Abstract
Serum microRNA-21 (miR-21) expression has been shown to be significantly up-regulated in breast cancer, which implies that it could be a biomarker to discriminate breast cancer patients from healthy controls. We therefore performed this meta-analysis to assess the diagnostic value of miR-21 for breast cancer. Relevant articles were collected from PubMed, Scopus, Embase, the Cochrane Library, BioMed Central, ISI Web of Knowledge, China National Knowledge Infrastructure, Wan Fang Data and Technology of Chongqing databases, from inception to June 10, 2014 by two independent researchers. Diagnostic capacity of miR-21 for breast cancer was assessed using pooled sensitivity and specificity, diagnostic odds ratio (DOR), area under the summary receiver operating characteristic (AUC) and Fagan’s nomogram. Meta-Disc software and Stata SE 12.0 were used to investigate the source of heterogeneity and to perform the meta-analysis. We used six studies with a total of 438 patients and 228 healthy controls in this meta-analysis. The pooled sensitivity, specificity and DOR were 0.79 [95 % confidence interval (CI) 0.66–0.87], 0.85 (95 % CI 0.75–0.91) and 19.46 (95 % CI 8.74–43.30), respectively; positive and negative likelihood ratios were 5 and 0.25, and AUC was 0.89 (95 % CI 0.86–0.91). In addition, heterogeneity was clearly apparent but was not caused by the threshold effect. This meta-analysis suggests that miR-21 is a potential biomarker for early diagnosis of breast cancer with high sensitivity and specificity, and its clinical application warrants further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is a common malignancy in women. It is one of the three most common cancers in the USA [1]. An estimated 522,000 females died from breast cancer globally during 2012, and it is the leading cause of cancer-related deaths for females in some Asia-Pacific countries [2]. However, the causes of BC are quite complex and heterogeneous [3]. Currently, mammography screening is a major public health intervention and is widely used in many westernized countries for early detection of breast cancer. For example, in Germany, the mammography screening program has served women aged 50–69 years since 2008 [2, 4]. Although mammography screening can reduce breast cancer mortality, overdiagnosis can lead to increased radiation. Screening for disease in healthy people inevitably leads to other risks such as false positives and psychological duress, and the long-term outcome for women is unknown [4–6]. Therefore, a simple and minimally invasive diagnostic method for BC is needed. Although some tumor biomarkers, such as carcinoembryonic antigen, cancer antigen 153 and tissue polypeptide antigen are widely used to screen BC, these single tumor markers have low sensitivity and specificity for early-stage BC [7–10].
MicroRNAs (miRNAs) are endogenous single-stranded noncoding RNA molecules, ~23 nucleotides in length. Found widely in animals and plants, miRNAs regulate gene expression by pairing to mRNAs of protein-coding genes [11]. Many studies have reported that miRNA dysregulation can affect cancer initiation, invasion and metastasis [12, 13]. Abnormal miRNA expressions have been found in a variety of human solid tumors. Furthermore, as extracellular miRNAs can circulate in body fluids, circulating miRNAs show great promise for diagnosis and prognosis of cancer [14]. miRNAs as biomarkers have significant advantages over conventional biomarkers, including minimal invasiveness, stability and high predictive value [15, 16].
MiR-21 is one of the most commonly studied oncomiRNAs. It has played a significant role in diagnosis of lung carcinoma, gastric cancer and colorectal cancer [17–19] and is reportedly up-regulated in serum of BC patients compared with healthy controls. miR-21 could therefore potentially serve as an indicator of BC [15, 20]. On the other hand, the study of Wang et al. [21] showed that serum miR-21 levels were associated with hormone receptor status and histologic grade. However, other studies reported no significant association between serum miR-21 expression and clinicopathologic features such as hormone receptors, histologic grade and lymph node metastasis [22–25]. Thus, the relationship among serum miR-21, BC diagnosis and other factors needed to be clarified beyond the limits of these single studies. We therefore designed this systematic review and meta-analysis to confirm whether miR-21 could serve as a diagnostic marker for BC.
