycophenolate mofetil with gastrointestinal side effects, an increase in liver enzymes, and myelotoxicity [88, 92, 95]. In the only comparative clinical trial, anti-tumor necrosis factor-α infliximab had a higher percentage of associated side effects than adalimumab. Adalimumab, on the other hand, was found to have a higher safety profile in childhood AU, based on the absence of severe complications (with the only reaction at the injection site) [97], whereas the reversibility upon discontinuation of therapy shifted the risk benefit in favor of infliximab.
A five-step protocol based on sequential therapeutic stages was recently discussed [103]. It recommends the invariable use of topical, periocular, and systemic corticosteroids in the treatment of non-infectious uveitis. Immunosuppressive drugs may enhance their anti-inflammatory effects and act as steroid-sparing agents. However, among the immunosuppressive drugs, cyclosporin A and methotrexate should be used as second-line treatment; azathioprine, mycophenolate mofetil, and tacrolimus as third-line treatment; anti-tumor necrosis factor-α monoclonal antibodies (such as adalimumab or infliximab) as fourth-line treatment, with cyclophosphamide and chlorambucil administered only if the other drugs failed to provide improvement.
Two final points should be underscored. The first is a potentially higher risk of malignancies in AU patients receiving systemic immunosuppressive therapy [104]. Nonetheless, given that the clinical advantages largely outweigh the risks, immunosuppressive therapy should be administered whenever it is deemed beneficial, and the potential risk for the later occurrence of a tumor should not be a hindrance to its use. However, for a more reliable risk assessment, multicenter, prospective, cohort studies on a large number of patients with a long and accurate follow-up are required.
The second point is the need, of careful reviewing all current and previous drugs that the AU patient has been given, in which systemic, periocular, intravitreal, and topical medications have long been recognized as potentially responsible for drug-induced ocular inflammation [48–50]. The prompt recognition of drug-induced uveitis and the consequent discontinuation of the inciting drug usually result in regression of the ocular inflammatory process, thus avoiding the administration of additional, unnecessary drugs and the possible irreversible impairment of the visual function.
Several main issues remain unresolved: (1) The classification of AU is poorly defined, such that most studies consist of heterogeneous groups of patients with more than one specific diagnosis, thus making it difficult to assess their therapeutic responses. (2) Additional large randomized controlled prospective trials are required to evaluate the safety and efficacy of immunosuppressive drugs, given that most studies have been retrospective and few studies have compared the efficacy of the various therapeutic agents, thus accounting for the lack of a standard definition of success for biologics. (3) Information about the risks and benefits of systemic treatment for AU not associated with systemic diseases is still scanty, especially for pediatric patients. (4) A critical assessment of the individual systemic therapies currently available is strictly related to a number of problems which have been recently reviewed [105] and include the small target population, the already-mentioned heterogeneous disease groups, the poorly defined phenotypes, and the diagnostic inconsistencies in the classification of the various types of uveitis between clinicians. (5) Delivery systems to administer potent drugs locally remain to be developed; their availability would avoid the risk of the severe complications that may occur following systemic treatment of patients whose disease is confined to the eye [106].
References
Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California epidemiology of uveitis study. Ophthalmology. 2004;111:491–500.
Prete M, Guerriero S, Dammacco R, Fatone MC, Vacca A, Dammacco F, Racanelli V. Autoimmune uveitis: a retrospective analysis of 104 patients from a tertiary reference center. J Ophthalmic Inflamm Infect. 2014;24(4):17. doi:10.1186/s12348-014-0017-9.
Khairallah M, Attia S, Zaouali S, et al. Pattern of childhood-onset uveitis in a referral center in Tunisia, North Africa. Ocul Immunol Inflamm. 2006;14:225–31.
Kitamei H, Kitaichi N, Namba K, Kotake S. Goda: clinical features of intraocular inflammation in Hokkaido, Japan. Acta Ophthalmol. 2009;87:424–8.
Keino H, Nakashima C, Watanabe T, et al. Frequency and clinical features of intraocular inflammation in Tokyo. Clin Exp Ophthalmol. 2009;37:595–601.
Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K, Auer H. Uveitis-a rare disease often associated with systemic diseases and infections-a systematic review of 2619 patients. Orphanet J Rare Dis. 2012;7:57.
Yang P, Zhang Z, Zhou H, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res. 2005;30:943–8.