Methods
Search strategy
Two reviewers independently searched several databases, including PubMed, Scopus, Embase, the Cochrane Library, BioMed Central, ISI Web of Knowledge, China National Knowledge Infrastructure, Wan Fang Data and Technology of Chongqing databases. The following search terms were used to retrieve articles and abstracts: (miR-21 or microRNA-21 or has-miR-21) and (breast or mammary) and (cancer or cancers or tumor or neoplasm or carcinoma) and (plasma or serum or sera or serums or blood). We conducted a computerized search between inception and June 10, 2014. Publication languages were limited to English or Chinese.
Study selection and exclusion criteria
Further eligibility criteria for this meta-analysis included (1) all the patients with BC must have been confirmed by pathological examination; (2) miR-21 expression was measured by real-time polymerase chain reaction or real-time quantification PCR (RT-qPCR) method; (3) healthy controls had no history of cancer; (4) the study included clear sensitivity, specificity and cutoff values and described how they were derived; and (5) all blood samples were collected for miR-21 analysis before any treatment.
We excluded duplicate publications; studies with insufficient data; meeting, review and meta-analysis articles; animal and cell studies; and studies with fewer than 30 patients.
Data extraction and quality assessment
Two independent reviewers screened publications for the following information: first author, publication year, disease type, ethnicity and number of patients and controls, cutoff values, and true and false positives and negatives. We contacted corresponding authors to obtain any missing information, if they did not respond, their study was excluded. We used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist to assess the quality of the studies we selected [26].
Statistical analysis
We evaluated the diagnostic value of miR-21 by calculating the pooled sensitivity, pooled specificity, DOR and corresponding 95 % CI. Summary receiver operator characteristics (SROC) and DOR were used to evaluate the performance of diagnostic tests. Fagan’s nomogram was used to describe the diagnosis value of miR-21 for BC. Funnel plots with Begg’s test and Egger’s test were performed to test publication bias. The Spearman correlation coefficient was used to test the diagnostic threshold effect, which may produce significant heterogeneity. We performed meta-regression to explore sources of heterogeneity. Finally, we conducted sensitivity analysis to assess whether this meta-analysis especially depended on one study. All statistical analyses used Meta-Disc statistical software [27] and Stata SE12.0 (Stata Corporation).
Results
Study selection and quality assessment
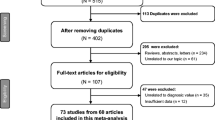
Our study flowchart is illustrated in Fig. 1. We retrieved a total of 292 articles after searching the above databases, and excluded 259 articles, of which 114 were duplicated, 59 were reviews, meta-analyses or meeting reports, and 86 were not relevant. After screening full texts of the remaining 33 articles, we excluded 27 articles that failed to satisfy our inclusion criteria, of which 15 failed to meet our diagnostic criteria, seven were based on tissue samples and five did not include complete data. Finally, six high-quality articles were used in this meta-analysis [22–25, 28, 29].
The six studies used in our study included a total of 438 BC patients and 228 healthy controls, and all diagnoses were confirmed independently by at least two pathologists. The studies’ first authors, years of publication, subject ethnicities, numbers of patients and healthy controls, cutoff values, reference controls, RNA extraction kits, sensitivity and specificity are shown in Table 1. The QUADAS scores of studies ranged from 11 to 13, which indicates that the quality of the included studies were satisfactory.
Data analysis
A forest plot of sensitivity and specificity of miR-21 is shown in Fig. 2. Pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) were 0.79 (95 % CI 0.66–0.87), 0.85 (95 % CI 0.75–0.91), 4.77 (95 % CI 2.76–8.27) and 0.27 (95 % CI 0.17–0.41), respectively, which indicates that miR-21 is a great indicator for BC diagnosis. However, the I 2 value of sensitivity, specificity, PLR and NLR were 85.11 % (95 % CI 74.41–95.81 %; P = 0.00), 70.36 % (95 % CI 45.20–95.51; P = 0.00), 63.4 % (P = 0.018) and 80.8 % (P = 0.000), indicating significant heterogeneity in our study, we therefore selected the random effects model.
Forest plots of sensitivity and specificity for miR-21 test in breast cancer. a The pooled sensitivity was 0.79 (95 % CI 0.66–0.87; I 2 = 85.11 %, n = 6); b The pooled specificity was 0.85 (95 % CI 0.75–0.91; I 2 = 70.36 %, n = 6); c The pooled PLR was 4.77 (95 % CI 2.76–8.27; I 2 = 63.4 %, n = 6); d The pooled NLR was 0.27 (95 % CI 0.17–0.41; I 2 = 80.8 %, n = 6)
Diagnostic accuracy was evaluated by the pooled DOR and the area under the curve (AUC), which were 19.46 (95 % CI 8.74–43.30; Fig. 3) and 0.89 (95 % CI 0.86–0.91; Fig. 4), respectively, indicating that miR-21 has high diagnostic accuracy for BC.
Analysis diagnostic threshold effect
Threshold effect is an important cause of heterogeneity in diagnostic tests and is indicated by a “shoulder–arm”-shaped distribution in the SROC curve. The SROC curve (Fig. 4) showed no “shoulder–arm”-shaped distribution. The corresponding Spearman correlation coefficient was 0.314 (P = 0.544), suggesting that there was no threshold effect.
Meta-regression analysis
As the forest plot indicated obvious heterogeneity in the six studies, we performed a meta-regression analysis to investigate the sources of this heterogeneity. We selected ethnicity, RNA extraction kits, reference controls and measurements to confirm sources of heterogeneity, but the data showed no significant heterogeneity among these factors.
Publication bias
We used a funnel plot to test for publication bias (Fig.S1). The shape of the funnel plot showed no significant asymmetry. Begg’s test and Egger’s test were also performed to estimate publication bias, and their results were 0.452 and 0.223, respectively, which indicate no significant publication bias appeared. However, considering the limited number of studies, publication bias still may exist in the present study.
Clinical utility and index test
Fagan’s nomogram was used to describe the diagnosis value of miR-21 for BC (Fig. 5). When 20 % was selected as the pretest probability, the data showed posttest probability to increase to 56 %, the PLR of 5 indicates that a person with BC is five times more likely to have a positive diagnosis than a healthy woman. Similarly, the probability would decrease to 6 %, and the NLR was 0.25, suggesting that miR-21 is a promising indicator for the diagnosis of BC.
Discussion
BC is a common malignancy in women. Since early diagnosis is associated with long-term survival and decreased mortality, efforts to promote early detection continue to be the major focus in fighting BC. However, there are few biomarkers suitable for large-scale screening or early diagnosis. By now, carcinoembryonic antigen, cancer antigen 153 and tissue polypeptide antigen are widely used to screen BC, however, because of these single tumor markers with low sensitivity and specificity, the diagnostic effect for early-stage BC is compromised [7–10]. Recently, many studies found that circulating miRNAs exhibit altered expression in patients with cancer. Dysregulated expression of miRNAs plays an important role in the pathogenesis, metastasis and prognosis for BC patients [30, 31]. Some reports even imply that miRNAs have potential therapy uses [32]. Compared with healthy controls, patients with BC have higher serum miR-21 expression. Thus, miRNA is a potential biomarker for diagnosis and prognosis for BC [33, 34]. In this study, we used a meta-analysis to show the diagnostic value of miR-21 for BC.
In this meta-analysis, pooled sensitivity was 0.79 (95 % CI 0.66–0.87) and pooled specificity was 0.85 (95 % CI 0.75–0.91), suggesting its potential diagnostic capability. The area under SROC (AUC) and DOR were used to represent diagnostic test performance. The value of DOR ranged from 0 to infinity, and higher values indicate better test discrimination [35]. The ideal SROC curve position is near the upper-left corner, which would indicate a perfect test [36]. The DOR and AUC of miR-21 were 19.46 (95 % CI 8.74–43.30) and 0.89 (95 % CI 0.86–0.91), respectively, indicating miR-21 has excellent test performance.