Al-Mezaine HS, Kangave D, Abu El-Asrar AM. Patterns of uveitis in patients admitted to a University Hospital in Riyadh, Saudi Arabia. Ocul Immunol Inflamm. 2010;18:424–31.
Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Investig. 2010;120:3073–83.
Commodaro AG, Bueno V, Belfort R Jr, Rizzo LV. Autoimmune uveitis: the associated proinflammatory molecules and the search for immunoregulation. Autoimmun Rev. 2011;10:205–9.
Guerriero S, Giancipoli E, Ciraci L, et al. Masquerade syndrome of multicentre primary central nervous system lymphoma. Case Rep Ophthalmol Med. 2011;2011:329857.
Grange LK, Kouchouk A, Dalal MD, et al. Neoplastic masquerade syndromes in patients with uveitis. Am J Ophthalmol. 2014;157:526–31.
Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–5.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
Khairallah M. Are the Standardization of the Uveitis Nomenclature (SUN) Working Group criteria for codifying the site of inflammation appropriate for all uveitis problems? Limitations of the SUN Working Group classification. Ocul Immunol Inflamm. 2010;18:2–4.
Selmi C. Diagnosis and classification of autoimmune uveitis. Autoimmun Rev. 2014;13:591–4.
Mendes D, Correia M, Barbedo M, et al. Behcet’s disease—a contemporary review. J Autoimmun. 2009;32:178–88.
Ehrenfeld M. Geoepidemiology: the environment and spondyloarthropathies. Autoimmun Rev. 2010;9:A325–9.
Keck KM, Choi D, Savage LM, Rosenbaum JT. Insights into uveitis in association with spondyloarthritis from a large patient survey. J Clin Rheumatol. 2014;20:141–5.
Tugal-Tutkun I, Quartier P, Bodaghi B. Disease of the year: juvenile idiopathic arthritis-associated uveitis-classification and diagnostic approach. Ocul Immunol Inflamm. 2014;22(1):56–63.
Nashtaei EM, Soheilian M, Herbort CP, Yaseri M. Patterns of uveitis in the middle East and Europe. J Ophthalmic Vis Res. 2011;6:233–40.
Kazokoglu H, Onal S, Tugal-Tutkun I, et al. Demographic and clinical features of uveitis in tertiary centers in Turkey. Ophthalmic Epidemiol. 2008;15:285–93.
Kianersi F, Mohammadi Z, Ghanbari H, Ghoreyshi SM, Karimzadeh H, Soheilian M. Clinical patterns of uveitis in an Iranian tertiary eye-care center. Ocul Immunol Inflamm. 16 Apr 2014. (Epub ahead of print).
Pan J, Kapur M, McCallum R. Noninfectious immune-mediated uveitis and ocular inflammation. Curr Allergy Asthma Rep. 2014;14:409.
Brezin AP, Monnet D, Cohen JH, Levinson RD. HLA-A29 and birdshot chorioretinopathy. Ocul Immunol Inflamm. 2011;19:397–400.
Nussenblatt RB, Mittal KK, Ryan S, Green WR, Maumenee AE. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol. 1982;94:147–58.
Damico FM, Kiss S, Young LH. Sympathetic ophthalmia. Semin Ophthalmol. 2005;20:191–7.
Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008;67:955–9.
Lambert JR, Wright V. Eye inflammation in psoriatic arthritis. Ann Rheum Dis. 1976;35:354–6.
Accorinti M, Iannetti L, Liverani M, Caggiano C, Gilardi M. Clinical features and prognosis of HLA B27-associated acute anterior uveitis in an Italian patient population. Ocul Immunol Inflamm. 2010;18:91–6.
Monnet D, Breban M, Hudry C, Dougados M, Brezin AP. Ophthalmic findings and frequency of extraocular manifestations in patients with HLA-B27 uveitis: a study of 175 cases. Ophthalmology. 2004;111:802–9.
Loh AR, Acharya NR. Incidence rates and risk factors for ocular complications and vision loss in HLA-B27-associated uveitis. Am J Ophthalmol. 2010;150:534–42.
Yurdakul S, Yazici H. Behcet’s syndrome. Best Pract Res Clin Rheumatol. 2008;22:793–809.
Mendes D, Correia M, Barbedo M, et al. Behcet’s disease—a contemporary review. J Autoimmun. 2009;32:178–88.
Foeldvari I. Ocular involvement in juvenile idiopathic arthritis: classification and treatment. Clin Rev Allergy Immunol. 1 Aug 2014. (Epub ahead of print).
Ravelli A, Felici E, Magni-Manzoni S, Pistorio A, Novarini C, Bozzola E, Viola S, Martini A. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum. 2005;52(3):826–32.
Gregory AC 2nd, Kempen JH, Daniel E, Kaçmaz RO, Foster CS, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study Research Group: risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Study. Ophthalmology. 2013;120(1):186–92.
Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143(5):840–6.
Jamilloux Y, Kodjikian L, Broussolle C, Sève P. Sarcoidosis and uveitis. Autoimmun Rev. 2014;13:840–9.
Birnbaum AD, Huang W, Sahin O, Tessler HH, Goldstein DA. Ocular sarcoidosis misdiagnosed as primary intraocular lymphoma. Retina. 2010;30:310–6.
Pan D, Hirose T. Vogt-Koyanagi-Harada syndrome: review of clinical features. Semin Ophthalmol. 2011;26:312–5.
Sakata VM, da Silva FT, Hirata CE, de Carvalho JF, Yamamoto JH. Diagnosis and classification of Vogt-Koyanagi-Harada disease. Autoimmun Rev. 2014;13:550–5.
Mohsenin A, Huang JJ. Ocular manifestations of systemic inflammatory diseases. Conn Med. 2012;76:533–44.
Lim LL, Scarborough JD, Thorne JE, et al. Uveitis in patients with autoimmune hepatitis. Am J Ophthalmol. 2009;147:332–8.
Allegri P, Rissotto R, Herbort CP, Murialdo U. CNS diseases and uveitis. J Ophthalmic Vis Res. 2011;6:284–308.
Cury DB, Moss AC. Ocular manifestations in a community-based cohort of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1393–6.
Mollazadegan K, Kugelberg M, Tallstedt L, Ludvigsson JF. Increased risk of uveitis in coeliac disease: a nationwide cohort study. Br J Ophthalmol. 2012;96:857–61.
Moorthy RS, London NJ, Garg SJ, Cunningham ET Jr. Drug-induced uveitis. Curr Opin Ophthalmol. 2013;24:589–97.
London NJ, Garg SJ, Moorthy RS, Cunningham ET. Drug-induced uveitis. J Ophthalmic Inflamm Infect. 2013;25(3):43. doi:10.1186/1869-5760-3-43.
Cordero-Coma M, Salazar-Mendez R, Garzo-Garcia I, et al. Drug-induced uveitis. Expert Opin Drug Saf. 2015;14(1):111–26.
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45.
Lee RW, Dick AD. Current concepts and future directions in the pathogenesis and treatment of non-infectious intraocular inflammation. Eye. 2012;26:17–28.
Horai R, Caspi RR. Cytokines in autoimmune uveitis. J Interferon Cytokine Res. 2011;31:733–44.
Sugita S, Takase H, Taguchi C, et al. Ocular infiltrating CD4 + T cells from patients with Vogt-Koyanagi-Harada disease recognize human melanocyte antigens. Investig Ophthalmol Vis Sci. 2006;47:2547–54.
Damico FM, Cunha-Neto E, Goldberg AC, et al. T-cell recognition and cytokine profile induced by melanocyte epitopes in patients with HLA-DRB1*0405-positive and -negative Vogt-Koyanagi-Harada uveitis. Investig Ophthalmol Vis Sci. 2005;46:2465–71.
Agarwal RK, Silver PB, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Biol. 2012;900:443–69.
Yu CR, Lee YS, Mahdi RM, Surendran N, Egwuagu CE. Therapeutic targeting of STAT3 (signal transducers and activators of transcription 3) pathway inhibits experimental autoimmune uveitis. PLoS ONE. 2012;7:e29742.
Qin T. Upregulation of DR3 expression in CD4(+) T cells promotes secretion of IL-17 in experimental autoimmune uveitis. Mol Vis. 2011;17:3486–93.
Jawad S, Liu B, Agron E, Nussenblatt RB, Sen HN. Elevated serum levels of interleukin-17A in uveitis patients. Ocul Immunol Inflamm. 2013;21:434–9.
Yoshimura T, Sonoda KH, Miyazaki Y, et al. Differential roles for IFN-gamma and IL-17 in experimental autoimmune uveoretinitis. Int Immunol. 2008;20:209–14.