Exploring the sources of heterogeneity is critical to a meta-analysis. Our test clearly shows heterogeneity in our study, and we attempted to explain its sources. Threshold effect is a primary cause of heterogeneity in test accuracy studies [27], but the Spearman correlation coefficient for the present study was 0.314 (P = 0.544), which suggests that the threshold effect was not a factor here. Sensitivity analysis was next used to see if the heterogeneity came from any individual study. It indicated obvious influence came from the study of Sun et al. [24], When Sun study was removed, the I 2 of specificity, PLR and DOR were 0.0 %, which indicated no further heterogeneity in the other five studies, indicating that the Sun study was one source of heterogeneity. A meta-regression was implemented to explore other factors that caused heterogeneity. In our study, RT-qPCR was widely used to test miR-21 expression. However, different studies used different measures to extract and quantify miR-21, such as different RNA extraction kits, reference controls and RNA measurement methods, all which may influence the heterogeneity. Unfortunately, we failed to find other sources.
MiR-21 appears to be a diagnostically valuable biomarker for BC. However, our meta-analysis has several limitations. First, as the diagnostic value of miR-21 has been explored only very recently, sample sizes have been rather small—for example, the study of Li et al. [29] included only 33 BC patients. As a result, a small-study effect may appear. Second, to the best of our knowledge, no publication bias in English or Chinese used Begg’s test, Egger’s test or Deeks’ funnel plot (although our limitations to English or Chinese language may have led to a publication bias). Also, the study of Sota et al. had two cutoff values [22], we selected the one with higher sensitivity and specificity, which may also have led to bias. Third, our explanation of associations between serum miR-21 expression levels and clinicopathologic features (Table S1) have been constrained by the limited number and size of available studies.
In conclusion, as a novel minimally invasive biomarker, miR-21 shows great potential in early diagnosis for BC and warrants further study to explore its clinical application.
References
DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–71. doi:10.3322/caac.21235.
Danny RY, Susanna MC, Cheng HY, Baade PD, et al. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med. 2014;11:101–15. doi:10.7497/j.issn.2095-3941.2014.02.005.
James SL, Water HG, Noel JW. Viruses and human breast cancer. Future microbiol. 2006;1(1):33–51.
Ismail J, John RB. Novel approaches to the diagnosis and treatment of breast cancer. Future Oncol. 2014;10(4):515–8.
Heywang-Kobrunner SH, Hacher A, Sedlacek S. Advantages and disadvantages of mammography screening. Breast Care. 2011;6:199–207. doi:10.1159/000329005.
Euler-Chelpin MV, Louise MR, Brian LT, et al. Risk of breast cancer after false-positive test results in screening mammography. J Natl Cancer Inst. 2012;104(9):682–9. doi:10.1093/jnci/djs176.
Mahendar P, Nagulu M, Uday KV, et al. evaluation of tumor markers in southern Indian breast cancer patients. Asian Pac J Cancer Prev. 2010;11:157–9.
Fiorella G, Patrizia F, Sandro C, et al. A re-evaluation of carcinoembryonic antigen (CEA) as a serum marker for breast cancer : a prospective longitudinal study. Clin Cancer Res. 2001;7:2357–62.
Nicolini A, Colombini C, Luciani L, et al. Evaluation of serum CA15-3 determination with CEA and TPA in the post-operative follow-up of breast cancer patients. Br J Cancer. 1991;64:154–8.
Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52(3):345–51. doi:10.1373/clinchem.2005.059832.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi:10.1016/j.cell.2009.01.002.
Weiland M, Gao XH, Zhou L, et al. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol. 2012;9(6):850–9. doi:10.4161/rna.20378.
Zhang J, Zhao H, Gao Y, et al. Secretory miRNAs as novel cancer biomarkers. Biochim Biophys Acta. 2012;1826(1):32–43. doi:10.1016/j.bbcan.2012.03.001.
Shen J, Stass SA, Jiang F. MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett. 2013;329(2):125–36. doi:10.1016/j.canlet.2012.11.001.
Heneghan HM, Miller N, Lowery AJ, et al. Circulating micrornas as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251(3):499–505. doi:10.1097/SLA.0b013e3181cc939f.