Caspi RR. Understanding autoimmunity in the eye: from animal models to novel therapies. Discov Med. 2014;17:155–62.
Chang JH, McCluskey P, Wakefield D. Expression of toll-like receptor 4 and its associated lipopolysaccharide receptor complex by resident antigen-presenting cells in the human uvea. Investig Ophthalmol Vis Sci. 2004;45:1871–8.
Pratap DS, Lim LL, Wang JJ, et al. The role of toll-like receptor variants in acute anterior uveitis. Mol Vis. 2011;17:2970–7.
Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90:103–8.
Wang J, Lu H, Hu X, et al. Nuclear factor translocation and acute anterior uveitis. Mol Vis. 2011;17:170–6.
Grajewski RS, Silver PB, Agarwal RK, et al. Endogenous IRBP can be dispensable for generation of natural CD4+ CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. J Exp Med. 2006;203:851–6.
Keino H, Takeuchi M, Usui Y, et al. Supplementation of CD4+ CD25+ regulatory T cells suppresses experimental autoimmune uveoretinitis. Br J Ophthalmol. 2007;91:105–10.
Nanke Y, Kotake S, Goto M, Ujihara H, Matsubara M, Kamatani N. Decreased percentages of regulatory T cells in peripheral blood of patients with Behcet’s disease before ocular attack: a possible predictive marker of ocular attack. Mod Rheumatol. 2008;18:354–8.
Chen L, Yang P, Zhou H, et al. Diminished frequency and function of CD4 + CD25high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome. Investig Ophthalmol Vis Sci. 2008;49:3475–82.
Sun M, Yang P, Du L, Zhou H, Ren X, Kijlstra A. Contribution of CD4+ CD25+ T cells to the regression phase of experimental autoimmune uveoretinitis. Investig Ophthalmol Vis Sci. 2010;51:383–9.
Ruggieri S, Frassanito MA, Dammacco R, Guerriero S. Treg lymphocytes in autoimmune uveitis. Ocul Immunol Inflamm. 2012;20:255–61.
Manickam B, Jha P, Matta B, Liu J, Bora PS, Bora NS. Inhibition of complement alternative pathway suppresses experimental autoimmune anterior uveitis by modulating T cell responses. J Biol Chem. 2011;286:8472–80.
Manickam B, Jha P, Hepburn NJ, et al. Suppression of complement activation by recombinant Crry inhibits experimental autoimmune anterior uveitis (EAAU). Mol Immunol. 2010;48:231–9.
Read RW, Szalai AJ, Vogt SD, McGwin G, Barnum SR. Genetic deficiency of C3 as well as CNS-targeted expression of the complement inhibitor sCrry ameliorates experimental autoimmune uveoretinitis. Exp Eye Res. 2006;82:389–94.
Dick AD, Broderick C, Forrester JV, Wright GJ. Distribution of OX2 antigen and OX2 receptor within retina. Investig Ophthalmol Vis Sci. 2001;42:170–6.
Copland DA, Calder CJ, Raveney BJ, et al. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am J Pathol. 2007;171:580–8.
Yang MM, Lai TY, Tam PO, et al. Complement factor H and interleukin gene polymorphisms in patients with non-infectious intermediate and posterior uveitis. Mol Vis. 2012;18:1865–72.
Yang MM, Lai TY, Tam PO, et al. Association of C2 and CFB polymorphisms with anterior uveitis. Investig Ophthalmol Vis Sci. 2012;53:4969–74.
Whitcup SM, DeBarge LR, Caspi RR, Harning R, Nussenblatt RB, Chan CC. Monoclonal antibodies against ICAM-1 (CD54) and LFA-1 (CD11a/CD18) inhibit experimental autoimmune uveitis. Clin Immunol Immunopathol. 1993;67:143–50.
Devine L, Lightman SL, Greenwood J. Role of LFA-1, ICAM-1, VLA-4 and VCAM-1 in lymphocyte migration across retinal pigment epithelial monolayers in vitro. Immunology. 1996;88:456–62.
Martin AP, de Moraes LV, Tadokoro CE, et al. Administration of a peptide inhibitor of alpha4-integrin inhibits the development of experimental autoimmune uveitis. Investig Ophthalmol Vis Sci. 2005;46:2056–63.
Luna JD, Chan CC, Derevjanik NL, et al. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-1beta-mediated breakdown. J Neurosci Res. 1997;49:268–80.