Patrick SM, Rachael KP, Evan MK, et al. Circulating microRNAs as stable blood-basedmarkers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–8. doi:10.1073/pnas.0804549105.
Li T, Leong MH, Harms B, et al. MicroRNA-21 as a potential colon and rectal cancer biomarker. World J Gastroenterol. 2013;19(34):5615–21. doi:10.3748/wjg.v19.i34.5615.
Yang XR, Du Gao YN, et al. Serum microRNA-21 as a diagnostic marker for lung carcinoma: a systematic review and meta-analysis. PloS One. 2013;9(5):e97460. doi:10.1371/journal.pone.0097460 eCollection 2014.
Zeng ZY, Wang JG, Zhao LY, et al. Potential role of microRNA-21 in the diagnosis of gastric cancer: a meta-analysis. PLoS One. 2013;8(9):e73278. doi:10.1371/journal.pone.0073278.
Si HY, Sun XM, Chen YJ, et al. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol. 2013;139(2):223–9. doi:10.1007/s00432-012-1315-y.
Wang F, Zheng Z, Guo J, et al. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol Oncol. 2010;119(3):586–93. doi:10.1016/j.ygyno.2010.07.021.
Asaga S, Kuo C, Nguyen T, et al. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57(1):84–91. doi:10.1373/clinchem.2010.151845.
Gao J, Zhang Q, Xu J, et al. Clinical significance of serum miR-21 in breast cancer compared with CA153 and CEA. Chin J Cancer Res. 2013;25(6):743–8. doi:10.3978/j.issn.1000-9604.2013.12.04.
Sun Y. Novel serum biomarkers in breast cancer : detection and clinical significance [Master]: National Center for Clinical Laboratory. 2012.
Wang B, Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol. 2012;138(10):1659–66. doi:10.1007/s00432-012-1244-9.
Penny W, Anne WR, Johannes BR, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3(25).
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6(31). doi:10.1186/1471-2288-6-31.
Mar-Aguilar F, Mendoza-Ramirez JA, Malagon-Santiago I, et al. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Marker. 2013;34(3):163–9. doi:10.3233/dma-120957.
Li XF, Xu JJ, Zhang QY. Establishment of real-time PCR for detecting serum micriRNA-21 and its preliminary application in breast cancer. Chin J Lab Med. 2011;34(10):920–5.
Götte M. MicroRNAs in breast cancer pathogenesis. Minerva Ginecol. 2010;62(6):559–71.
Rahul S, Berna SS, Alex HM, et al. MicroRNA control of invasion and metastasis pathways. Front Genet. 2011;2:1–5. doi:10.3389/fgene.2011.00058.
Marilena V, Iorio PC, Piovan C, Braccioli L, Tagliabue E. Breast cancer and microRNAs: therapeutic impact. Breast. 2011;20(S3):S63–70. doi:10.1016/S0960-9776(11)70297-1.
Aoife JL, Nicola M, Roisin EM, et al. MicroRNAs as prognostic indicators and therapeutic targets: potential effect on breast cancer management. Clin cancer Res. 2008;14:360–5. doi:10.1158/1078-0432.CCR-07-0992.
Cathy AA, Brian MN, Thompson EA, et al. MicroRNA signatures: clinical biomarkers for the diagnosis and treatment of breast cancer. Cell. 2011;17(6):313–9. doi:10.1016/j.molmed.2011.01.006.
Afina S, Jeroen GL, Martin HP, Bossuyt PM, et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–35. doi:10.1016/S0895-4356(03)00177-X.
Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2002;21:1237–56. doi:10.1002/sim.1099.
Acknowledgments
The authors would like to thank Bin Qin for supporting more datum that not reported in their articles. And thanks to the anonymous reviewers for their suggestions to improve the quality of the paper.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, S., Yang, X., Yang, J. et al. Serum microRNA-21 as a potential diagnostic biomarker for breast cancer: a systematic review and meta-analysis. Clin Exp Med 16, 29–35 (2016). https://doi.org/10.1007/s10238-014-0332-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-014-0332-3