Tappeiner C, Heinz C, Specker C, Heiligenhaus A. Rituximab as a treatment option for refractory endogenous anterior uveitis. Ophthalmic Res. 2007;39:184–6.
Davatchi F, Shams H, Rezaipoor M, et al. Rituximab in intractable ocular lesions of Behcet’s disease; randomized single-blind control study (pilot study). Int J Rheum Dis. 2010;13:246–52.
Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab). Rheumatology. 2011;50:1390–4.
Larson T, Nussenblatt RB, Sen HN. Emerging drugs for uveitis. Expert Opin Emerg Drugs. 2011;16:309–22.
Gomes BM, Sepah YJ, Do DV, et al. New treatment options for noninfectious uveitis. Dev Ophthalmol. 2012;51:134–61.
Samson CM, Waheed N, Baltatzis S, Foster CS. Methotrexate therapy for chronic non-infectious uveitis: analysis of a case series of 160 patients. Ophthalmology. 2001;108:1134–9.
Durrani K, Papaliodis GN, Foster CS. Pulse IV cyclophosphamide in ocular inflammatory disease: efficacy and short-term safety. Ophthalmology. 2004;111:960–5.
Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. 2009;148:500–9.
Tappeiner C, Roesel M, Heinz C, Michels H, Ganser G, Heiligenhaus A. Limited value of cyclosporine A for the treatment of patients with uveitis associated with juvenile idiopathic arthritis. Eye. 2009;23:1192–8.
Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116:2188–98.
Kacmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117:576–84.
Pujari SS, Kempen JH, Newcomb CW, et al. Cyclophosphamide for ocular inflammatory diseases. Ophthalmology. 2010;117:356–65.
Daniel E, Thorne JE, Newcomb CW, et al. Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol. 2010;149:423–32.
Goebel JC, Roesel M, Heinz C, Michels H, Ganser G, Heiligenhaus A. Azathioprine as a treatment option for uveitis in patients with juvenile idiopathic arthritis. Br J Ophthalmol. 2011;95:209–13.
Simonini G, Taddio A, Cattalini M, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res. 2011;63:612–8.
Okada AA, Goto H, Ohno S, Mochizuki M. Multicenter study of infliximab for refractory uveoretinitis in Behcet disease. Arch Ophthalmol. 2012;130:592–8.
Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119:1575–81.
Dobner BC, Max R, Becker MD, et al. A three-centre experience with adalimumab for the treatment of non-infectious uveitis. Br J Ophthalmol. 2013;97:134–8.
Davatchi F, Shams H, Shahram F, et al. Methotrexate in ocular manifestations of Behcet’s disease: a longitudinal study up to 15 years. Int J Rheum Dis. 2013;16:568–77.
Kruh JN, Yang P, Suelves AM, Foster CS. Infliximab for the treatment of refractory noninfectious uveitis: a study of 88 patients with long-term follow-up. Ophthalmology. 2014;121:358–64.
Gallego-Pinazo R, Dolz-Marco R, Martínez-Castillo S, Arévalo JF, Díaz-Llopis M. Update on the principles and novel local and systemic therapies for the treatment of non-infectious uveitis. Inflamm Allergy Drug Targets. 2013;12:38–45.
Yates WB, McCluskey PJ, Wakefield D. Are patients with inflammatory eye disease treated with systemic immunosuppressive therapy at increased risk of malignancy? J Ophthalmic Inflamm Infect. 2013;3(48):2013. doi:10.1186/1869-5760-3-48.
Denniston AK, Dick AD. Systemic therapies for inflammatory eye disease: past, present and future. BMC Ophthalmol. 2013;13:18. doi:10.1186/1471-2415-13-18.
Lin P, Suhler EB, Rosenbaum JT. The future of uveitis treatment. Ophthalmology. 2014;121:365–76.
Acknowledgments
The authors are grateful to Mrs. Laura Di Pietro for her excellent secretarial assistance. This work was supported by grants from the University of Bari and the Italian Association for Cancer Research (AIRC), Milano.
Conflict of interest
All authors declare that they have no financial relationships with any organizations that might have an interest in the submitted work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prete, M., Dammacco, R., Fatone, M.C. et al. Autoimmune uveitis: clinical, pathogenetic, and therapeutic features. Clin Exp Med 16, 125–136 (2016). https://doi.org/10.1007/s10238-015-0345-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0345-6